INTRODUCTION
In 2003, a new methicillin-resistant Staphylococcus aureus (MRSA) clone emerged in livestock, particularly pigs, and in individuals in contact with livestock [Reference Armand-Lefevre, Ruimy and Andremont1, Reference Voss2]. Subsequently, this livestock-associated MRSA (LA-MRSA) clone has been identified throughout Europe [3], Canada [Reference Khanna4], and the USA [Reference Smith5]. The analysis of LA-MRSA isolates identified four features that are characteristic for LA-MRSA: (i) they are non-typable by pulsed-field gel electrophoresis (PFGE) using SmaI [Reference Bens, Voss and Klaassen6]; (ii) they belong to clonal complex (CC) 398 by multi-locus sequence typing (MLST) [Reference Enright7]; (iii) the staphylococcal chromosomal cassette (SCC)mec types IV and V predominate but are structurally different from the corresponding types carried by MRSA genotypes endemic in community and healthcare settings [Reference Li8]; and (iv) most isolates lacks toxins such as Panton–Valentine leukocidin (PVL) and other enterotoxins [Reference Hallin9].
LA-MRSA seems to be less virulent and less transmissible than community- and healthcare-associated MRSA [Reference van Rijen, Van Keulen and Kluytmans10–Reference Bootsma12], although secondary transmission to household members, people and animals in contact has been reported [Reference Denis13]. In a Danish case-control study, a relatively high proportion of case-patients reported skin and soft tissue infections (10/21), one patient developed sinusitis, and another developed bacteraemia after knee surgery [Reference Lewis14].
In Europe, geographical variation in the number of human LA-MRSA cases (both asymptomatic carriage and infections) is directly linked to pig and veal calf densities [Reference van Cleef15]. In areas where LA-MRSA is endemic in the agricultural setting, the carriage rate in pig farm workers is higher than in any other population, including patients with predisposing risk factors such as exposure to foreign healthcare facilities [Reference van Rijen, Van Keulen and Kluytmans10]. In countries with a low incidence of MRSA, this epidemiological change threatens existing control policies developed to prevent spread of MRSA in the community and into hospitals. For example, a Dutch survey conducted during 2002–2006 found that 32% of patients carried MRSA on admission to a hospital located in an area with a high pig density compared to a nationwide incidence of 0·03% in 2000 [Reference van Rijen, Van Keulen and Kluytmans10].
In this study, we assessed the prevalence of LA-MRSA in veterinarians from two countries, Belgium and Denmark, representing areas with high and low proportions (>tenfold difference) of positive pig farms, respectively, as previously reported [3]. We hypothesized that veterinarians working with livestock constitute an occupational risk group due to frequent contact with livestock and that the individual risk for LA-MRSA carriage is correlated with the proportion of LA-MRSA in the agricultural setting. For appropriate adaptation of control and protective measures specific for veterinarians (and indirectly for their household members), a variety of potential risk factors modified according to their profession were also examined.
MATERIALS AND METHODS
Study design and population
A cross-sectional prevalence study was conducted from February to April 2010 in Belgium and from February to October 2010 in Denmark. A total of 1215 veterinarians (Belgium, n=800; Denmark, n=415) were invited to participate in the study after receiving full information from the investigators. In Belgium, veterinarians were randomly selected from two registers, Nederlandstalige Orde der Dierenartsen and Conseil regional francophone de l'Ordre de Médecin Vétérinaires. In Denmark, two groups of veterinarians were invited to participate in the study: all veterinary practitioners with specialization for particular animals (i.e. pigs, cattle, horses, companion animals) registered with the Danish Veterinary Association (n=279) and all veterinary meat inspectors, responsible for inspection of live animals before slaughter, registered with the Danish Veterinary and Food Administration (n=136).
The study was approved by the Ethics Committee of the ULB-Erasme Hospital (protocol no. P2009/244) or the Danish National Committee on Biomedical Research Ethics (protocol no. H-4-2009-112), and the Danish Data Protection Agency (protocol no. 2009-54-0821). The volunteers signed an informed consent form and were asked to agree to self-administered nasal swabbing and to answer a standard questionnaire, which was designed to capture demographic, lifestyle, and environmental data as well as information on known/identified risk factors for MRSA (including medical history, antibiotic usage, contact sports, and travel) and animal-related exposures (including living or working on a farm, living with a farm worker, intensity and type of exposure to animals, and hygienic/protective measures). Nasal swabs (Venturi Transystem, Copan Innovation, Italy) were taken by the veterinarians themselves according to the manufacturer's instructions and were sent to Erasme Hospital (Brussels, Belgium) or to Statens Serum Institut (Copenhagen, Denmark) in transport medium.
