Implications
An early and high intake of colostrum is a major determinant of piglet survival in the first days after birth and is essential for ensuring the health and growth of piglets at least until weaning. However, both colostrum yield and composition are highly variable among sows. Better knowledge of endocrine and nutritional factors that participate in this variability is needed to define new strategies to manage reproductive sows to increase yield and quality of colostrum.
Introduction
Colostrum intake is essential for piglet survival within the few days after birth and for piglet health and growth performance until weaning (Quesnel et al., Reference Quesnel, Farmer and Devillers2012). Long-term effects were also reported, with colostrum intake being positively associated with piglet weight at weaning, at onset and end of the fattening period and being negatively correlated with piglet mortality during the nursery period (Declerck et al., Reference Declerck, Dewulf, Sarrazin and Maes2016). Colostrum provides newborn piglets with the energy necessary for thermoregulation and body growth, with passive immunity needed for protection against pathogens, and with growth factors that stimulate growth and maturation of tissues and organs, especially the gastrointestinal tract (Quesnel et al., Reference Quesnel, Farmer and Devillers2012). By transferring bioactive factors from the mother to the offspring, colostrum is crucial for the process of lactocrine signaling, which has an impact on the offspring much beyond the neonatal period. Bioactive components of colostrum include, for example, proteins, growth factors, peptides, oligosaccharides, fatty acid-derived molecules, steroids and microRNAs. The most striking example of a lactocrine-active factor is relaxin, which is a peptide hormone found in sow colostrum, that plays an important role in developmental programing of the female reproductive tract until puberty (Bartol et al., Reference Bartol, Wiley, George, Miller and Bagnell2017).
In swine, colostrum can be defined as the first secretion of the mammary glands excreted for approximately 24 h after the onset of parturition. In both swine and ruminants, the major difference between colostrum and milk resides in the high concentrations of colostral immunoglobulins (Ig), specifically immunoglobulins G (IgG) in swine (and IgG1 in ruminants). Immunoglobulins A, although more abundant in colostrum than in milk, are not specific to colostrum and are therefore also present in milk throughout lactation. The composition of sow colostrum, especially in terms of IgG content, is highly variable among sows (Quesnel, Reference Quesnel2011). Sow colostrum yield is estimated by summing the colostrum intakes of each piglet in the litter; it thus depends on the prediction model used to estimate individual colostrum intake. Based on Devillers’ model (Devillers et al., Reference Devillers, van Milgen, Prunier and Le Dividich2004), colostrum yield averages 3.5 kg and ranges from <1 kg to >6.0 kg (Quesnel et al., Reference Quesnel, Farmer and Devillers2012). Based on Theil’s model (Theil et al., Reference Theil, Flummer, Hurley, Kristensen, Labouriau and Sørensen2014a), it averages 6.0 kg with a range from 2.8 up to 8.5 kg (Vadmand et al., Reference Vadmand, Krogh, Hansen and Theil2015). Nevertheless, regardless of the method used to estimate colostrum yield, it is greatly variable. The amount of colostrum needed by an average piglet (weighing 1.4 kg at birth) was estimated at 250 g, or 180 g/kg birth weight (calculated with Devillers’ model; Quesnel et al., Reference Quesnel, Farmer and Devillers2012). This amount would significantly reduce the risk of mortality, provide passive immunity and allow a slight weight gain. Based on this recommendation, Quesnel et al. (Reference Quesnel, Farmer and Devillers2012) estimated that at least one-third of sows do not produce enough colostrum to fulfill the needs of their litter.
The amount and composition of colostrum produced can be influenced by sow characteristics, such as endocrine status, nutritional status, immune status and level of stress, and by litter characteristics, especially vitality at birth. Environmental factors also could alter colostrum yield and composition through effects on the sows (e.g., heat stress is likely to reduce feed intake and cortisol concentration in sows). The complex interactions between these factors make it difficult to decipher their respective influence in the regulation of colostrogenesis. The present review will focus on the effects of sow endocrine status and nutrition because hormones play a major role in regulating colostrum synthesis, and nutrition could be a tool to manipulate colostrum yield and composition in herds.
When does colostrogenesis take place?
