Introduction
Of the 9,672 extant species of bird, 1,111 are threatened by extinction, and 97 out of 108 bird extinctions since 1600 have been on islands (Collar et al. Reference Collar, Crosby and Stattersfield1994). Extinction seems to be inevitable for most Critically Endangered species, but sometimes the efforts of committed individuals are able to bring some from the brink of extinction. By the early 1950s, the population of the Hawaiian Goose Branta sandvicensis had fallen to about 30 individuals, but a reintroduction programme brought the bird's numbers back to a sustainable level (Black and Banko Reference Black, Banko, Olney, Mace and Feistner1994). The decline of the Mauritius Kestrel Falco punctatus was even more desperate, only four individuals remaining by the mid-1970s. Again, captive breeding programmes were instrumental in saving the bird, which recovered to a population of several hundred (Cade and Jones Reference Cade and Jones1993, Jones et al. Reference Jones, Heck, Lewis, Mungroo, Slade and Cade1995, Cade and Burnham Reference Cade and Burnham2003). Reintroductions of endangered species to their former ranges have helped restore declining bird populations worldwide (Rudolph et al. Reference Rudolph, Conner, Carrie and Schaefer1992, Armstrong et al. Reference Armstrong, Castro, Alley, Feenstra and Perrott1999). With recent increases in the numbers of species reintroduction projects, a recognizable field of reintroduction biology has emerged (Seddon et al. Reference Seddon, Armstrong and Maloney2007), which is part of a conservation strategy to save the Crested Ibis Nipponia nippon (Yu et al. Reference Yu, Chang, Li, Chen and Shi2009).
The ultimate success of reintroductions is critically dependent on the ability of the released animals to establish self-sustaining populations in the release habitat, involving fidelity to release area, long-term survival and breeding success (Armstrong et al. Reference Armstrong, Castro, Alley, Feenstra and Perrott1999). The number of birds released in a reintroduction programme also strongly correlates with release success (Green Reference Green1997, Wolf et al. Reference Wolf, Garland and Griffith1998). Habitat quality such as food richness, predator abundance and human disturbance may be even more important determinants of reintroduction success, particularly for long-term persistence (Armstrong and McLean Reference Armstrong and McLean1996, Wolf et al. Reference Wolf, Garland and Griffith1998). The long-term goal of any reintroduction projects should be the creation of viable populations that replicate as closely as possible the behaviour and ecology of the original wild population (Meretsky et al. Reference Meretsky, Snyder, Beissinger, Clendenen and Wiley2001).
Captive populations can exhibit more behavioural variation than their wild counterparts as a result of relaxed selective pressures in the captive environment. These variations can translate into decreased survival upon reintroduction to released habitats (Mcphee and Silverman Reference Mcphee and Silverman2004). Failure of released captive-bred animals to breed successfully in the wild may result from poor behavioural adjustment, lack of suitable breeding partners, genetic abnormalities, or poor ecological conditions. Demographic data such as breeding success are essential in determining the mechanisms responsible for reintroduction success or failure (Armstrong et al. Reference Armstrong, Davidson, Dimond, Perrott, Castro, Ewen, Griffiths and Taylor2002). Environmental and demographic stochasticity are also likely to have a long-term effect on very small reintroduced populations (Simberloff Reference Simberloff1988, May 1991). Moreover, all biological factors able to play a role in population viability do so by acting on survival and fecundity rates, whatever their genetic, behavioural or environmental origin. Therefore, field study on demography of a species is crucial to assessing the possible effects of all factors affecting reintroduction success (Sarrazin and Barbault Reference Sarrazin and Barbault1996). In that way, comparative studies of breeding ecology can provide useful information for the management of a released population.
Crested Ibis was once one of eastern Asia’s most widespread birds. However, deforestation and destruction of its habitat, and illegal hunting led to successive extirpations of Russian, Korean, and Japanese populations in the 20th century (Yamashina Reference Yamashina1967, Archibald et al. Reference Archibald, Lantis and Munetchika1980). The Crested Ibis population, which had been presumed extinct since the 1960s, was rediscovered in 1981 in a small mountain village (Yao Jiagou) in Yangxian County of Shaanxi Province. At that time, the world population of Crested Ibis stood at only seven, including two pairs and three chicks (Liu Reference Liu1981). During the past 30 years, a great deal of conservation effort both in situ and ex situ, has been successfully undertaken. The protection measures taken include local legislation, effective management of nesting and foraging habitats, periodic surveys and monitoring, and public education (Shi and Cao Reference Shi and Cao2001, Ding Reference Ding2004). The wild population has increased from seven to approximately 800. From 1981 to 1987, six wild nestlings were captured and a first attempt at captive breeding of the Crested Ibis was conducted in Beijing Zoo (Li Reference Li1991). To date, more than 1,000 captive-bred individuals reside in twelve conservation breeding centres or zoos located in China, Japan and Korea. The successful breeding programme for this species has made it possible to reintroduce this rare bird to its historical range.
