Introduction
As a result of rapid industrialization and urbanization, heavy metal pollution in agricultural soils has become an important global concern with serious threats to human health and agricultural production (Devi et al., Reference Devi, Behera, Raza, Mangal, Altaf, Kumar, Kumar, Tiwari, Lal and Singh2022; Hou et al., Reference Hou, O'Connor, Igalavithana, Alessi, Luo, Tsang, Sparks, Yamauchi, Rinklebe and Ok2020). This is particularly important for agricultural soils irrigated with wastewater that usually contains high concentrations of heavy metals (Rattan et al., Reference Rattan, Datta, Chhonkar, Suribabu and Singh2005; Khan et al., Reference Khan, Cao, Zheng, Huang and Zhu2008; Hou et al., Reference Hou, O'Connor, Igalavithana, Alessi, Luo, Tsang, Sparks, Yamauchi, Rinklebe and Ok2020), because heavy metals originated from fertilizers and/or wastewater irrigation can accumulate in the tissues of numerous plant species (Amin et al., Reference Amin, Hussain, Alamzeb and Begum2013; Yan et al., Reference Yan, Wu, Fan, Zhang, Paw U, Zheng, Qiang, Guo, Zou, Xiang and Wu2020; Sun et al., Reference Sun, Zhang, Luo, Guo, Ma, Fan, Chen and Geng2022).
Heavy metal accumulation in plants varies among species, and even among populations and ecotypes of the same species (Rascio and Navari-Izzo, Reference Rascio and Navari-Izzo2011). However, previous studies have reported a significant accumulation of some heavy metals in vegetables, grains, and other edible plant structures consumed by humans as part of their daily diet (Rattan et al., Reference Rattan, Datta, Chhonkar, Suribabu and Singh2005; Sharma, Agrawal, and Marshall, Reference Sharma, Agrawal and Marshall2007; Khan et al., Reference Khan, Cao, Zheng, Huang and Zhu2008; Amin et al., Reference Amin, Hussain, Alamzeb and Begum2013). The consumption of food contaminated by heavy metals can deplete some essential nutrients in the body causing various diseases and result in a number of serious health problems in humans, including a high prevalence on gastrointestinal cancer, comma, renal damage, infertility, stroke, epilepsy, and neurodegenerative diseases, among others; with effects on children’s even more severe than adults (Khan et al., Reference Khan, Cao, Zheng, Huang and Zhu2008; Amin et al., Reference Amin, Hussain, Alamzeb and Begum2013; Alengebawy et al., Reference Alengebawy, Abdelkhalek, Qureshi and Wang2021). Consequently, there has been an increasing interest in developing technologies to reduce the accumulation of heavy metals in crops, including physical, chemical, and biological methodologies (Khalid et al., Reference Khalid, Shahid, Niazi, Murtaza, Bibi and Dumat2017). Among these, biological methodologies (i.e., bioremediation) have gained great attention because they are based on sustainable processes and more economically viable strategies (Khalid et al., Reference Khalid, Shahid, Niazi, Murtaza, Bibi and Dumat2017; Yaashikaa et al., Reference Yaashikaa, Kumar, Jeevanantham and Saravanan2022). One approach involves the use of biological materials as adsorbents for these metals, effectively decreasing their concentrations in soils and in cultivated plants, but limited information is available on this topic (Eltlbany et al., Reference Eltlbany, Baklawa, Ding, Nassal, Weber, Kandeler, Weber, Kandeler, Neumann, Ludewig, Van Overbeek and Small2019; Vukelić et al., Reference Vukelić, Racić, Bojović, Ćurčić, Mrkajić, Jovanović and Panković2020). However, the use of microorganisms such as filamentous fungi has shown advantages over biological adsorbents for bioremediation of soils and for the reduction of heavy metal concentration in crops due to their efficient ability to accumulate metals in their cell walls and cellular structures (i.e., biosorption), as well as for their ability to establish stable interactions with plants (i.e., endophytic fungi) (Audet and Charest, Reference Audet and Charest2007; Hildebrandt, Regvar, and Bothe, Reference Hildebrandt, Regvar and Bothe2007; Verma and Kuila, Reference Verma and Kuila2019; Anand et al., Reference Anand, Pal, Yadav, Singh, Tripathi, Choudhary, Shukla, Sunita, Kumar, Bontempi, Ma, Kolton and Singh2023; Hou et al., Reference Hou, O'Connor, Igalavithana, Alessi, Luo, Tsang, Sparks, Yamauchi, Rinklebe and Ok2020). These fungi are also known for their resilience and ability to thrive under extreme pH, temperature, and nutrient availability conditions, allowing their use under different environmental conditions in agricultural lands (Deng et al., Reference Deng, Fu, Hu, Lu, Wu and Bryan2020; Chugh et al., Reference Chugh, Kumar, Bhardwaj, Bharadvaja, Maulin, Rodriguez-Couto and Riti2022). Additionally, they can also enhance plant growth and productivity in contaminated soils (O'Sullivan et al., Reference O'Sullivan, Bonnett, McIntyre, Hochman and Wasson2019; Devi et al., Reference Devi, Behera, Raza, Mangal, Altaf, Kumar, Kumar, Tiwari, Lal and Singh2022).