In the present review paper, colostrogenesis is defined as englobing both the synthesis of milk-specific constituents and the transfer of IgG into lacteal secretions. However, in order to efficiently investigate strategies to alter colostrogenesis in sows, it is imperative to know precisely when these two events occur. The synthesis of milk components takes place during late gestation, with the first lipid droplets being visible at 105 days of gestation (the gestational term of the sow is 115 days) (Kensinger et al., Reference Kensinger, Collier and Bazer1986) and the synthesis of lactose starting not before 3 to 4 days before parturition (Hartmann et al., Reference Hartmann, Whitely and Willcox1984). The transfer of IgG from sow plasma to lacteal secretions slowly begins approximately 10 days before farrowing, increases 3 or 4 days before farrowing and is maximal on the day of farrowing (Huang et al., Reference Huang, Hu, Hasler-Rapacz and Rapacz1992). This suggests that colostrum synthesis, when defined by IgG transfer, is not entirely finished before parturition in sows. This is somewhat different from what occurs in dairy cows, with IgG1 transfer beginning several weeks before parturition, peaking a week prepartum and ceasing abruptly before calving (Barrington et al., Reference Barrington, McFadden, Huyler and Besser2001). Nevertheless, recent findings suggest that the transfer of IgG in dairy cows can persist at least until parturition (Gross et al., Reference Gross, Kessler, Bjerre-Harpoth, Dechow, Baumrucker and Bruckmaier2014).
During colostrogenesis, tight junctions between mammary epithelial cells (MECs) are ‘leaky,’ thereby allowing the paracellular transfer of constituents from maternal plasma to the alveolar compartment, notably of hormones, growth factors and Ig. The uptake of IgG by mammary glands in pigs could also be mediated by a Fc-specific receptor, the neonatal Fc receptor (FcRn), as it is the case in ruminants (Barrington et al., Reference Barrington, McFadden, Huyler and Besser2001). Expression of the FcRn protein was demonstrated in porcine mammary tissue in the peripartum period, from 3 days before up to 1 day after parturition (Schnulle and Hurley, Reference Schnulle and Hurley2003; Palombo et al., Reference Palombo, Loor, D’Andrea, Vailati-Riboni, Shazad, Krogh and Theil2018). However, the role of the FcRn in IgG transfer has not been demonstrated in swine. The uptake of IgA in a polymeric form (pIgA) could also be achieved via transcytosis mediated by the polymeric immunoglobulin receptor (pIgR), whose transcript was shown in sow’s mammary tissue (Schnulle and Hurley, personal communication). The relative importance of the passive v. the active transport of IgG and IgA transfer into sow colostrum is not known.
The composition of colostrum gradually changes between the onset of parturition (T0) and 24 h later (T24; Foisnet et al., Reference Foisnet, Farmer, David and Quesnel2010a). Lactose and fat contents increase while protein content decreases because of the drop in IgG and IgA concentrations (Theil et al., Reference Theil, Lauridsen and Quesnel2014b). In parallel, concentrations of K increase while Na concentrations decrease, as well as the Na:K ratio (Quesnel, Reference Quesnel2011). The decrease in the Na:K ratio is indicative of the development of tight junctions between cells in the mammary epithelium. Concentrations of IgG in colostrum at T24 are positively correlated with the Na:K ratio in colostrum at T24, whereas the drop in IgG concentrations in colostrum between T0 and T24 is negatively correlated to that same variable (Quesnel, Reference Quesnel2011). These relations are consistent with the fact that the cessation of IgG transfer into lacteal secretions and the closure of tight junctions are part of the process, marking the end of colostrogenesis and the initiation of lactation.
Sow v. piglets, who drives?
In swine, it is well known that milk production is stimulated by the action of suckling from piglets, and thus depends on litter size and weight of piglets. Moreover, the estimation of colostrum yield actually corresponds to the consumption of colostrum by the litter. Therefore, it has long been argued that colostrum yield mainly depends on litter characteristics. However, there is now a large body of evidence that even though colostrum yield may be influenced by the global vitality of the litter at birth, it also largely depends on the capacity of the sow to produce colostrum (Farmer and Quesnel, Reference Farmer and Quesnel2009).