A reintroduction programme was conducted at Zhaigou village, Ningshan County in Shaannxi Province China in 2007 in order to establish a new population within the former range. The ultimate goal is to increase numbers in the wild to a healthy population size at which the species may be eligible for downlisting. The programme achieved one of its first goals when two pairs successfully fledged three young in the first breeding season (Yu et al. Reference Yu, Chang, Li, Chen and Shi2009).
To establish a new population, it is essential for the released birds to survive during the establishment phase, to settle in to the reintroduction area, to pair and nest successfully, and to recruit sufficient young individuals to balance mortality. In the present study, the released birds were monitored using radio-telemetry and colour-banding to examine the reproductive potential of captive-reared ibis in this habitat. Specifically, we sought to (1) determine whether captive-reared birds were capable of pairing and breeding in the wild; (2) document behaviour and breeding success of the reintroduced individuals; (3) compare reproductive parameters between the released population and the wild population in Yangxian County studied by Yu et al. (Reference Yu, Liu, Xi and Lu2006); (4) calculate survival rates for released birds, breeders and first year fledglings; (5) compare growth rates for the reintroduced and natural populations (Lu et al. Reference Lu, Fu, Zhai, Xi, Zhang and Huang2000, Ding Reference Ding2004 ); and (6) determine factors limiting population growth in the newly established population. The results have implications for both Chinese Crested Ibis recovery and other reintroduction programmes.
Methods
Study area
We conducted a four year study (2008–2011) of the breeding ecology of released Crested Ibis at Ningshan County (33° 07'–33°50'N, 108°02'–108°56'E) on the south slope of the Qinling Mountains in Shaanxi province in central China (Figure 1). Reproductive data were collected from February to July and survival data obtained from January to December each year. This area is used by ibis for breeding and foraging and the landscape mosaic is characterised by intermontane basins, often developed for rice fields, interspersed with hills. Numerous shallow rivers and streams flow southward through this area into Hanjiang River, a large tributary of the Yangtze River. Forest patches provide 80% of the coverage used by ibis for nesting. These patches are primarily mixed coniferous-broadleaf forest where the dominant tree species include Cork Oak Quercus variabilis, Chinese Pine Pinus tabulaeformis, Chinese Red Pine P. massoniana, and Oriental Arborvitae Platycladus orientalis. Almost all nest sites are found in pine and Cork Oak forest characterised by trees with diameter at breast height (DBH) ranging from five to 40 cm, and canopy heights from 10 to 25 m, planted on a slope near local farm settlements. Breeding pairs will reuse the same nesting territory year after year if not disturbed. Breeding territories are typically small, with a diameter of 50–100m, so distances between nests range from dozens of meters to several kilometres (Shi and Cao Reference Shi and Cao2001).
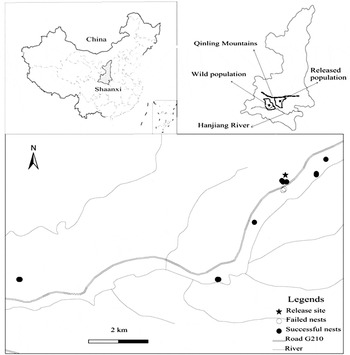
Figure 1. Map of release region showing the location of release site (pentagram), failed (hollow circle) and successful nests (solid circle) for reintroduced population.
The study area is in a warm, temperate zone and is relatively humid. The annual temperature averages 12.50C with a frost-free period of 215 days. The mean temperature in January is 0.6°C (with a minimum of -12.80C) and 23.40C in July (with a maximum of 36°C). Temperatures are warm enough to leave open water in most winters after the release except in January 2007. The annual precipitation is 899 mm with July to September being the wettest time of the year (latest 10-year period data from Ningshan County meteorological station).