There are several fungal species identified in previous studies for bioremediation of heavy metals in soils and/or reduction of heavy metal concentration in crops, including the species in the genera Acremonium, Alternaria, Aspergillus, Cladosporium, Fusarium, Ganoderma, Geotrichum, Monilia, Mortierella, Mucor, Penicillium, Rhizopus, Trichoderma, among other arbuscular mycorrhizal fungi (Priyadarshini et al., Reference Priyadarshini, Priyadarshini, Cousins and Pradhan2021; Devi et al., Reference Devi, Behera, Raza, Mangal, Altaf, Kumar, Kumar, Tiwari, Lal and Singh2022; Yaashikaa et al., Reference Yaashikaa, Kumar, Jeevanantham and Saravanan2022). Nevertheless, fungal species may be specific toward metal ions and plant species because of the variation in cellular compositions of microbial systems, the functional sites responsible for metal binding, and different cellular and molecular mechanisms employed to tolerate metals (Hildebrandt, Regvar, and Bothe, Reference Hildebrandt, Regvar and Bothe2007; Priyadarshini et al., Reference Priyadarshini, Priyadarshini, Cousins and Pradhan2021; Devi et al., Reference Devi, Behera, Raza, Mangal, Altaf, Kumar, Kumar, Tiwari, Lal and Singh2022). For cultivated plants, environmental and management conditions can also influence the effectiveness of heavy metal reduction by various fungal species (Priyadarshini et al., Reference Priyadarshini, Priyadarshini, Cousins and Pradhan2021; Devi et al., Reference Devi, Behera, Raza, Mangal, Altaf, Kumar, Kumar, Tiwari, Lal and Singh2022). Hence, suitable fungal species need to be identified for specific heavy metals and cultivated plants exposed to high heavy metal concentrations in different agricultural lands.
The Mezquital Valley in Hidalgo, Mexico encompasses 80,000 hectares of land dedicated to cultivating various crops such as alfalfa, maize, wheat, oats, chili peppers, cabbage, cilantro, spinach, parsley, and beans. These crops have traditionally been irrigated with untreated domestic and industrial wastewater from Mexico City for over a century (Hernández et al., Reference Hernández, Kampfner, Rodríguez and Meneses2017; García-Salazar, Reference García-Salazar2019), and several studies have reported the presence of heavy metals in the soil of Mezquital Valley (Lara et al., Reference Lara-Viveros, Ventura-Maza, Muhammad, Rodríguez, Vargas and Landero-Valenzuela2015; Ziegler-Rivera et al., Reference Ziegler-Rivera, Prado, Mora, Ponce de León-Hill and Siebe2022). The elevated levels of heavy metals found in the Mezquital Valley soil can be attributed to their low mobility in alkaline or neutral pH soils (Plant and Raiswell, Reference Plant, Raiswell and Thornton1983). Moreover, wastewater generated by industries utilizing Pb-containing compounds is subsequently used for crop irrigation in the region, further contributing to contamination.
Among the crops produced in this valley and despite the changes in dietary habits in the last decades, beans (Phaseolus vulgaris L.) along with maize (Zea mays) continue to be essential protein sources for more than 300 million people in Mexico. For instance, in 2020, the total area of bean cultivation reached 1,676,355 hectares, with an average national yield of 0.79 tons per hectare, resulting in an approximate per capita consumption of 8.4 kg per year (Gálvez and Salinas, Reference Gálvez and Salinas2015; OECD, 2019; SIAP, 2020; Mesele et al., Reference Mesele, Dibaba, Garbaba and Mendesil2022). Given that beans have a protein content ranging from 14 to 35%, 52 to 76% of carbohydrates, 1.5 to 6.5% of lipids, 14 to 20% of fiber, 3 to 5% of minerals, and a significant content of micronutrients, such as iron, zinc, thiamine, and folic acid, their cultivation play a critical role in national food security (Abbade and Dewes, Reference Abbade and Dewes2014; Petry et al., Reference Petry, Boy, Wirth and Hurrell2015; Chávez-Servia et al., Reference Chávez-Servia, Heredia-García, Mayek-Pérez, Aquino-Bolaños, Hernández-Delgado, Carrillo-Rodríguez, Hill-Langarica, Vera-Guzmán and Goyal2016; Chávez and Sánchez, Reference Chávez and Sánchez2017). Moreover, beans are also one of the most produced and consumed food grain legumes in the world (FAO, 1997).
Previous studies focused on evaluate phytotoxicity and atmospheric deposition of heavy metals on bean plants, found significant negative effects on plant physiology and crop productivity, and high deposition rates of atmospheric heavy metals in different plant structures (Aldoobie and Beltagi, Reference Aldoobie and Beltagi2013; Gjorgieva et al., Reference Gjorgieva, Kadifkova, Ruskovska, Bačeva and Stafilov2013; Cannata et al., Reference Cannata, Bertoli, Carvalho, Augusto, Bastos, Freitas and Carvalho2015; De Temmerman et al., Reference De Temmerman, Waegeneers, Ruttens and Vandermeiren2015; Hammami et al., Reference Hammami, Parsa, Bayat and Aminifard2022). In these studies, it was also found that the ability of bean plants to accumulate different heavy metals depends on the physicochemical conditions, heavy metal concentrations, and management of soil. However, in our knowledge there are not studies evaluating the effect of fungal inoculation on the reduction of heavy metal concentration in bean plants cultivated under a wastewater irrigation regime. Hence, it is crucial to conduct studies on microbial inoculation in bean crops to understand the impact of microorganisms on the accumulation of heavy metals in different parts of the plant, particularly in the grains, which represent the plant structure used for human consumption.