Endocrine control of colostrogenesis
Role of endogenous hormones
In swine, the prepartum peak of prolactin is essential for colostrogenesis (Farmer et al., Reference Farmer, Robert and Rushen1998) and is brought about by the drop in progesterone concentrations (Taverne et al., Reference Taverne, Bevers, Bradshaw, Dieleman, Willemse and Porter1982). In primiparous sows, delays in the decrease in progesterone and in the increase in prolactin relative to the onset of parturition were greatly associated with a reduced yield of colostrum (Foisnet et al., Reference Foisnet, Farmer, David and Quesnel2010a). Furthermore, the relative concentrations of prolactin and progesterone during the prepartum period were recently shown to influence colostrum yield. Primiparous sows that had lower progesterone concentrations associated with a tendency for greater prolactin concentrations (high prolactin to progesterone ratio) during the 2 days before and the day of parturition produced more colostrum (+0.6 kg) than sows that had a low prolactin to progesterone ratio (Loisel et al., Reference Loisel, Farmer, van Hees and Quesnel2015). There were no concomitant changes in the concentrations of macronutrients in colostrum, indicating an absence of dilution effect. The greater colostrum concentrations of progesterone at the onset of parturition were also negatively related with colostrum yield in sows from parities one to eight, with sows having concentrations of progesterone >4.5 ng/ml producing 1.6 kg less colostrum than those having progesterone concentrations of <4.5 ng/ml (Hasan et al., Reference Hasan, Junnikkala, Peltoniemi, Paulin, Lyyski, Vuorenmaa and Oliviero2018). It is however important to mention that even though lower peripartum concentrations of prolactin are observed in primiparous than in multiparous sows (Quesnel et al., Reference Quesnel, Ramaekers, van Hees and Farmer2013), this does not necessarily translate into a lower production of colostrum. Indeed, significant discrepancies exist between studies on the effect of sow parity on colostrum yield (Table 1). Greater colostrum yield was reported in young sows compared with older sows (Decaluwé et al., Reference Decaluwé, Maes, Declerck, Cools, Wuyts, De Smet and Janssens2013; Quesnel, unpublished data), while Devillers et al. (Reference Devillers, Farmer, Le Dividich and Prunier2007) reported that sows of parities two and three tended to produce more colostrum than primiparous and older sows. Finally, Boonraungrod et al. (Reference Boonraungrod, Sutthiya, Kumwan, Tossakui, Nuntapaitoon, Muns and Tummaruk2018) recently reported that multiparous sows produced more colostrum than primiparous sows.
Table 1 Colostrum yield according to sow parity

1 Number of sows.
ab Average colostrum yield with different letters differed (P<0.05).
Besides progesterone and prolactin, other hormones also undergo drastic changes peripartum. Among them, estrogens and glucocorticoids whose concentrations increase during the week and the day before parturition, respectively (Foisnet et al., Reference Foisnet, Farmer, David and Quesnel2010a), are considered to be involved in the regulation of lactogenesis in rodents and ruminants. To the best of our knowledge, however, a role for estrogens and glucocorticoids in the control of colostrogenesis has not yet been described in swine. Oxytocin, which is essential for fetus expulsion, peaks during parturition. The peak of oxytocin may be too late to participate in the regulation of colostrum nutrient synthesis. However, its amplitude and duration may influence the transfer of IgG into lacteal secretions by delaying the closure of tight junctions between mammary epithelial cells.
Finally, a link between colostrum yield and farrowing kinetics was reported in primiparous sows (Quesnel, Reference Quesnel2011). The rhythm of piglet births during the onset of parturition was lower in sows with a low colostrum production (<3 kg) than in sows with a medium (3–4 kg) or high (>4 kg) production. It can be suggested that abnormalities in the endocrine status of sows in late pregnancy may affect the processes of both parturition and colostrogenesis, and these two processes being partly regulated by common hormones, such as progesterone and maybe estrogens and glucocorticoids.
Colostrum yield was shown to be positively correlated with plasma concentrations of IGF-I in prepartal sows (Foisnet et al., Reference Foisnet, Farmer, David and Quesnel2010a). Accordingly, it was suggested that the 20% increase in colostrum yield observed when sows were fed a fermented potato protein product for 7 days before expected farrowing could be due to greater IGF-I concentrations in sow plasma (Wavreille et al., Reference Wavreille, Planchon, Renaville, Forier, Agneessens and Bartiaux-Thill2010). It can be hypothesized that IGF-I could stimulate lactogenesis because it can act as a mitogenic factor or an anabolic hormone that favors nutrient uptake by MEC, or both. However, these observations are insufficient to conclude for a stimulatory effect of IGF-I on colostrum production.