MRSA isolation and characterization
Nasal swabs were analysed individually. MRSA isolation was performed using standard methods described previously [Reference Denis13]. Identification of all S. aureus isolates, including presence of the mecA gene (conferring resistance to β-lactam antibiotics) and lukF-lukS genes (encoding PVL), was performed using a multiplex PCR assay [Reference Larsen, Stegger and Sørum16]. Isolates were further characterized by MLST typing [Reference Enright7], spa typing [Reference Harmsen17], and SCCmec typing [Reference Kondo18]. The presence of 10 resistance genes [tet(K), tet(M), aac(6′)-Ie+aph(2″), ant(4′)-Ia, aph(3′)-IIIa, aadC, ermA, ermC, msrA/mrsB, vatB] was investigated in all isolates, using multiplex PCR assays and primers that have been described previously [Reference Vanhoof19–Reference Ng21].
Antimicrobial susceptibilities
The antimicrobial susceptibility profiles (spectinomycin, gentamicin, kanamycin, tobramycin, rifampin, trimethoprim-sulfamethoxazole, clindamycin, erythromycin, linezolid, chloramphenicol, mupirocin, ciprofloxacin, minocycline, tetracycline, fusidic acid) of all isolates were determined by the Kirby–Bauer disk diffusion method using Neo-Sensitabs (Rosco, Denmark), in accordance with the Clinical Laboratory Standards Institute (CLSI) guidelines with modifications for interpretation according to the manufacturer's instructions (details can be found in the manufacturer's database) [22]. Vancomycin susceptibility was determined onto brain heart infusion agar (BHIA). Briefly, 10 μl of a 0·5 McFarland suspension was spotted onto BHIA supplemented with 2 and 3 μg/ml vancomycin and incubated at 35°C for 48 h. Any growth was interpreted as positive. Multidrug resistance was defined as resistance to ⩾4 non-β-lactam antimicrobial classes.
Statistical analysis
In a first model, it was attempted to identify risk factors using a univariable analysis. Therefore a single-factor exact logistic regression was performed for the presence or absence of MRSA/ST398 as binary outcome. The variables with a P value ⩽0·25 were withheld for the multivariable regression model. The multivariable model was built forwards, gradually including the variables to the model and only retaining the significant factors. Interactions were checked for all significant main factors in the model. A median-unbiased estimator (MUE) was used to estimate odds ratios in case of null values.
For both models, the significance level was set at 5% (P⩽0·05). All models were built in Stata version 10.0 (Stata Corporation, USA).
RESULTS
Study population
A total number of 289 veterinarians signed the informed consent form and returned the nasal swab and questionnaire. The response rates were 18·3% (146/800) and 34·4% (143/415) for Belgium and Denmark, respectively. In Belgium, volunteering rates differed between occupational groups and were lower for veterinarians lacking exposure to livestock. The proportions of veterinarians working with livestock (defined in this study as professional contact on a daily basis with pigs, ruminants, and poultry) were 71·9% and 67·8% for veterinarians from Belgium and Denmark, respectively.
Molecular and phenotypic characterization
Of the 289 veterinarians, 16 carried MRSA (Belgium, n=14; Denmark, n=2) of which 13 belonged to MLST type ST398 (Belgium, 11/14; Denmark, 2/2), whereas the remaining three isolates from Belgian veterinarians belonged to other MLST types (ST1, ST5, ST45) (Table 1). All isolates lacked the lukF-lukS genes (encoding PVL). The spa and SCCmec types as well as resistance gene and antibiotic susceptibility profiles were heterogeneous within ST398 (Table 1). An important characteristic of these MRSA ST398 isolates was uniform resistance to tetracycline (13/13) due to the presence of the tet(M) determinant (13/13). Of note, the proportion of multidrug-resistant isolates was higher in ST398 MRSA isolates (7/13) than in non-ST398 MRSA isolates (0/3). However, this difference was not statistically significant (P=0·0901).
Table 1. Molecular and phenotypic characteristics of methicillin-resistant Staphylococcus aureus isolates from veterinarians in Belgium and Denmark
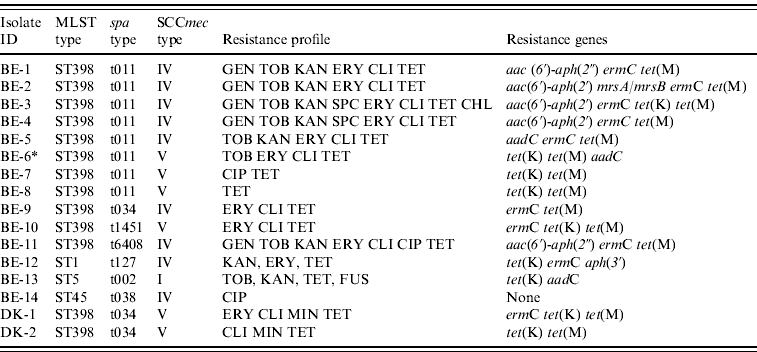
BE, Belgium; DK, Denmark; MLST, multi-locus sequence typing; SCCmec, staphylococcal chromosomal cassette mec; GEN, gentamicin; TOB, tobramycin; KAN, kanamycin; SPC: spectinomycin, ERY, erythromycin; CLI, clindamycin; CHL, chloramphenicol; CIP, ciprofloxacin; MIN, minocycline; TET, tetracycline; FUS, fusidic acid.