Trichoderma species are found in all types of soils and are commonly isolated from different ecosystems, since they are able to establish endophytic associations with plants (Woo et al., Reference Woo, Hermosa, Lorito and Monte2023; Racić et al., Reference Racić, Vukelić, Kordić, Radić, Lazović, Nešić and Panković2023). They have been used in previous studies for bioremediation because of their ability to tolerate, translocate and to act as biosorbents of different heavy metals (Babu et al., Reference Babu, Shim, Bang, Shea and Oh2014; Govarthanan et al., Reference Govarthanan, Mythili, Selvankumar, Kamala-Kannan and Kim2018; Khan et al., Reference Khan, Ali, Aftab, Shakir, Qayyum, Haleem and Tauseef2019; Maldaner et al., Reference Maldaner, Steffen, Missio, Saldanha, de Morais and Nicoloso2021; Talukdar et al., Reference Talukdar, Jasrotia, Sharma, Jaglan, Kumar, Vats, Kumar, Mahnashi and Umar2020; Racić et al., Reference Racić, Vukelić, Kordić, Radić, Lazović, Nešić and Panković2023). Previous studies have also suggested that the use of a combination of Trichoderma species or the combination with other fungal species can increase the effectiveness of metal mobilization in soil solutions (Kacprzak et al., Reference Kacprzak, Rosikon, Fijalkowski and Grobelak2014). Based on this, the aim of this study was to evaluate the potential reduction of heavy metal concentration in bean plants cultivated in soils irrigated with untreated wastewater in Hidalgo, Mexico by inoculating different Trichoderma spp. and combinations of these species, in order to identify strategies to ameliorate the health impacts related to the consumption of heavy metal contaminated crops, as well to identify fungal strains that can potentially be used for bioremediation of contaminated soils in future studies.
Materials and methods
Study site
The study was conducted on an open experimental field at the Universidad Politécnica de Francisco I. Madero, located in Tepatepec, Francisco I. Madero, Hidalgo, Mexico (20° 13′ 27″ N, 99° 05′ 21″ W) at 1977 masl. Francisco I. Madero is one of the 28 municipalities conforming the Mezquital Valley, a semiarid region with a mean temperature of 16 ± 7°C and an average annual rainfall of 578 mm at elevations around 2000 m (Friedel et al., Reference Friedel, Langer, Siebe and Stahr2000). This valley, located 80 km North of Mexico City, is a large agricultural production area irrigated with untreated wastewater from Mexico City by flooding soil surface with 150 to 200 mm according to crop requirements and water availability throughout the year (Guédron et al., Reference Guédron, Duwig, Prado, Point, Flores and Siebe2014).
Fungal material
T. harzianum and T. viride cultures were generously provided by CEPLAC (Comissão Executiva do Plano da Lavoura Cacaueira) from Brazil. T. asperellum was isolated from agricultural soil in Mezquital Valley, Hidalgo, which had been irrigated with wastewater. Soil samples were collected using a widely accepted five-point sampling technique. Isolation of T. asperellum was conducted using the serial dilution method (ranging from 10-1 to 10-4) as described by Dhingra and Sinclair (Reference Dhingra and Sinclair1981). The samples were prepared in sterile distilled water and aliquots of the suspension were transferred onto potato dextrose agar (PDA) culture plates. The plates were then incubated at a controlled temperature of 28 ± 2°C for a period of 10 days to facilitate sporulation. The identification process followed the guidelines of Samuels et al. (Reference Samuels, Chaverri, Farr and McCray2009). The preserved strains were maintained at the Agrobiotechnology Laboratory at the Center for Research in Applied Chemistry (CIQA, Mexico).
Soil sampling
To analyze the soil in the open field of the study site where the bean crop was grown, a composite sample was collected from five sampling points, totaling 1 kg of soil taken from a depth of 1–30 cm. The sample was prepared following the guidelines of the Mexican Official Standard NMX-AA-132-SCFI (2006), which specifies the method for soil sampling to identify and quantify metals and metalloids, as well as sample handling. The collected soil samples were then dried in a forced-air oven at 75°C for 72 h, ground into fine particles, and sieved through a mesh sieve with an opening size of 0.707 mm.
Determination of physicochemical soil properties
To assess the physicochemical properties of the soil, various parameters were measured, including the pH, organic matter content, texture, and cation exchange capacity. All soil parameters were analyzed using the methods described in the Mexican Official Standard NOM-021-RECNAT (2000). The pH was determined using the 1:5 soil-to-water ratio method; organic matter content was quantified using the Walkley–Black method. The electrical conductivity was measured in the saturated extract, while the cation exchange capacity was determined using the ammonium acetate method (Jain and Taylor, Reference Jain and Taylor2023). The soil texture was evaluated using the Bouyoucos method (Mwendwa, Reference Mwendwa2022).