Release and monitoring
Captive breeding provided an adequate number of individuals for release (Figure 1). Of the 56 individuals released, 20 were transported from the Shaanxi Louguantai conservation centre for endangered species (Zhouzhi County), 20 from the Shaanxi Crested Ibis conservation centre (Yangxian County), and 16 from the Crested Ibis captive breeding centre (Ningshan County). All individuals were held together for 6–12 months before release in a large aviary (a circular nylon cage 20 m high and 50 m in diameter) containing roost trees and man-made foraging habitat, allowing training in foraging and flying.
Prior to release, each bird was led into a relatively small aviary by a daily feeding crew and acclimatised for 24 hours in a wooded hacking aviary (measured 60 x 40 x 40 cm). Birds were provided with fresh food and water while in the hacking aviary. In addition to their normal captive diet, they were treated with a vermifuge such as albendazole and multivitamins. All individuals were uniquely marked with numbered colour bands for field identification. Of the 30 males, 11 were fitted with lightweight radio transmitters weighing 15g, approximately 1% of the body weight (model RI-2D; frequency 216.368-216.691 MHz; battery life ∼18 months; Holohil Systems, Carp, Ontario, Canada). The radio transmitters were attached to the neck using a harness of elasticated nylon string, leaving room for growth of the pectoral muscles.
Up to the present study, a total of 56 captive-bred ibises have been released: from May to October in 2007 (13 females, 13 males); 2008 (four females, two males); 2009 (six females, eight males), and 2011 (three females, seven males), respectively. Among the 14 individuals released in 2009, six birds accidentally escaped from an aviary destroyed by heavy snow on 15 November 2009. All captive-reared birds were banded when they were born in captivity. The mean age of individuals at release was 5.2 ± 2.5 years (n = 26) for females, and 6.4 ± 2.9 years (n = 30) for males. Twenty of them paired and 12 females laid eggs in captivity. In the winter following release, birds were provided with supplementary food (mainly loach Misgurnus anguillicaudatus) at the foraging sites they frequently visited. All the observed breeding pairs were monitored during four breeding seasons of 2008 to 2011 from January through to July.
Nest success
Nests were located by observing behavioural cues of the pair. Crested Ibis fed and roosted extensively near human settlements (Li et al. Reference Li, Ma, Ding, Zhai and Li2002) and all released birds dispersed ≤ 15 km making it possible to find all nests. The frequent hoarse calls, especially by the male, in early stages of breeding were used to locate breeding birds. Easily accessed nests (≤ 3 km from the release site) were monitored every two days, and more remote nests (≥ 10 km from the release site) were monitored every four days. We observed 10 nests for a total of 480 h during building, 6 nests for 672 h during incubation and 6 nests for 960 h during brooding.
The following data were recorded for a total of 21 clutches: (1) identity (age, sex and bands) of all individuals observed as well as breeders; (2) location of the nest (including the town or village where the nest was located, and the longitude, latitude and altitude of the nest-site); (3) date of egg laying; (4) clutch size; (5) egg losses from each nest; (6) number hatching from each clutch; (7) nestling losses in each clutch; (8) number of chicks fledging from each brood; and (9) species, height and DBH of the nest-tree.
Because the males and females in this species are monomorphic, the sex of each of the pair was determined by observations of copulation behavior during the courtship period. Nest contents were determined using a telescope (Nikon 40х) where possible or through adult and nestling behaviour for inaccessible nests. Transition dates (e.g. egg laying, start of incubation, hatching, and fledging) were observed directly or calculated from the beginning of a known stage in the nesting cycle.
Survival
We used the staggered entry design of the Kaplan-Meier method (Pollock et al. Reference Pollock, Winterstein, Bunck and Curtis1989) to obtain estimates of adult survival (SA) and the first year survival (SJ). We selected this method because newly collared Crested Ibis were introduced into our study area during four separate releases, and it allowed us to take into account emigration or radio loss. The growth rate of the reintroduced population can be calculated approximately as λ= SA + (SJ * F/2), where the F is the average number of young produced/year/pair.
We monitored radio-tagged and band-marked adults 10–15 days/month after release, and as well as the first year fledglings. Nestlings were banded with a unique combination of bands at 24–26 days old when their tarsi were fully developed but before they were able to leave the nest. Simultaneously, a radio transmitter set (RI-2D) was placed on the strongest nestlings (typically the eldest) at each nest. In case of adult or nestling death, carcasses were necropsied to determine cause of death.
