Brain-derived neurotrophic factor (BDNF) is a member of the nerve growth factor-related family(Reference Fujinami, Ohta and Obayashi1) and is a homodimeric protein that has been highly conserved in structure and function during evolution(Reference Jones and Reichardt2, Reference Maisonpierre, Le Beau and Espinosa3). BDNF is a secreted protein that, in human subjects, is encoded by the BDNF gene(Reference Maisonpierre, Le Beau and Espinosa3). It is involved in development, maintenance and function of the central nervous system(Reference Tessarollo4, Reference Yamamoto, Sobue and Yamamoto5). BDNF is found in a wide range of tissues(Reference Maisonpierre, Le Beau and Espinosa3) and it can be self-regulated(Reference Cunha, Angelucci and D'Antoni6, Reference Cunha, Brambilla and Thomas7). BDNF has several documented short- and long-term functional roles. It is now well-known that BDNF serves as a target-derived survival and differentiation factor for neuronal sub-populations in prenatal stages(Reference Grothe and Unsicker8–Reference Snider10). Likewise, BDNF promotes long-term potentiation by potentiating excitatory neurotransmitter activity in the hippocampus(Reference Boulanger and Poo11) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor insertion post-synaptically(Reference Li and Keifer12). These actions, along with BDNF's ability to stabilise dendritic spines(Reference Tanaka, Horiike and Matsuzaki13), underlie the molecule's important role in learning, memory and behaviour(Reference Cunha, Brambilla and Thomas7). In addition, BDNF levels increase in response to certain forms of injury, such as ischaemic–hypoxic(Reference Han and Holtzman14) and infectious insults(Reference Bifrare, Kummer and Joss15), presumably by blocking apoptosis. Various studies have shown a link between BDNF and certain health conditions, such as depression(Reference Brunoni, Lopes and Fregni16, Reference Dwivedi17), obsessive–compulsive disorder(Reference Maina, Rosso and Zanardini18), Alzheimer's disease(Reference Zuccato and Cattaneo19), dementia(Reference Arancio and Chao20) and Parkinson's disease(Reference Fumagalli, Racagni and Riva21, Reference Chun, Son and Son22).
Despite its name, BDNF is found in a variety of tissues and cell types, not just in the brain. It is also expressed in cardiovascular, immune, reproductive and endocrine tissues(Reference Tessarollo4, Reference Yamamoto, Sobue and Yamamoto5, Reference Seifer, Feng and Shelden23). Intact BDNF readily crosses the blood–brain barrier via a high-capacity transporter system(Reference Pan, Banks and Fasold24). Interestingly, exercise has been shown to increase the expression of BDNF in human subjects(Reference Kramer, Colcombe and McAuley25–Reference Kramer, Hahn and Cohen27) and a similar effect was observed after caffeine application in vitro (Reference Connolly and Tamy28) and in vivo (Reference Costa, Botton and Mioranzza29). Caffeine has been recently proposed as a potential candidate for maintaining physiological levels of BDNF, as it is capable of positively affecting cognition(Reference Costa, Botton and Mioranzza29, Reference Alhaider, Aleisa and Tran30). In the present study, we tested polyphenol-rich natural products containing different amount of caffeine to see if a single dose could lead to an increase in plasma levels of BDNF. Three natural products containing varying amounts of caffeine were selected for the present pilot study: green coffee caffeine (N677), green coffee bean extract (N625) and whole coffee fruit concentrate powder (WCFC)(Reference Heimbach, Marone and Hunter31). A grape seed extract (N31) containing high levels of polyphenols, but not caffeine(Reference Yashin, Nemzer and Ryzhnev32), was also tested.
Materials and methods
Materials
Extract powders tested in the present study were provided by FutureCeuticals, Inc. WCFC is a patented extract of whole coffee fruit (coffee berries) from Coffea arabica. Chemical composition and polyphenol profiles of each tested extract appear in Table 1. Dulbecco's PBS and water were purchased from Sigma Chemical Company. Protein low binding microtubes were obtained from Eppendorf and RC DC Protein Assay Kit II was purchased from Bio-Rad. Human BDNF Quantikine ELISA kits were from R&D Systems. Heparin blood collection tubes were obtained from Ram Scientific, Inc. and lancets were purchased from Medlance®. Silica oxide used as placebo was also purchased from Sigma Chemical Company.