Bean cultivation
The bean crop was established using disease-free and pest-free seeds of the ‘Michigan’ variety (emergence and early vegetative growth) sourced from the Universidad Politecnica de Francisco I. Madero in Hidalgo, Mexico. The seeds were carefully selected to ensure their quality. Planting was conducted in an open experimental field of the study site with a total of 500 plants spread across a 100 m2 area, ensuring proper spacing. To replicate the typical cultivation practices followed in the Mezquital Valley region, agronomic management of the crop adhered to the methods employed by local farmers. This involved the use of agricultural machinery for soil preparation and creating optimal conditions for growth. Irrigation was carried out through periodic flooding every 20 days, using wastewater sourced from Mexico City.
Inoculation of bean roots with Trichoderma spp
The experiment was conducted using a comprehensive randomized design encompassing eight distinct treatment conditions. These treatments were uniquely distinguished as follows: T. harzianum (T1 and T4), T. viride (T2 and T5), and T. asperellum (T3 and T6), each at two spore concentrations, 3 × 106 and 2 × 106 spores ml−1. Additionally, two combinations were studied: T. harzianum with T. viride (T7) and T. harzianum with T. asperellum (T8), both administered at spore concentrations of 3 × 106 spores ml−1. Finally, a control group was included, characterized by a spore concentration of zero.
For fungal inoculation in each experimental treatment, Trichoderma spp. were cultivated on PDA culture medium at an incubation temperature of 25°C for 10 days. Each colony was then gently flooded with 15 ml of sterile distilled water and the surface was lightly scraped using a glass rod to release the spores without disturbing the mycelium. The resulting suspension was filtered through a sterile cotton mesh to remove debris. Aliquots (0.5 ml) were collected from each plate and transferred to a Neubauer chamber for conidial counting. The spore concentration in the suspension was adjusted to either 2 × 106 or 3 × 106 spores ml−1, depending on the specific treatment, using known absorbance values obtained from a UV-visible spectrophotometer (Eppendorf BioSpectrometer 6135000923, Hamburg, Germany). This ensured consistent spore concentrations for subsequent inoculation.
For the inoculation process, 50 ml of the prepared spore suspension was applied to the roots of randomly selected bean plants grown in the open field 20, 30, and 40 days after sowing. This multi-stage inoculation approach was aimed at ensuring the successful establishment of Trichoderma spp. on bean roots. The methodology used in this study followed the procedure outlined by Landero et al. (Reference Landero, Lara-Viveros, Rodríguez-Ortega, Pérez-Vite and Ortíz-Hernández2019).
Percentage of bean roots colonized by Trichoderma spp
After the third inoculation (40 days later), four random root samples were selected from each of the nine treatments. To remove excess soil, roots were washed with tap water. For each root, ten 0.5 cm cuts were made, resulting in a total of 40 cuts per treatment. The cut sections were then transferred to Petri dishes, with 10 cuts placed on each dish. Each treatment had three dishes. To ensure cleanliness, cut sections were disinfected with 3% sodium hypochlorite for 1 min. Subsequently, they were rinsed with sterile distilled water and dried using sterile absorbent paper. The dried sections were transferred to Petri dishes. The Petri dishes were incubated at a constant temperature of 25°C and the development of the samples was evaluated every 24 h. Root colonization was considered effective when fungal growth in vitro reached 50% or more, based on a previous study (Landero-Valenzuela et al., Reference Landero, Lara-Viveros, Rodríguez-Ortega, Pérez-Vite and Ortíz-Hernández2019).
Plant tissues sampling
For the analysis of tissues of bean plants, four samples were collected at the harvest stage, specifically 100 days after planting. Each treatment had 10 replicated samples. These samples included leaf, grain, and pod sections. Each section was separated for individual processing. Similar to the soil samples, the plant sections were dried and sieved using a previously described method.
Determination of heavy metals in leaf, pod, grain, and soil
Heavy metals were extracted from plant tissues and soil using an acid digestion method, as described by Lara-Viveros et al. (Reference Lara-Viveros, Ventura-Maza, Muhammad, Rodríguez, Vargas and Landero-Valenzuela2015). The process involved taking 100 mg of each sample and adding 4 ml of nitric acid. The samples were then heated at 65°C for 60 min, followed by an increase in temperature to 120°C for an additional 30 min. Subsequently, 1.6 ml of hydrogen peroxide was added to the mixture, which was then allowed to cool to room temperature (25 ± 2°C). The resulting digested samples were filtered using Whatman 42 filter paper and adjusted to a final volume of 25 ml using deionized water (18 MΩ/cm). The quantification of Cd, Cr, Cu, and Pb was performed using the flame atomic absorption technique with an atomic absorption spectrometer (Perkin Elmer Inc., AANALYST 700, Massachusetts, USA). The wavelengths used were 228.8, 357.9, 324.8, and 283.3 nm for Cd, Cr, Cu, and Pb, respectively. The samples were measured in triplicate, including blank and blind samples. The limits of quantification (LOQ) and limits of detection (LOD) for metals were as follows: Pb (LOQ = 0.25 mg l−1 and LOD = 0.85 mg l−1); for Cd (LOQ = 0.15 mg l−1 and LOD = 0.017 mg l−1); for Cr (LOQ = 0.73 mg l−1 and LOD = 0.13 mg l−1); and for Cu (LOQ = 0.07 mg l−1 and LOD = 0.20 mg l−1). For the analysis of heavy metals, specifically Pb, Cd, Cr, and Cu, the Mexican Standard NMX-AA-132-SCFI (2006) was followed. The results were obtained by calculating the mean value based on three sets of duplicate repetitions, and the standard deviations (s.d.) were also considered.