Statistical analysis
Calculation of survival standard errors followed the method of Cox and Oakes (in Pollock et al. Reference Pollock, Winterstein, Bunck and Curtis1989). We used the log-rank test to determine annual differences in survival distribution. We used the Z-test statistic to examine differences in annual survival rates. We report means ± SD unless otherwise noted and statistical tests were considered significant at P < 0.05.
Results
Breeding behaviour
Of all released birds, 35.7% (20 birds) paired and 21.4% (12 females) laid eggs in captivity. All individuals monitored throughout the breeding season following their release paired and had active nests (n = nine females and eight males; Table 1). Of the paired birds, nine of them including five females and four males bred in their first season post-release and two males paired in the third year after release (Table 1). We observed 10 breeding pairs including six captive-captive pairs, two captive-bred males paired with two wild females that immigrated from the wild population in Yangxian County, and one captive-bred male and female paired with sexually mature birds fledged in 2008, respectively. Two males once paired in captivity chose other females for mates and one male remained with its former mate after release. One female and one male ibis reformed the pair bond in the next breeding season when they lost their previous mate (Table 1).
Table 1. Summary of breeding pairs recorded post release of the reintroduced population in Ningshan County.

We found 21 nests of 10 pairs (two in 2008, five in 2009, seven in 2010 and seven in 2011). Among them, 17 were successful (at least one nestling fledged), with nest-fate determined by fledging of young. Nests of released birds were exclusively found in trees (20 on Chinese Red Pine and one on David’s Poplar Populus davidiana). The nests of wild birds were built on Chinese Red Pine (n =175), Cork Oak (n =30), Siberian Elm Ulmus pumila (n = 19), Chinese Pine (n = 5), Chinese White Poplar Populus tomentosa (n = 1) and Dye-tree Platycarya strobilacea (n = 1) (Yu et al. Reference Yu, Liu, Xi and Lu2006). The nests were large and untidy dish-shaped structures built of sticks in a fork close to the trunk (n = 20) or on a fork of a horizontal branch (n = 1) of the nest tree. Captive-bred ibis built their nests with dry sticks of Cork Oak, Chinese Red Pine and David’s Poplar. The lining materials consisted of dry grass Poa spp., grass roots, leaves of trees and ferns, feathers and human garbage from nearby villages (e.g. pieces of paper or plastic). All the nests in our study were qualitatively similar in construction and site characteristics to those made by wild Crested Ibis (Table 2).
Table 2. Summary of breeding parameters for wild Crested Ibis in Yangxian County in 1981–2004 studied by Yu et al. (Reference Yu, Liu, Xi and Lu2006) and for captive-bred, released birds in Ningshan County in 2008–2011 (present study).

a Data from Shi and Cao (Reference Shi and Cao2001).
b Mean number of days after 14 March.
c Percentage of eggs that hatched out of the total number of eggs.
Nest building lasted 17.5 ± 2.6 days (n = 21) until the first egg was laid, but collection of nest materials occasionally occurred in the following period, especially in the early nestling stage. Males were the primary nest builders (510 of 545 observations of collection of nest materials were by males), and females and males developed brood patches, incubated, and brooded young. Mean daily incubation time spent by males (450.6 ± 75.4 min, n = 8) was not significantly higher than that by females (460.5 ± 54.5 min, n = 9) (t = 0.313, P = 0.759). Similarly, the mean daily brooding time was not significantly different between males and females (380.5 ± 65.5 min, n = 8; 365.8 ± 56.5 min, n = 9; t = 0.497, P = 0.626). The number of daily feeding bouts delivered to nestlings by males (45.6 ± 22.5, n = 8) was similar to those by females (43.4 ± 20.6, n = 9, t = 0.211, P = 0.836).
A single clutch of 1-5 eggs (usually four) by each pair was laid in two-day intervals. The egg-laying period lasted approximately three months (2 March to 8 May), compared with 14 March to 8 April for the wild population (Yu et al. Reference Yu, Liu, Xi and Lu2006). Clutch size was one in one nest, two in six nests, three in four nests, four in nine nests, and five in one nest. Mean clutch size (3.14 ± 1.06, n = 21) was not significantly larger than that of the wild population (2.84 ± 0.77, n = 271, Yu et al. Reference Yu, Liu, Xi and Lu2006) (t = 1.669, P = 0.100). Egg viability, measured as the percentage of eggs that hatched out of the total number of eggs that were laid and incubated to term (excluding abandoned or depredated eggs) was 68.2% (n = 66 eggs in 21 clutches). Incubation beginning at the first laying date lasted 28–30 days (28.5 ± 1.5 days; n = 19). Nestlings fledged 38–41 days after hatching (39.5 ± 2.0 days; n = 19 nests). The Crested Ibis exhibited a similar pattern of behaviour in the released and wild populations (Shi and Cao Reference Shi and Cao2001).