Table 1 Amount of caffeine, polyphenols and procyanidins in extracts tested in the present study
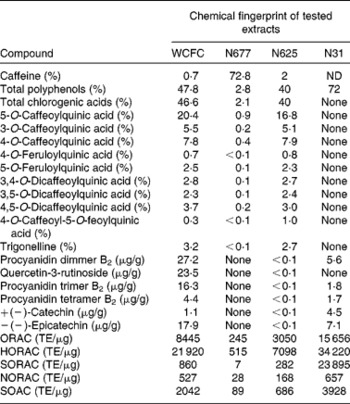
WCFC, whole coffee fruit concentrate powder; N677, green coffee caffeine powder; N625, green coffee bean extract powder; N31, grape seed extract powder; ND, not determined; ORAC, oxygen radical absorbance capacity; TE, Trolox™ equivalents; HORAC, hydroxyl oxygen radical absorbance capacity; SORAC, superoxide radical absorbance capacity; NORAC, peroxynitrite radical absorbance capacity; SOAC, singlet oxygen radical absorbance capacity.
Study description
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Institutional Review Board at Vita Clinical S.A. Avenida Circunvalacion Norte #135, Guadalajara, JAL, Mexico 44 270 (study protocol no. 12-06 ABC-BDNF). The present pilot acute clinical study was performed on healthy fasted subjects treated once with a single dose of tested material, placebo (silica oxide) or vehicle only (water). All study subjects were generally healthy, non-smokers and did not use any type of medication or supplement for a period of 15 d prior to the start of the study. The inclusion criteria required participants to be between the ages of 18 and 55 years and have a BMI between 18·0 and 25·0 kg/m2(Reference Borecki, Higgins and Schreiner33). At the time of the study, participants were free of rhinitis, influenza and other symptoms of upper respiratory infection. To minimise confounding effects, all subjects remained in the testing facility during experimentation to avoid the possibility of blood BDNF increase due to physical activity and exercise(Reference Zolodz, Majerczak and Grandys34). In addition, all subjects were tested during the same time of day to minimise any differences in blood BDNF due to diurnal effect(Reference Piccini, Marazziti and Del Debbio35). Participants were excluded if they had diabetes mellitus, a known allergy to any of the test ingredients or were using any anti-inflammatory, analgesic, anti-allergy, anti-depressant medication or multivitamins. Participants received oral and written information about the experimental procedures before giving their written consent.
For the present study, twenty-five(Reference Kramer, Colcombe and McAuley25) subjects were randomly divided into groups of five to receive one of the five treatments: N677, N625, N31, WCFC or placebo (silica oxide). In follow-up studies performed under the same experimental conditions, five participants received WCFC, chlorogenic acid or water as vehicle (no treatment group). In all cases, subjects fasted for 12 h prior to the first blood collection. Other than consuming one of the study materials or placebo, patients had no intake per os during the study period. Blood was collected at baseline (T 0) and subsequent samples were collected at every 30 min (T 30, T 60, T 90 and T 120) after the treatment.
Brain-derived neurotrophic factor detection and quantification
For the isolation of plasma, 100 μl finger blood were collected by finger puncture and placed in Safe-T-Fill® Capillary blood collection tubes (Ram Scientific, Inc.) and centrifuged at 1000 g for 10 min. Blood was transferred to protein low binding tubes and kept at − 80°C until use. BDNF was measured using a quantitative sandwich ELISA immunoassay (R&D Systems) following the instructions provided in the kit, using the buffers and calibrators specific for plasma. Final reactions were measured using a spectrophotometer (Molecular Devices) at 450 and 540 nm wavelengths, and final concentrations were calculated from a standard curve.
Chemical analyses
Chlorogenic acid, procyanidins, flavanols and flavonols of WCFC, N625 and N31 were characterised by LC–MS(n) and quantified by UV absorbance(Reference Mullen, Nemzer and Ou36). Total polyphenol content was determined by spectrophotometry according to the Folin–Ciocalteu method(37), and was calibrated against gallic acid standard (Sigma-Aldrich). Results were expressed as grams of gallic acid equivalents.
The caffeine and trigonelline contents were characterised by HPLC Agilent 1100 (Agilent Technologies) equipped with a diode array detector and quantified by UV absorbance (W Mullen, B Nemzer, M Clifford, et al., unpublished results). Antioxidant capacities of coffee fruit extract, coffee bean extract and N31 were characterised by the ability of the samples to scavenge peroxyl radicals (oxygen radical absorbance capacity), hydroxyl radicals (hydroxyl oxygen radical absorbance capacity), peroxynitrite (peroxynitrite radical absorbance capacity), superoxide anions (superoxide radical absorbance capacity) and singlet oxygen (singlet oxygen radical absorbance capacity)(Reference Huang, Ou and Hampsch-Woodill39–Reference Zhang, Huang and Kondo41).