Data analysis
The experimental design consisted of a fully random design with eight experimental treatments and one control treatment. This experimental design ensured a robust statistical approach, with each treatment being subjected to three repetitions. Within each repetition, two replicates were performed. This stringent methodology was employed to minimize potential sources of variability and to yield statistically reliable data for a comprehensive assessment of the effects of Trichoderma spp. treatments on bean plants. Rows at the edges were excluded from the analysis to reduce statistical bias.
Statistical Analysis System (SAS) version 9 was used for data analysis. The normality and homogeneity of the data were checked using the Kolmogorov–Smirnov test and Bartlett test. Subsequently, Analysis of Variance (ANOVA) was performed to determine whether there were any significant differences between the treatments. The Tukey method was used to compare means. All statistical tests were performed at a significance level of α = 0.05. In the case of colonization percentage, the data underwent a square root transformation before the analysis.
Results
Soil physicochemical properties
Chemical characteristics in the studied soil were: pH 7.7 ± 0.4, 1.72% of organic matter content, 27.86 cmol kg−1 of cation exchange capacity and a texture corresponding to silty loam soils. Meanwhile, the concentrations of Pb and Cd in the soil of the Mezquital Valley, Hidalgo, Mexico, irrigated with wastewater were higher than the allowable levels of these heavy metals in soils according to the World Health Organization, whereas the Cu and Cr concentrations were below the allowable limits (Table 1).
Table 1. Concentration of heavy metals (Pb, Cr, Cu, Cd) in soils irrigated with wastewater in the Mezquital Valley, Hidalgo, Mexico

Three replicates were used to measure heavy metal levels. Pb, lead; Cr, chromium; Cu, copper; Cd, cadmium. The concentrations are expressed in mg/kg based on the dry weights of the samples. VPMPS represents the allowable levels of heavy metals in soils (mg kg−1), according to the World Health Organization (WHO, 1996).
Root colonization by Trichoderma spp. in bean plants
In all experimental treatments, including those with the evaluation of the combination of two different species, Trichoderma spp. achieved a root colonization rate of over 75%. The highest percentage of root colonization (86.66%) was observed in T. harziamun 3 × 106 spores ml−1 (T1) and T. viride 3 × 106 spores ml−1 (T2) treatments, whereas the lowest percentage was for T. asperellum 3 × 106 spores ml−1 (T6) and T. harzianum + T. asperellum (T8) treatments (76.66%; Fig. 1). Control treatment showed 0% of colonization. There was no significant difference (P = 1.0) among the eight experimental treatments (Fig. 1), but colonization by Trichoderma spp. in all experimental treatments was significantly different from the control group (P < 0.001), indicating that all bean plants were successfully colonized by Trichoderma spp.