Nesting success
Of 21 active nests with known fates, 81% (17 nests) fledged at least one young. Four eggs in two clutches of the same pair (W08 vs 094, Table 1) in 2009 and 2010 failed to hatch due to the abnormal behaviour (long-time absence during incubation) of the male (094). Similar to wild birds, we confirmed that two reasons accounted for nestling death. A shortage of food in the breeding season was the primary cause, resulting in starvation of the nestlings. Eight of 14 fatalities occurred at the early chick stage (one week after hatching). In asynchronous hatching species, the first chicks have usually been the sole beneficiaries of the parents’ feeding efforts for a time and certainly receive much more food before younger hatchlings arrive; hence the younger chicks (especially the youngest one) are usually smaller and weaker, and they often die. The corpses of the nestlings were always found under the nest, thrown out by parent birds. Secondly, predators such as snakes (especially King Rat Snake Elaphe carinata) were observed to prey on Crested Ibis nestlings. Four of 14 chicks were lost in this way since 2008. In addition, one nestling died in the broken eggshell during hatching for unknown reasons.
In total, 33 nestlings successfully fledged during the four breeding seasons. There are significant differences in the altitude of nest site, height of nest tree and nest above the ground, and egg-laying date between the released and wild population (Table 2). However, the mean number of fledglings per active nest was 1.57 ± 1.03 (n = 21), and mean number of fledglings per successful nest was 2.00 ± 0.87 (range: 1–4; n = 17). In comparison, wild birds did not fledge a significantly different number of birds per active nest (1.96 ± 1.06, n = 231) and per successful nest (2.24 ± 0.80, n = 201) (Yu et al. Reference Yu, Liu, Xi and Lu2006; Table 2).
Survival
Overall, 32 of 56 released birds survived their first winter in the area of reintroduction. Since the release, we have extensively monitored the reintroduced population and paid particular attention to the birds’ ability to survive during the following winter. We omitted 24 individuals due to signal loss because of transmitter failure or bird emigration from the study area. Mean annual survival rate was 0.572 ± 0.064 (n = 56) for all released birds, and 0.815 ± 0.054 (n = 17) for breeders (Figure 2). Although 11 out of 15 (73.3%) fledglings monitored from 2008 to 2010 survived at least the first winter in their post-fledging dispersal (Yu et al. unpubl. data), the overall survival rate was quite low (0.515 ± 0.058, n = 33). No comparable data are available for wild Crested Ibis. The survival rate for each cohort may be underestimated because some of the released birds and fledglings emigrated outside our study area. Annual survival distributions and survival rates differed significantly among seasons for all released birds (χ2 = 4.56, df = 3, P = 0.004; Z = 1.06, P < 0.01), breeders (χ2 =3.12, df = 3, P = 0.006; Z = 3.14, P < 0.05) and first year fledglings (χ2 = 2.56, df = 3, P = 0.001; Z = 4.06, P < 0.001), respectively. The average number of young produced/year/pair was 1.57 ± 1.03 (n = 21). Then the growth rate (λ) of the reintroduced population was 1.2193, compared with that of the wild population (λ1=1.0783, 1981–1993, λ2 = 1.2014, 1994–1997, and λ3 = 1.3571, 1998–2003) (Lu et al. Reference Lu, Fu, Zhai, Xi, Zhang and Huang2000, Ding Reference Ding2004).
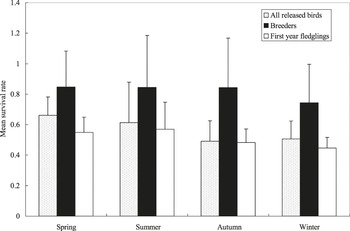
Figure 2. Mean survival rate (average ± SE) for all released birds (n = 56), breeders (n = 17) and first year fledglings (n = 33). Spring (1 March to 31 May), summer (1 June to 31 August), autumn (1 September to 30 November), winter (1 December to 28 February).