Effects of exogenous hormones
Inducing parturition
A key factor for successful intensive farrowing management is farrowing supervision, hence natural and synthetic prostaglandin F2α (PGF2α) are commonly used in pig farms to induce parturition. Although one study reported that inducing parturition on day 114 of gestation (i.e., one day before the average term) decreased colostrum yield, other studies showed no significant effect (Table 2). Nevertheless, farrowing induction on day 113 of gestation induced transient alterations in plasma hormonal concentrations and colostrum composition (Foisnet et al., Reference Foisnet, Farmer, David and Quesnel2011). Increases in prolactin and cortisol concentrations were observed 1 h after the injection of PGF2α. Colostrum collected at the onset of parturition (T0) contained more lactose and less IgA when parturition was induced, whereas colostrum composition at T24 did not differ between sows with natural or induced farrowing (Foisnet et al., Reference Foisnet, Farmer, David and Quesnel2011). Farrowing induction had no effect on colostral concentrations of IgG (Foisnet et al., Reference Foisnet, Farmer, David and Quesnel2011; Otto et al., Reference Otto, Machado, Moreira, Bernardi, Coutinho, Vaz, Wentz and Bortolozzo2017). It is apparent that if farrowing induction is performed not earlier than one day before the expected farrowing date (based on individual monitoring or on the average farrowing date in the herd), no negative impact on colostrum yield is expected. However, the effect of farrowing induction on IgA concentrations and on the subsequent occurrence of neonatal diarrhea warrants further investigation.
Table 2 Colostrum yield and gestation length in sows with farrowings induced on day 113 or 114 of gestation
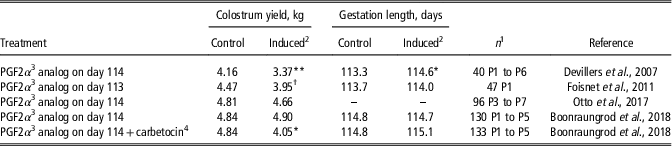
1 Total number of sows and parity (P).
2 Treatment effect: **P<0.01, *P<0.05, † P<0.10.
3 Prostaglandin F2α.
4 Carbetocin is an oxytocin-like molecule with long-acting properties; it was injected 24 h after the PGF2α analog.
Preventing premature parturition
In a retrospective study, based on 61 000 farrowing records, a 10% occurrence of early parturition (<114 days) was observed (Vanderhaeghe et al., Reference Vanderhaeghe, Dewulf, Jourquin, De Kruif and Maes2011). Premature parturition may affect the birth weight, survival and growth of piglets and it interferes with the management procedures on the farm. It is thus important to prevent premature parturition in herds where its incidence is high. This can be achieved by administering an analog of progesterone (altrenogest) to maintain plasma concentrations of progesterone during the last few days of pregnancy. Administration of altrenogest from days 109 to 112 or 113 of gestation did not affect colostrum yield, likely because the treatment delayed farrowing and main hormonal changes without affecting the relative chronology of these changes (Foisnet et al., Reference Foisnet, Farmer, David and Quesnel2010b). Such a treatment was tested on a large scale in commercial herds, with no report of negative impact on piglet survival until weaning (Vanderhaeghe et al., Reference Vanderhaeghe, Dewulf, Jourquin, De Kruif and Maes2011), which is consistent with the lack of negative effect on colostrum yield.
Injecting oxytocin
As mentioned earlier, the composition of colostrum is affected by the status of tight junctions between mammary epithelial cells. Therefore, the ability to manipulate mammary tight junctions in the late colostral phase could allow Ig concentrations to be maintained at higher levels for a longer period of time, thereby increasing the quality of early milk. Injecting a supraphysiological dose of oxytocin to sows on day 2 of lactation (i.e., between 12 and 20 h after birth of the last piglet) increased the Na:K ratio (0.48 v. 0.28) and concentrations of IGF-I (78.1 v. 37.2 ng/ml), IgG (18.8 v. 6.6 mg/ml) and IgA (4.1 v. 2.7 mg/ml) in milk collected 8 h after the injection (Farmer et al., Reference Farmer, Lessard, Knight and Quesnel2017). The injection of oxytocin in the early postpartum period therefore delayed the occurrence of tightening of mammary tight junctions and prolonged the colostral phase, thereby having beneficial effects on the composition of early milk.