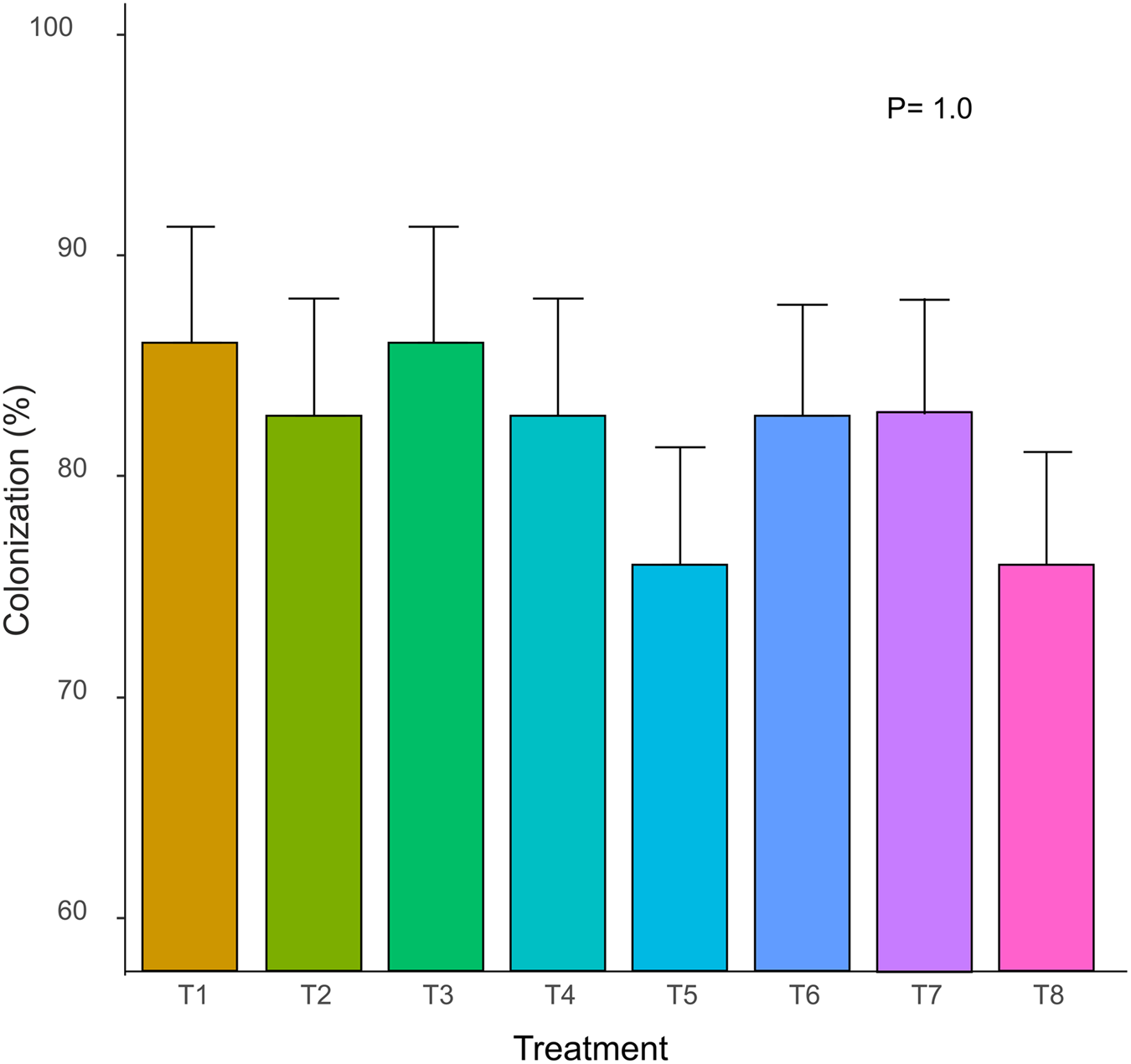
Figure 1. Effect of Trichoderma spp. strains on root colonization of bean plants. T1: inoculation with T. harzianum 3 × 106 spores ml−1; T2: inoculation with T. harzianum 2 × 106 spores ml−1; T3: inoculation with T. viride 3 × 106 spores ml−1; T4: inoculation with T. viride 2 × 106 spores ml−1; T5: inoculation with T. asperellum 3 × 106 spores ml−1; T6: inoculation with T. harzianum 2 × 106 spores ml−1; T7: inoculation with T. harzianum + T. viride 3 × 106 spores ml−1; T8: inoculation with T. harzianum + T. asperellum 3 × 106 spores ml−1. Mean (± s.e.) of the percentages of root colonization are shown. Differences between treatments were determined using Tukey's test.
Effect of Trichoderma spp. on the accumulation of heavy metals in bean plants
In general, Trichoderma spp. inoculation resulted in a significant decrease in the accumulation of the four heavy metals in the evaluated structures (leaf, pod, and grain) of bean plants compared with the control, with the exception of Pb concentration in leaves in treatment T4, Cu concentration in leaves in treatments T3 and T8, and Cu concentration in pods in treatment T8 (Fig. 2, Table 2). Specifically, Pb concentration was higher in grains (38.5–76.00 mg kg−1), followed by pods (30.08–64.16 mg kg−1) and leaves (3.91–26 mg kg−1) in the experimental treatments (Fig. 2). Meanwhile, Cd and Cu showed less variation among the plant structures (11.08–15.05, 5.42–29.42 mg kg−1, respectively; Fig. 2). Cr concentration ranged from 0 to 48.08 mg kg−1, with the greatest values for T5 and T6 treatments for pods (48.08 mg kg−1) and leaves (42.75 mg kg−1), respectively (Fig. 2).
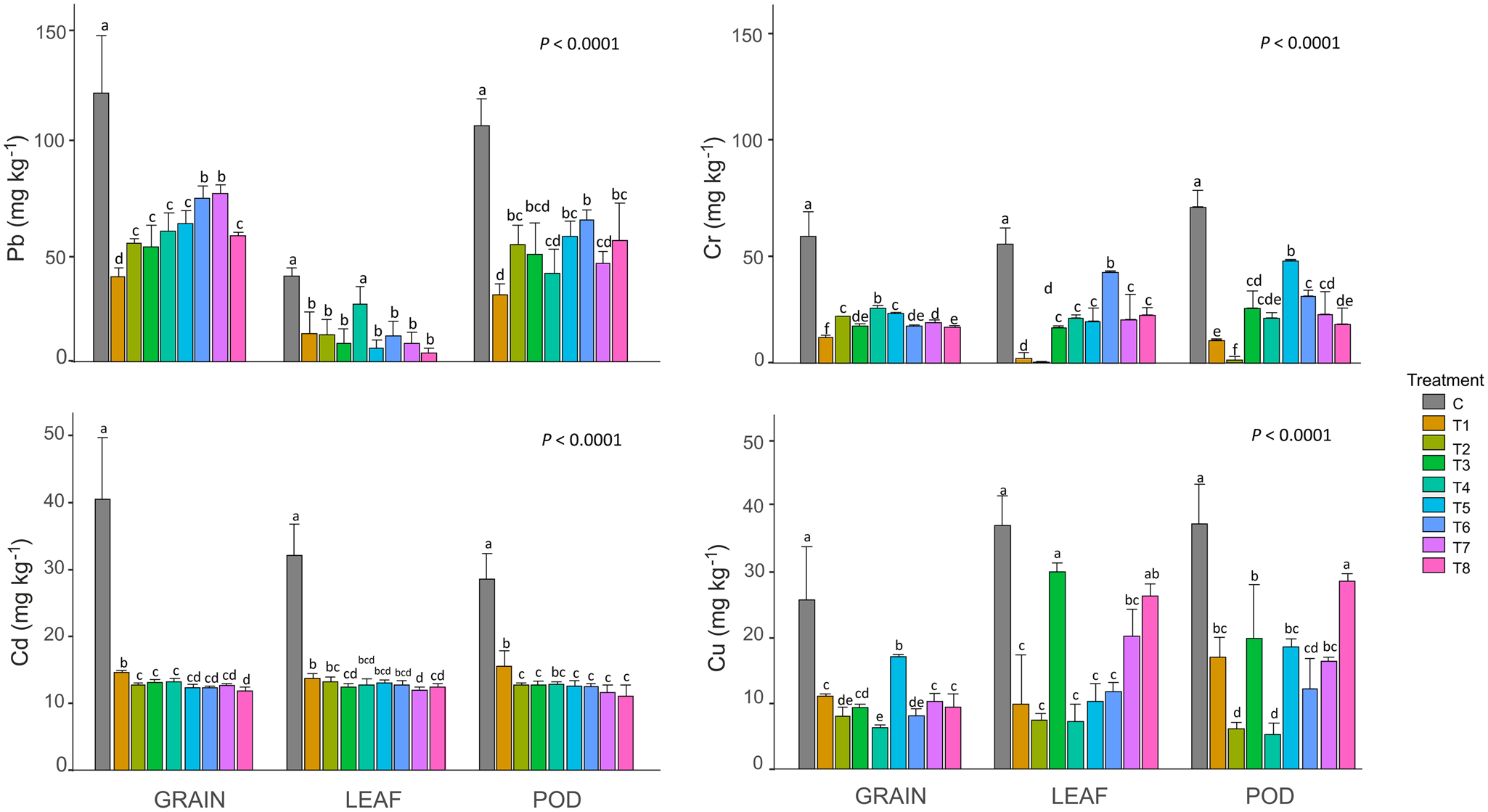
Figure 2. Concentration of lead, cadmium, chromium, and copper in bean leaf, pod, and bean grain under different treatments with Trichoderma spp. C, control treatment. The experimental treatments included: T1: inoculation with T. harzianum 3 × 106 spores ml−1; T2: inoculation with T. harzianum 2 × 106 spores ml−1; T3: inoculation with T. viride 3 × 106 spores ml−1; T4: inoculation with T. viride 2 × 106 spores ml−1; T5: inoculation with T. asperellum 3 × 106 spores ml−1; T6: inoculation with T. harzianum 2 × 106 spores ml−1; T7: inoculation with T. harzianum + T. viride 3 × 106 spores ml−1; T8: inoculation with T. harzianum + T. asperellum 3 × 106 spores ml−1. Pb: lead; Cd: cadmium; the concentrations of heavy metals are expressed in mg/kg based on the dry weight of the samples. Means ± standard deviations (s.d.) are provided. Means with the same letter are not significantly different according to Tukey's test (P < 0.05, n = 51).
Table 2. Treatment effect statistics for heavy metal concentration of different tissues of bean plants