* Erythromycin and clindamycin resistance could not be detected with the PCR used.
Risk factors for LA-MRSA carriage
The MRSA and LA-MRSA carriage rates were 9·5% (95% CI 5·3–15·6) and 7·5% (95% CI 3·8–13·1) for MRSA and LA-MRSA, respectively, in Belgium and 1·4% (95% CI 0·17–5·05) in Denmark (all Danish MRSA isolates belonged to the LA-MRSA genotype) which supports that Belgian veterinarians have a higher risk of MRSA carriage in general and of LA-MRSA carriage in particular than Danish veterinarians (Table 2).
Table 2. Carriage rates of methicillin-resistant Staphylococcus aureus isolates among veterinarians from Belgium and Denmark
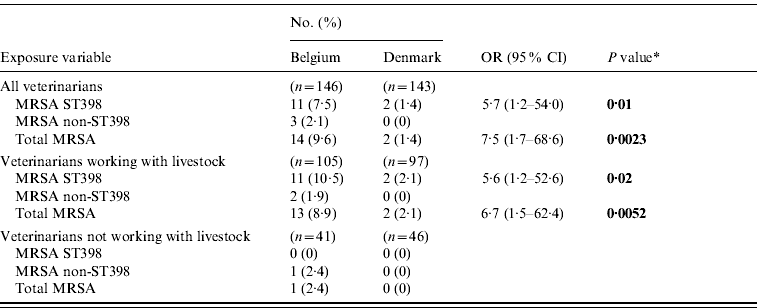
OR, Odds ratio; CI, confidence interval.
* Significant exposure variables are shown in bold (based on a P value of ⩽0·05).
Due to a lack of variance in the descriptive results for the Danish veterinarians combined with their low carriage prevalence found, further analysis was limited to the Belgian data. The female/male ratio and the mean age of Belgian participants were 0·52 and 45·1 years, respectively. Univariable analysis, with nasal carriage of LA-MRSA as the endpoint, showed that working with livestock was a significant risk factor (Table 3). In contrast, neither exposure to other animals nor risk factors defined by demographic, lifestyle, environmental characteristics, medical history, or antimicrobial exposure were found to be significantly associated with nasal carriage of LA-MRSA, except for male sex and living with a healthcare worker. Veterinarians working with pigs were at higher risk of LA-MRSA carriage than those working with cattle. The highest risk was associated with exposure to live pigs. Interestingly, exposure to cattle within 24 h before sampling was a significant risk factor, whereas exposure to pigs within 24 h before sampling was not associated with carriage. No association was found to exposure to other farm animals, sheep, goats, poultry, neither to horses nor to companion animals. None of the following non-related occupational exposure variables, like smoking, living in the countryside, living with a farm worker, travel abroad, contact with healthcare institutions in the last 12 months, use of antimicrobials drugs during the last month before sampling and skin disorders could be associated with LA-MRSA carriage. The univariable analysis could not demonstrate that hygiene and protective measures (e.g. hand washing, use of gloves and mask) had a protective effect due to incomplete data for this variable. Statistically, working in a small animal veterinary clinic was protective. In the multivariable analysis, a negative association was observed between LA-MRSA acquisition and working in a small animal veterinary clinic (OR 0·15, 95% CI 0–1·0, P=0·05); however, the limited sample size does not allow us to clearly show a direct association. The strongest significant direct association found was between LA-MRSA acquisition and working with live pigs (OR 12·1, 95% CI 1·6–548·5, P=0·01).
Table 3. Statistically significant associations by univariable analysis for MRSA ST398 carriage in Belgian veterinarians

MUE, Median unbiased estimates.
* Significant exposure variables (based on a P value of ⩽0·05).