Effects of nutrition on colostrogenesis
The development of strategies to improve colostrum composition through maternal feeding has been largely explored, with a special focus on colostral fat and Ig because both are sensitive to nutritional changes. In contrast, very few attempts were made to increase colostrum yield, which is most likely due to the difficulty in measuring the amount of colostrum produced. Most recent attempts focused on maternal feeding during late gestation, when milk-specific constituents are synthetized. Alternatively, long-term strategies might aim at increasing mammary gland development. Although it is well known that milk yield depends on the number of mammary epithelial cells present at the onset of lactation (Head and Williams, Reference Head and Williams1991), it is not known whether this is also the case for colostrum production. Nutritional strategies to improve colostrum synthesis might also aim at managing sow body reserves and metabolic status during late pregnancy and this is covered in more detail by Quesnel et al. (Reference Quesnel, Farmer and Theil2015). However, the fact remains that the relation between sow metabolic state during gestation and colostrum yield is not clear, with discrepancies between findings.
Sow nutrition and colostrum yield
Manipulating circulating concentrations of prolactin
Given the effects of prepartum prolactin concentrations on colostrum yield, the development of strategies to influence prolactin concentrations through nutritional approaches is warranted. Silymarin, an extract from the milk thistle plant (Silybum marianum), is known for its hyperprolactinemic properties. Yet an attempt to induce hyperprolactinemia in sows before parturition by providing silymarin was not successful (Loisel et al., Reference Loisel, Farmer, Ramaekers and Quesnel2014). To the best of our knowledge, there are no other known nutritional means to increase prolactin concentrations in swine.
Manipulating circulating concentrations of progesterone
Circulating concentrations of progesterone can be influenced by the level of feeding. Indeed, in gilts and ewes, progesterone concentrations were reduced with a high feeding level, likely because of an increase in metabolic clearance rate (Banchero et al., Reference Banchero, Milton, Lindsay, Martin and Quintans2015). Supplementing ewes with energy during the last week of pregnancy accelerates the decline in circulating progesterone that, in turns, increases colostrum yield (Banchero et al., Reference Banchero, Milton, Lindsay, Martin and Quintans2015). However, increasing sow feed intake for the last 2 weeks of pregnancy did not affect circulating concentrations of progesterone and piglet weight gain during the first 2 days postpartum, indicating that colostrum yield was unaltered (Miller et al., Reference Miller, Foxcroft and Aherne2004). Authors suggested that this absence of effect could be tied to the stimulatory effect of increased feeding level (via greater insulin and IGF-I concentrations) on the production of progesterone by corpora lutea, occurring concurrently with the increased clearance rate of progesterone. This problem may be overcome by imposing a high level of feeding for a shorter period of time at the beginning of luteolysis (i.e., 2 days before parturition) in order to accelerate the decrease in progesterone without stimulating its synthesis.
Manipulating colostrum yield
The few published experiments where nutritional attempts were made to stimulate colostrum yield used various strategies based on feeding level, source or level of fat and dietary fiber, and feeding specific nutrients (Table 3). A high feeding level (three times 1.5 kg daily compared with 1.5 kg/day) during the last week of gestation tended to increase colostrum yield from 3.5 to 4.0 kg (Decaluwé et al., Reference Decaluwé, Maes, Cools, Wuyts, De Smet, Marescau, De Deyn and Janssens2014). This effect may be related to the greater lactose content of colostrum from overfed sows, lactose being the major osmotic factor that favors water transfer into lacteal secretions. Interestingly, Decaluwé et al. (Reference Decaluwé, Maes, Cools, Wuyts, De Smet, Marescau, De Deyn and Janssens2014) also showed that sow body condition had more impact on colostrum yield than feeding level. The highest colostrum yield was achieved when sows entered the farrowing unit with a moderate body condition (i.e., with a backfat thickness between 17 and 23 mm) and were fed the high plane of nutrition for a week before farrowing.
Table 3 Nutritional manipulation of colostrum yield and effects on macrochemical composition of colostrum in sows
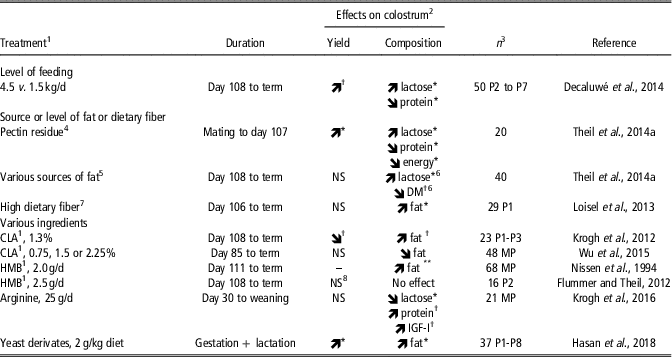
1 CLA: Conjugated linoleic acid; HMB: ß-hydroxy ß-methyl butyrate.
2 Treatment effect: **P<0.01, *P<0.05, † P<0.10, NS: not significant.
3 Total number of sows and parity (P, when known). MP: multiparous sows.
4 A high-fiber diet formulated with pectin residue (32% DM, insoluble dietary fiber) was compared with a high-fiber diet rich in potato pulp (40% DM, soluble dietary fiber).