The statistical analysis evaluated the concentration of lead (Pb), chromium (Cr), cadmium (Cd) and copper (Cu) among the control treatment and the eight experimental treatments: T1: inoculation with T. harzianum 3 × 106 spores ml−1; T2: inoculation with T. harzianum 2 × 106 spores ml−1; T3: inoculation with T. viride 3 × 106 spores ml−1; T4: inoculation with T. viride 2 × 106 spores ml−1; T5: inoculation with T. asperellum 3 × 106 spores ml−1; T6: inoculation with T. harzianum 2 × 106 spores ml−1; T7: inoculation with T. harzianum + T. viride 3 × 106 spores ml−1; T8: inoculation with T. harzianum + T. asperellum 3 × 106 spores ml−1 for each plant structure.
The concentration of fungal spores did not show a significant effect on Pb concentration in the leaves of plants inoculated with T. harzianum (T1 vs. T2; Fig. 2, Table 2), pods and grains of plants inoculated with T. viride (T3 vs. T4; Fig. 2, Table 2), or leaves and pods of plants inoculated with T. asperellum (T5 vs. T6; Fig. 2, Table 2). However, there was a significant effect associated with the increase in fungal spores on Pb concentration in the pods and grains of plants inoculated with T. harzianum (T1 vs. T2; Fig. 2, Table 2), leaves of plants inoculated with T. viride (T3 vs. T4; Fig. 2, Table 2), and grains of plants inoculated with T. asperellum (T5 vs. T6; Fig. 2, Table 2), showing lower concentrations of Pb in the structures of plants inoculated with 3 × 106 vs 2 × 106 spores ml−1 . In addition, there was no significant effect of increasing the number of fungal spores on the Cd concentration of leaves from plants inoculated with T. harzianum, T. viride and T. asperellum, or pods and grains from plants inoculated with T. viride and T. asperellum (Fig. 2, Table 2), although the increase in the concentration of fungal spores showed a significantly higher concentration of Cd in pods and grains from plants inoculated with 3 × 106 vs 2 × 106 spores ml−1 of T. harzianum (Fig. 2, Table 2). A similar pattern was observed for Cu, with no significant effect of fungal spore concentration in the leaves of plants inoculated with T. harzianum and T. asperellum, as well as pods of plants inoculated with the former fungal species, and a significantly higher concentration of Cu in the structures of plants inoculated with 3 × 106 vs 2 × 106 spores ml−1such as: pods and grains for T. harzianum inoculation; leaves, pods, and grains for T. viride inoculation; and grains for T. asperellum inoculation (Fig. 2, Table 2). A more variable pattern of the effect of the fungal spore concentration was found for Cr, with no significant effect on leaves from plants inoculated with T. harzianum (T1 and T2) and T. viride (T3 and T4), as well as for pods from plants inoculated with T. harzianum (T1 and T2), but with a significant effect on pods and grains of T1 vs T2, grains of T3 vs T4, and the three evaluated plant structures of T5 vs T6 (Fig. 2, Table 2). Nevertheless, in this case, inoculation with 3 × 106 spores ml−1 resulted in an increase (pods from plants inoculated with T. harzianum and T. asperellum, and grains from plants inoculated with T. asperellum) and a decrease (grains from plants inoculated with T. harzianum and T. viride, and leaves from plants inoculated with T. asperellum) in Cr concentration in plant structures.