The main results of the field observations to date can be summarised as follows: three females and four males could not be relocated following the release for unknown reasons. Based on evidence found at recovery sites, 11 mortalities (four females, six males and one of indeterminate sex, its identity ring having been lost) were recorded as five from starvation, one due to electrocution by power lines, two due to predation or injuries attributable to predation (likely by Yellow-throated Marten Martes flavigula, a member of the Mustelidae, and three from unknown causes.
Discussion
Most reintroduction projects aimed at saving endangered animals in the 1970s and 1980s failed to establish viable populations, partly due to poor planning and subsequent monitoring (Griffith et al. Reference Griffith, Scott, Carpenter and Reed1989, Wolf et al. Reference Wolf, Griffith, Reed and Temple1996). The success of reintroduction programmes depended on the quality of the habitat in which the reintroductions took place, the adaptive ability of the birds to new conditions, as well as the number and age structure of the released birds (Griffith et al. Reference Griffith, Scott, Carpenter and Reed1989, Wolf. et al. 1996, Armstrong et al. Reference Armstrong, Davidson, Dimond, Perrott, Castro, Ewen, Griffiths and Taylor2002, Tweed et al. Reference Tweed, Foster, Woodworth, Monahan, Kellerman and Lieberman2006). Generally, it seems to be more difficult to reintroduce migratory species successfully than resident ones. For example, in a restoration programme, biologists had to teach Canada Geese Branta canadensis and Trumpeter Swans Cygnus buccinator a safe migration route using ultralight aircraft (Sladen et al. Reference Sladen, Lishman, Ellis, Shire and Rininger2002). In the migratory Northern Bald Ibis Geronticus eremita, there has been no successful reintroduction of fully independent individuals into the wild (Boehm et al. Reference Boehm, Bowden, Jordan and King2007). There are a number of reasons responsible for this, including complexities due to migratory behaviour (Akçakaya Reference Akçakaya1990, Lindsell et al. Reference Lindsell, Serra, Peške, Ghazy Al Qaim, Kanani and Wondafrash2009). The conservation of endangered migratory ibis poses greater challenges than do resident Crested Ibis (Serra et al. Reference Serra, Bruschini, Lindsell, Peske and Kanani2011). The present study provides evidence for the first successful release of captive-reared Crested Ibis, presenting a meaningful model for reintroduction programmes of other endangered species. In the present reintroduction programme, although the survival rate of reintroduced birds was not as high as that of some species such as Puaiohi Myadestes palmeri released to the wild (Tweed et al. Reference Tweed, Foster, Woodworth, Monahan, Kellerman and Lieberman2006), two pairs fledged young in their first breeding season after release (Yu et al. Reference Yu, Chang, Li, Chen and Shi2009) and new pair formations continued to occur and the number of fledglings produced continued to increase in the following years.
The reintroduced site is approximately 80 km from Yangxian County where the wild population survived (Figure 1). We have never found any wild individuals in this area during the pre-investigation of feasibility for release. Based on the definition of reintroduction by IUCN (1998), strictly speaking, this is a purely reintroduced population. However, it could also be classed as a restocking programme as two females from the wild population mated with released birds through natal dispersal.
There were no obvious differences in reproductive behaviour and general breeding patterns between the released (present study) and wild population (Shi and Cao Reference Shi and Cao2001), but there are a few differences in egg-laying date and clutch size. The relative extended duration of egg-laying (from 2 March to 8 May) for the released population may be the result of local ecological conditions such as food availability (Armstrong et al. Reference Armstrong, Davidson, Dimond, Perrott, Castro, Ewen, Griffiths and Taylor2002). The clutch size of the reintroduced population (3.14 ± 1.06, n = 21) was slightly higher than that of wild population (2.84 ± 0.77, n = 271). This may reflect a different age structure of the two populations. For the wild population, the clutch sizes achieved by 2–3 year-old and ≥10 year-old individuals were significantly lower than that of 4–10 year-old birds (Yu et al. Reference Yu, Lu, Lu and Liu2007).