5 Prefarrowing diets: standard (3% animal fat), 8% coconut oil, 8% sunflower oil, 8% fish oil or 4% fish oil + 4% octanoic acid.
6 Effects of dietary supplements of 8% fish oil or 4% fish oil + 4% octanoic acid versus 8% coconut oil.
7 A high-fiber diet with 23% insoluble and soluble dietary fiber was compared with a diet containing 13% insoluble and soluble dietary fiber.
8 Colostrum yield per piglet tended to be increased (512 v. 434 g) but colostrum yield for the whole litter did not differ (5.3 v. 5.0 kg).
Theil et al. (Reference Theil, Flummer, Hurley, Kristensen, Labouriau and Sørensen2014a) combined two feeding strategies by providing various levels and sources of dietary fiber during most of gestation (from mating to day 107) and then various levels and sources of fat during the last week of gestation (Table 3). The gestation diet, but not the prefarrowing diet, influenced colostrum yield, with sows fed pectin residue (rich in insoluble fiber) producing more colostrum than sows fed potato pulp (rich in soluble fiber; 6.2 v. 4.6 kg). It is not clear why sows fed pectin residue produced more colostrum but this was consistent with a greater lactose content in colostrum. However, when sows were fed sugar beet pulp (rich in soluble dietary fiber), their colostrum yield was intermediate compared with that of sows fed pectin residue or potato pulp, despite their higher lactose content in colostrum. Loisel et al. (Reference Loisel, Farmer, Ramaekers and Quesnel2013) found no effect of high dietary fiber providing both soluble and insoluble fibers (23% of dietary fiber originating from a mixture of soybean hulls, wheat bran, undecorticated sunflower meal and sugar beet pulp) on colostrum yield when compared with a low fiber diet (13% of dietary fiber). Nevertheless, this high-fiber diet increased by nearly 60% the colostrum intake of low birth weight-piglets (<900 g) and was associated with lower piglet mortality until weaning. Clearly the effects of dietary fiber source on colostrum yield or composition and their underlying mechanisms need further attention.
The potential effects of various specific ingredients, namely conjugated linoleic acid (CLA), the leucine metabolite ß-hydroxy ß-methyl butyrate (HMB) and arginine, on colostrum yield were also investigated, with no significant effects being reported (Table 3). A trend for lower colostrum yield was reported in response to CLA supplementation during the last week of gestation (Krogh et al., Reference Krogh, Flummer, Jensen and Theil2012).
Collectively, these data indicate that nutrition in late pregnancy is important for colostrum yield of sows, but that more investigations are needed to determine which nutrients or feeding strategies are most effective and which part of gestation (last week, last three weeks or last third) is most critical for colostrum production.
Sow nutrition and colostral fat content
The effects of sow nutrition, encompassing feed intake, energy intake and source, and protein intake, on macrochemical composition of colostrum, have been extensively reviewed, as well as the effects of vitamins and minerals on microchemical composition (Farmer and Quesnel, Reference Farmer and Quesnel2009). The present review therefore only covers most recent findings, with a special focus on fat because it is by far the most labile macronutrient of colostrum. The fatty acid content of colostrum greatly depends on the amount of lipids provided in the sow diet during late gestation, whereas the fatty acid profile is largely influenced by the type of lipid being fed to the pregnant sow (Farmer and Quesnel, Reference Farmer and Quesnel2009). Additional colostral fat may be used, to a certain extent, as supplementary energy by the piglet and it may also increase lipid accretion, thereby enabling piglets to maintain a constant body temperature. Moreover, specific fatty acids are important for piglet maturation, such as polyunsaturated fatty acids (PUFA) which are known to play a role in brain development and function. Various studies were therefore carried out with the goal of increasing the amount of polyunsaturated fats in sow colostrum. This was successfully achieved via the inclusion of various fish oils or linseed oil in the diet of pregnant sows (Rooke et al., Reference Rooke, Bland and Edwards1998; Reference Rooke, Sinclair, Edwards, Cordoba, Pkiyach, Penny, Penny, Finch and Horgan2001). Supplementation of soybean oil in the diet of sows during the last week of pregnancy also increased the fat content and the concentrations of polyunsaturated fatty acids in colostrum (Bai et al., Reference Bai, Wang, Zhao, Shi and Shan2017). Dietary CLA also affects the fatty acid composition of colostral fat in sows, increasing saturated fatty acids and decreasing monounsaturated fatty acids (Bee, Reference Bee2000).