Statistical analysis
BDNF levels were compared with a reference standard curve and each subject was normalised to their own value measured at time zero (T 0). Peak levels of plasma BDNF for each patient were used for comparisons. Results were pooled and standard error of the mean was used for each separate analysis. Plasma BDNF levels at 60 min after treatment were compared with baseline using a two-tail, independent Student's t test. Power analyses were run using G*Power Data Analysis (Heinrich-Heine-University Düsseldorf). Statistical power was run for the whole group and it was also calculated per-pair power for individual comparison. Descriptive analysis was run in GraphPad to derive the mean and standard deviation for each group.
Results and discussion
Four polyphenol-rich fruit extracts were tested in healthy subjects. Three of the extracts contained caffeine in varying amounts (WCFC: 0·7 % caffeine; N677: 72·8 % caffeine; and N625: 2 % caffeine) and one extract was caffeine free (N31) (see Table 1). Of the substances tested, WCFC increased BDNF plasma levels (Fig. 1) in patients by an average of 137 % with respect to baseline (range 65–222 %; P= 0·001 v. placebo). N677 showed an increase of 42 %, but was not statistically significant (P= 0·49). N625 did not cause a significant increase in BDNF. N31 increased BDNF levels in plasma by 30 % with respect to baseline, though not significantly (P= 0·65).

Fig. 1 Blood levels of brain-derived neurotrophic factor (BDNF) collected from subjects treated with whole coffee fruit concentrate powder (WCFC), green coffee bean extract powder (N625), green coffee caffeine powder (N677), grape seed extract powder (N31) and placebo (silica). Data represent average percentage difference from administration (T 0). * Mean value was significantly different compared with placebo by Student's t test (P< 0·05).
It is important to note that a power analysis of the entire sample indicated that forty subjects would be required to reach a power of 80 %. Our sample size was twenty-five, therefore one must interpret the lack of BDNF effect of N677, N625 and N31 with caution. Larger groups are required to make definitive conclusions for these treatments. However, the result of this power analysis does not diminish the statistical significance of WCFC on plasma BDNF levels. Indeed, a separate per-pair power analysis of WCFC and placebo showed a sample size of ten to have a greater than 99 % power.
Quite unexpectedly, treatment with placebo (silica dioxide) resulted in a 34 % reduction in BDNF blood levels (P= 0·09). Silica oxide was used as placebo, as it is generally considered to be an inert material. It has been previously reported that stress can decrease plasma levels of BDNF in some human and animal models(Reference Elzinga, Molendijk and Oude Voshaar42–Reference Advani, Koek and Hensler44). However, the reduction of plasma BDNF due to the intake of silica/placebo has not been reported. Consequently, a second experiment was performed to verify the reproducibility of the effect of WCFC on BDNF and to test the effect of extended fasting (untreated control) on the baseline level of BDNF in blood.
A total of five additional healthy subjects who met the same inclusion criteria were treated with 100 mg WCFC. As before, all groups fasted for 12 h prior to testing, but the control group did not receive any treatment besides water as vehicle (200 ml) as it was used for treatment with WCFC. As shown in Fig. 2, BDNF plasma levels were increased in subjects treated with 100 mg of WCFC (148 % increase; P= 0·002). However, blood BDNF level in the untreated group was not statistically changed (15 % increase by average).
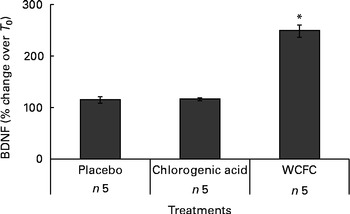
Fig. 2 Blood levels of brain-derived neurotrophic factor (BDNF) measured in non-treated subjects (placebo), subjects receiving 50 mg of chlorogenic acid or 100 mg whole coffee fruit concentrate powder (WCFC). Data are presented as the average percentage difference compared with baseline (T 0). * Mean value was significantly different compared with placebo by Student's t test (P< 0·05).
Pooling all ten subjects from the two studies, treatment with 100 mg of WCFC caused a 143 % increase in BDNF plasma levels. The stimulatory effect of caffeine on BDNF has been previously observed(Reference Costa, Botton and Mioranzza29, Reference Alhaider, Aleisa and Tran30, Reference Capiotti, Menezes and Nazario45, Reference Bairam, Kinkead and Lajeunesse46). However, as presented in Fig. 1, single-dose treatment with caffeine-containing extracts resulted in an increased level of plasma BDNF in a caffeine concentration-independent manner. As shown in Table 1, N677 is mostly comprised of caffeine (72·8 % by weight), yet caused only modest increases in plasma BDNF levels. The most profound increases in plasma BDNF were observed after treatment with WCFC, although this extract contains only 0·7 % caffeine by weight.