DISCUSSION
The MRSA and LA-MRSA carriage rates were significantly higher for Belgian veterinarians (9·6% and 7·5%, respectively) than for Danish veterinarians (1·4%), with LA-MRSA accounting for 78·6% and 100·0% of the cases, respectively. The difference in carriage rate may be explained by geographical variations in MRSA–animal host associations and animal population structures. Moreover, considering the MRSA status of the Belgium food production system, it seems that large differences exist between both countries. In 2008, it was reported that the proportion of positive pig farms was tenfold higher for Belgium than for Denmark (40·0% and 35·9% vs. 0·0% and 3·5% for breeding and production holdings, respectively) [3]. Comparative data from Belgium and Denmark on MRSA prevalence in veal farms are currently lacking; however, in a recent study it has been shown that LA-MRSA was highly prevalent in Belgium veal calf farms (64%) [23]. Therefore, it seems that Belgium has a large potential LA-MRSA reservoir in its production system that may in turn represent a threat to people in contact with it. The MRSA carriage rates observed in this study are in accord with previous findings of veterinarians from other European countries, with carriage rates ranging from 3% to 17·9% depending on the country and the type of practice [Reference Moodley24–Reference Wulf26]. Moreover, the carriage rate of LA-MRSA in veterinarians working with livestock compared to pig farm workers was lower in both Belgium (10·5%, 95% CI 5·2–18·7 vs. 37·8, 95% CI 25·6–50) [Reference Denis13] and Denmark (2·1%, 95% CI 0·3–7·5 vs. 3·1%, 95% CI 1·7–5·0) [27]. Furthermore, the observed carriage rates were about tenfold higher than those in the general population (Belgium, 0·5%; Denmark, 0·2%) ([Reference Nulens28], R. Skov, unpublished data) and were comparable to the levels found in Belgian nursing-home residents (19·5%) [Reference Denis29]. Thus, from a healthcare point of view, this segment of the population can be considered as a group of concern and measures to protect them and the healthcare system should be considered.
In the univariable analysis, the predominant factors affecting nasal carriage of LA-MRSA among Belgian veterinarians were exposure to pigs and, to a lesser extent, cattle. This is in accord with previous findings [Reference Denis13, Reference Lewis14, Reference Graveland30]. However, in the multivariable analysis, exposure to cattle was not retained as a risk factor and only exposure to live pigs was positively associated. Beside this, our results suggest that working with small animals in a veterinary clinic is negatively associated with LA-MRSA acquisition. This observation is in agreement with a recent Belgium study in which LA-MRSA ST398 could not be detected in dogs [Reference Vanderhaeghen31] and suggests that the small animal population does not seem to constitute a significant LA-MRSA reservoir. However, those findings should be viewed with caution and require further studies including larger populations. In our study the sample size was limited to a small number and the proportion of participants in the category ‘working with small animals in a veterinary clinic’ was about twofold lower compared to the category of veterinarians working with livestock.
The LA-MRSA isolates identified in this study shared characteristics with the main genotypes reported in Europe [Reference Li8, Reference Moodley24–Reference Wulf26, Reference Graveland30]. The majority of LA-MRSA isolates (53·8%) showed a multidrug-resistant phenotype, which supports the hypothesis that this clone has adapted to the antimicrobial pressure encountered in intensive animal husbandry [Reference Kadlec32, Reference Fessler, Kadlec and Schwarz33]. In particular, the fact that genes resistant to a large variety of antibiotics are present in zoonotic bacteria is of great concern for public health since it may lead to limit and failure of human therapy when treating infection.
The non-ST398 MRSA strains found in this study belong to genotypes commonly associated with hospital- or community-acquired infections in humans, which have recently been reported in pigs and poultry. However, the limited number of isolates does not allow us to suggest any association.
This study has a limitation. Since veterinary practices appear to differ within and between countries, comparison of MRSA prevalence by animals species could not be accurately evaluated and MRSA prevalence was determined by category, livestock contact vs. no livestock contact, therefore prevalence has to be interpreted with caution.
In conclusion, veterinarians are at high risk of acquiring LA-MRSA through exposure to pigs, and particularly to live pigs, rather than other animal species. The fact that carriage was not dependent on a recent exposure to pigs may suggest that veterinarians could become chronic carriers and therefore they could be implicated in the spread of LA-MRSA between farms. Long-term epidemiological studies are currently underway to investigate this hypothesis.
ACKNOWLEDGEMENTS
The authors thank the participants from Belgium and Denmark for kindly agreeing to take part in the study. We thank the Nederlandstalige Orde der Dierenartsen and the Conseil regional francophone de l'Ordre de Médecin Vétérinaires in Belgium and the Danish Veterinary Association and the Danish Veterinary and Food Administration for administrative support during recruitment of participants. Marie-Laurence Lambert is acknowledged for help during the initial study design.
This work was supported by the EU-HEALTH-Project PILGRIM (223050) of the Seventh Framework Programme. C. Garcia-Graells, J. Larsen, R. Skov and O. Denis are members of the EU-FP7 PILGRIM Consortium.
DECLARATION OF INTEREST
None.