Besides the addition of fat to the maternal diet, providing additional dietary fiber in late gestation may be another way of increasing the fat content of colostrum. Loisel et al. (Reference Loisel, Farmer, Ramaekers and Quesnel2013) demonstrated that feeding 23.4% compared with 13.3% dietary fiber to sows from day 106 of gestation until parturition increased colostral fat from 8.3% in control sows to 10.7% in treated sows. Such an effect could not be attributed to dietary lipid intake or mobilization of body lipids since they did not differ significantly between sows fed the low and the high level of dietary fiber. This effect may be due to the fact that dietary fiber increases plasma concentrations of short chain fatty acids, including acetate which can be used by the mammary gland as precursors for de novo synthesis of lipids (Linzell et al., Reference Linzell, Mepham, Annison and West1969).
Colostral fat content was also increased by supplementing the sow diet with the leucine metabolite HMB (2.0 g/day of Ca(HMB)2) in the last 3 to 4 days before parturition (6.9 v. 4.9%; Nissen et al., Reference Nissen, Faidley, Zimmerman, Izard and Fisher1994). The mechanism of action of HMB is likely to be linked to its lipolytic effect, causing fat mobilization and elevated plasma concentrations of non-esterified fatty acids that can be used for de novo fat synthesis (Theil et al., Reference Theil, Lauridsen and Quesnel2014b). However, Flummer and Theil (Reference Flummer and Theil2012) could not reproduce this effect of HMB supplementation on colostrum composition (Table 3). Lastly, supplementing the sow diet with glutamine and glutamate can also affect colostral fat concentration. Providing the two amino acids as Aminogut (1.5% w/w) during the last week of gestation increased colostral fat by 60% (7.4 v.4.6%, Santos de Aquinos et al., Reference Santos de Aquino, Dutra Junior, Manso, MansoFilho, Kutschenko, Nogueira and Watford2014). However, it is not known if glutamine or glutamate stimulate lipid synthesis or act as substrates for de novo lipogenesis.
Sow nutrition and immunoglobulin contents of colostrum
Numerous ingredients that presumably have immuno-modulating effects were tested for their potential beneficial effects on the Ig concentrations of swine colostrum. These ingredients belong to various families of components: specific fatty acids (such as CLA and fish oils), plant extracts (as essential oils), prebiotics (mannan oligosaccharides (MOS) or fructo oligosaccharides (FOS)) and probiotics (mainly Saccharomyces cerevisiae boulardii), or ingredients containing pre- or probiotics (fermented liquid feed). When fed to late pregnant sows, many of these ingredients increased IgG, IgA and (or) IgM concentrations in colostrum (Table 4).
Table 4 Nutritional manipulation of immunoglobulin concentrations in colostrum in sows, presumably through immunomodulating effects of active componentsFootnote 1
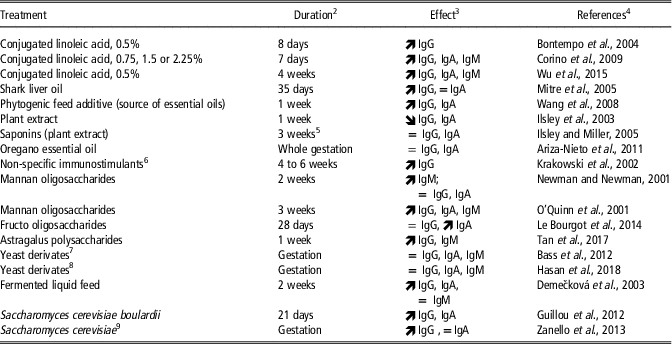
1 Published in part by Farmer and Quesnel (Reference Farmer and Quesnel2009).
2 Duration of treatment before parturition.
3 ![]() increased immunoglobulin (Ig) concentrations; = no significant effect on Ig concentrations;
increased immunoglobulin (Ig) concentrations; = no significant effect on Ig concentrations; ![]() reduced Ig concentrations.
reduced Ig concentrations.