Predation was one of the main causes of nest loss in both the reintroduced and wild populations (Shi and Cao Reference Shi and Cao2001, Yu et al. Reference Yu, Liu, Xi and Lu2006, present study). The important predators include King Rat Snake, which usually attacks eggs and chicks, and Yellow-throated Marten and Siberian Weasel Mustela sibirica, which may also prey on adults as well as their eggs and nestlings (Cao et al. Reference Cao, Yu and Zhai1995, Xi et al. Reference Xi, Lu, Geng, Zhai and Zhang1997, Zhang et al. Reference Zhang, Lu, Zhai, Xi and Wang2000). A total of 40 eggs and 15 chicks were taken by nest predators from 1981 to 2004 (Yu et al. Reference Yu, Liu, Xi and Lu2006). Four nestlings died from predation by King Rat Snake in our 4-year study period. These predation events occurred occasionally during the nestling stage, when nests are more conspicuous because of nestling begging calls, increased visitation by adults, and the scent of feces under nest tree. Corvids, such as Collared Crow Corvus torquatus, Black-billed Magpie Pica pica and Red-billed Blue Magpie Urocissa erythrorhyncha often nest on the same tree or in forest patches very close to the breeding territory of the Crested Ibis, and may be a threat. Observations on the wild birds showed that crows have preyed on two eggs of a clutch in only one case in 1981 (Shi and Cao Reference Shi and Cao2001,Yu et al. Reference Yu, Liu, Xi and Lu2006). However, incubation failure of the released ibis population on Sado Island in Japan in 2010 was completely due to egg predation by crows Corvus spp. (Nishimiya and Hayashi Reference Nishimiya and Hayashi2010). We interpret that egg loss from predation to imply there is a high density population of crows on Sado Island.
We conclude that snake predation is one of the serious problems affecting recovery of the Crested Ibis in release areas. A predation event usually occurs quickly and often under circumstances that make observation difficult. Since 2008, one King Rat Snake was captured and three were driven away from the nest. A series of approaches to protecting Crested Ibis and their young from snake predation using plastic cloth covering the trunks of nesting and adjacent trees, and bait (poisoned chicken eggs) has not been very effective. A total of 40 eggs and 15 chicks in the wild population were taken by nest predators from 1981 to 2004 (Yu et al. Reference Yu, Liu, Xi and Lu2006), and four nestling mortalities were due to snake predation in the released population (present study). Hence, there is an urgent need for a cost-effective management technique that can be applied at an ecosystem level.
The most promising result from our monitoring efforts was that captive-bred ibis successfully paired and fledged young in the wild within the first breeding season (Yu et al. Reference Yu, Chang, Li, Chen and Shi2009). The two fledglings produced in the 2008 breeding season (part of the first generation) paired with released individuals in 2009 and 2010 and fledged six young (the second generation) in the following breeding season. In total, 81% (17 out of 21) of nests fledged at least one young, and most of the fledglings survived to complete their post-fledging dispersal (Yu et al. unpubl. data). Furthermore, two captive-bred birds paired with two wild individuals that had dispersed long distances from the wild population in Yang Xian County.
Our results strongly lead us to recommend that: (1) The Crested Ibis was once a summer resident in the north of its former range, such as Siberia, north-eastern China and Hokkaido of Japan (Shi and Cao Reference Shi and Cao2001). Hence, further releases should be given priority in regions on the southern slope of the Qinling Mountains where the Crested Ibis was resident. Thus, the forthcoming Crested Ibis release programme in Henan Province of central China must focus on how to guide the released birds to complete their migration (Yu unpubl. data). (2) At the meta-population level (Armstrong and Seddon Reference Armstrong and Seddon2007), multiple releases should be conducted among different sites away from the core (wild) population in Yangxian County. Each reintroduced population would be expected to become a satellite population once the wild individuals dispersed to the reintroduced areas to mate with released individuals. (3) Birds that have previously bred in captivity, and their offspring, should be a high priority for release into the wild. (4) Although continued releases at one site are not always necessary (Armstrong and Ewen Reference Armstrong and Ewen2001), the study population would have benefited from additional releases, because new pair formations continued to occur after subsequent releases. (5) In the wild population, the mean weight of supplementary food per nest was significantly correlated with the overall breeding success of each year (Yu et al. Reference Yu, Liu, Xi and Lu2006). Food supplementation, especially in the breeding season, is therefore essential to guarantee the survival of more releases. (6) Public education should be implemented to inform local people of the purpose of ibis conservation and on how to get involved.
Acknowledgements
We are very grateful to the reintroduction base of Crested Ibis in Ningshan County for logistical help and research aid. We thank Cao Hai-xin, Shi Liang, Guo Jun-feng and Ma Xiao-chun for obtaining the breeding data, Ren Wei for editing the manuscript, Wang Yu-zhou for drawing the maps, and Ningshan County meteorological station for providing meteorological information. This work was completely supported by National Nature Science Foundation of China under grant number 31172103.