Previous reports have shown that procyanidins extracted from grape seeds are capable of stimulating neurotrophic factors in aged rats(Reference Xu, Rong and Xie47). These compounds have also been implicated in the regulation of metabolic disorders(Reference Fraga and Oteiza48, Reference Terra, Pallares and Ardevol49) that are BDNF dependent. The N31 used in the present study had relatively high polyphenol levels compared with the coffee fruit, coffee caffeine and coffee seed extracts, yet failed to significantly increase BDNF in blood.
This result suggests that the stimulatory effect of WCFC on the blood level of BDNF is not associated with the amount of polyphenols or caffeine per dose. Rather, the effect may be related to either the amount of procyanidins or to the unique coffee polyphenol profile of the WCFC material. According to the present analyses, WCFC shows a significant amount of procyanidins in comparison to N31, N625 and N677 (Table 1), suggesting that acute treatment with procyanidin-rich whole coffee fruit extracts (and possibly other procyanidin-rich extracts), may increase blood levels of BDNF in human subjects. Future work could include testing other procyanidin-rich extracts for their ability to raise plasma BDNF in order to confirm this hypothesis.
The amount of trigonelline also varied in each material tested (Table 1). As presented, WCFC and N625 contain the highest amount of this compound; however, the effect of WCFC on plasma BDNF is superior to the effect of N625 under the same experimental conditions, suggesting that this is not the primary agent responsible for increased BDNF levels in blood.
As WCFC contains high amounts of chlorogenic acid, it was hypothesised that this specific polyphenolic acid may cause an increase in blood level of BDNF. Consequently, we administered 50 mg of chlorogenic acid as a single dose to five healthy subjects. As presented in Fig. 2, chlorogenic acid did not increase blood level of BDNF in a statistically significant manner (P= 0·89), suggesting that this substance is not responsible for the ability of WCFC to increase BDNF.
The compounds tested in the present study had different concentrations of caffeine and polyphenols (Table 1). It is important to note that the present results show the percentage of caffeine within each tested compound, rather than an absolute mass of caffeine. Nevertheless, the present work suggests that procyanidins may have the ability to increase plasma BDNF levels and, perhaps, to a larger extent than caffeine itself. This is particularly interesting considering that recent research show that procyanidin oligomers play a role in neuroprotection from excitotoxic injury(Reference Narita, Hisamoto and Okuda50).
BDNF-dependent telomerase activity has been shown to promote neuron survival in developing hippocampal neurons(Reference Fu, Lu and Mattson51). Increased BDNF expression and telomerase activity after brain injury suggest that telomerase may play a role in BDNF-mediated neuroprotection. Furthermore, BDNF has been shown to up-regulate telomerase expression and activity in spinal motor neurons(52). These neurons, treated with BDNF, are more resistant to ex-cytotoxic injury, presumably from increased cellular resistance to apoptosis. It is interesting to speculate that WCFC possibly may also exert an anti-apoptotic effect through telomerase by increased BDNF activity.
WCFC was tested under experimental conditions reducing impact of possible confounding effects; for example, non-smokers were not included in the present study, since nicotine is known to increase blood levels of BDNF(Reference Wang, Liao and Yuan53). All subjects remained in the clinical site during the duration of the study to avoid any shifts in blood level of BDNF due to exercise and physical activity(Reference Zolodz, Majerczak and Grandys34). Finally, the present clinical study was performed in the morning starting at 08.00 hours, as it was reported that blood levels of BDNF are increased due to diurnal effect(Reference Piccini, Marazziti and Del Debbio35).
In summary, WCFC increased the blood level of BDNF during first 60 min after treatment with a dose of 100 mg. In order to confirm the results of the present pilot study, further clinical testing in a larger group is required.
Acknowledgements
The present study was funded by Futureceuticals, Inc. T. R.-I. conducted the experimental work, analysed the data and led the manuscript writing. C. S., L. H. and R. A. performed serum tests and helped in the data analysis. R. K. organised and helped execute clinical protocol of the study. B. N. designed and conducted all the chemical analysis. Z. P. designed and directed the study. We would like to thank Michael Sapko for his help in editing the manuscript. All authors declare that they have no conflicts of interest.