4 The list of references is given in Supplementary material S1.
5 From days 72 to 93 of pregnancy.
6 Factors that induce non-specific defense mechanisms of the organism toward the viral and bacterial pathogens.
7 Whole extract derived from P. guilliermondii.
8 Whole extract derived from S. cerevisiae.
9 Two strains were tested: S. cerevisiae strain CNCM I-4407 and S. cerevisiae var. boulardii strain CNCM I-3799. A third strain (S. cerevisiae strain LC 10-05) had no effect on colostral IgG and IgA.
As mentioned earlier, long-chain PUFA of the n-6 and n-3 series have received a lot of attention in sow nutrition because of their role in brain development. They may also have health-promoting properties through impacts on inflammatory and immune status. Conjugated linoleic acid which is the collective name for isomers of linoleic acid (an n-6 PUFA) was efficient in increasing Ig contents of colostrum, at various doses (Table 4). This effect was likely due to the increase in Ig concentrations in sow serum, itself potentially mediated through an increase in interleukin-2 synthesis in response to CLA supplementation (Wu et al., Reference Wu, Li, Bai, Liu, Lai, Thacker and Wang2015). The increase in colostral IgG observed when the sow diet was supplemented with shark-liver oil was also associated with greater Ig concentrations in sow plasma. Shark-liver oil is a source of two bioactive lipids, the alkylglycerols and n-3 PUFA, and the increased plasmatic Ig production by sows was mainly attributed to alkylglycerols. These supplementations improved the maternal transfer of passive immunity to the piglets, as indicated by greater IgG concentrations in piglet blood. Interestingly also, there are recent indications that feeding fish oil during the last month of gestation can affect the antioxidant capacity of sow colostrum (Shen et al., Reference Shen, Wan, Zhu, Fang, Che, Xu, Lin, Li and Wu2015), yet much remains to be learned on this topic.
A wide range of beneficial properties are also attributed to plant-derived compounds, among them antimicrobial activity, antioxidant property and effects on the immune system. However, both positive and negative effects on colostrum Ig content were reported (Table 4).
Finally, probiotics and prebiotics receive considerable attention currently because of many potential beneficial effects on health. In short, probiotics are living organisms which colonize the gastrointestinal tract while prebiotics are fermented compounds that induce changes in gut microbiota. Effects on gut microbiota may be accompanied by beneficial effects on the gut immune system with consequences on the whole organism. Providing living bacteria (Lactobacillus strains in fermented liquid feed) or living cells (as Saccharomyces cerevisiae) or prebiotics MOS or FOS was shown to increase IgG or IgA concentrations in colostrum (Table 4). In contrast, yeast extract provided throughout gestation had no effect on colostral Igs. Interestingly, however, a recent study reported no effects of prebiotics supplementation on IgG, IgA or IgM content in sow colostrum but positive effects on both fat content and colostrum yield were observed (Hasan et al., Reference Hasan, Junnikkala, Peltoniemi, Paulin, Lyyski, Vuorenmaa and Oliviero2018). Multiparous sows were fed a control diet or a control diet supplemented with 2 g of yeast derivatives throughout pregnancy. This extract contained a mix of mannan oligosaccharides and polysaccharides, glucomannoproteins and betaglucans. Protein and lactose contents of colostrum were not affected, whereas fat content was increased by 21% and colostrum yield by 24%.
In conclusion, the most promising results on colostrum Ig content were obtained with CLA, prebiotics in the form of MOS and FOS, and living yeast cells, while the effects of plant extracts and yeast extracts are far less convincing.
Conclusions
Colostrum is crucial for the survival, development and health of piglets so that knowledge about mechanisms that regulate or influence colostrogenesis is essential. Although progress has been made in understanding the factors that regulate colostrum yield and composition, including the endocrine regulation and the impact of sow nutrition, several essential questions remain unanswered. Namely, when is colostrum synthetized precisely? What are the impacts of sow nutrition and metabolic status during late gestation? Does mammary development affect colostrum yield? Promising results on colostrum yield or quality were obtained by manipulating levels and sources of dietary fat and fiber during late gestation, or by supplementing sow diets with pre- and probiotics. In the future, nutritional strategies should be tested in commercial farms, using large numbers of animals, in order to investigate whether, beyond their effects on colostrum yield or composition, they affect piglet performance in terms of survival, growth, health and robustness at weaning.
Acknowledgement
None.
Declaration of interest
The authors declare that they have no competing interests.
Ethics statement
Not applicable.
Software and data repository resources
No data were deposited in an official repository.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1751731118003555