The treatments with lowest concentrations of heavy metals in bean leaves were the combined treatments T7 (12 mg kg−1) and T8 (3.91 mg kg−1) for Cd and Pb, respectively (Fig. 2), and T4 (7.42 mg kg−1) and T2 (0 mg kg−1) for Cu and Cr (Fig. 2). On thother hand, for bean pods the lowest concentration was observed in the treatment with T. harzianum at 3 × 106 spores ml−1 (T1; 30.08 mg kg−1) for Pb, the combined T. harzianum + T. asperellum treatment for Cd (T8; 11.08 mg kg−1), T viride at 2 × 106 spores ml−1 (T4; 5.42 mg kg−1) for Cu, and T. harzianum at 2 × 106 spores ml−1 (T2; 1.41 mg kg−1). Finally, T1, T8, T4, and T2 treatments showed the maximum decrease in the concentration of Pb (38.50 mg kg−1), Cd (11.83 mg kg−1), Cu (6.50 mg kg−1) and Cr (12.16 mg kg−1) in bean grains, respectively (Fig. 2).
Discussion
A growing number of studies have indicated that plant endophytic fungi possess high metal tolerance and alleviation capabilities in heavy metal-contaminated soils (Lin et al., Reference Lin, Xiao, Ye, Wu, Hu and Shi2020; Hao et al., Reference Hao, Zhang, Hao, Diao, Zhang, Bao and Guo2021; Priyadarshini et al., Reference Priyadarshini, Priyadarshini, Cousins and Pradhan2021; Devi et al., Reference Devi, Behera, Raza, Mangal, Altaf, Kumar, Kumar, Tiwari, Lal and Singh2022). According to this, the results of this study indicate that inoculation of beans with different species of the fungus Trichoderma effectively diminishes the concentrations of Pb, Cr, Cu, and Cr in plant tissues, since in general, the plants of the experimental treatments had significantly lower concentration (47–70%) of the heavy metals than the plants in the control treatment. This is consistent with the results reported by Kredrics et al. (Reference Kredrics, Dóczi, Antal and Manczinger2001), who demonstrated that some strains of Trichoderma can effectively bind heavy metals, thereby reducing their toxicity to the environment.
Colonization of plants by endophytic fungi is key to the beneficial effects of these microorganisms on their host plants (Yan et al., Reference Yan, Zhu, Zhao, Shi, Jiang and Shao2019; Poveda et al., Reference Poveda, Eugui, Abril-Urías and Velasco2021). The colonization of roots by Trichoderma spp. was higher than 75% in all experimental treatments, indicating the successful colonization of roots. Importantly, no significant differences were observed among the experimental treatments; thus, the variation in the effects on the concentration of heavy metals among some experimental treatments could not be related to root colonization by the endophytic fungus. High root colonization by Trichoderma spp. under experimental conditions has been reported in several plants, including Arabidopsis thaliana, Brassica napus, Solanum lycopersicum, and Capsicum annum (Morán-Diez et al., Reference Morán-Diez, Rubio, Domínguez, Hermosa, Monte and Nicolás2012; Poveda, Reference Poveda2021; Sánchez-Cruz et al., Reference Sánchez-Cruz, Mehta, Atriztán-Hernández, Martínez-Villamil, del Rayo Sánchez-Carbente, Sánchez-Reyes, Lira Ruan, González-Chávez, Tabche-Barrera, Bárcenas-Rodríguez, Batista-García, Herrera-Estrella, Balcázar-López and Folch-Mallol2021), and under natural conditions, Trichoderma spp. are ubiquitous soil fungi commonly found in a great diversity of plant roots (Woo et al., Reference Woo, Hermosa, Lorito and Monte2023). The ability of Trichoderma to colonize plant roots may be linked to its capacity to tolerate various secondary metabolites present in the roots, facilitated by a KELCH-type protein (Poveda et al., Reference Poveda, Hermosa, Monte and Nicolás2019), and it takes some time for plants to undergo extensive genetic reprogramming in response to colonization, which likely allows the fungus to establish successful root colonization (Morán-Diez et al., Reference Morán-Diez, Rubio, Domínguez, Hermosa, Monte and Nicolás2012). Other factors that can explain the variation in the response of bean plants to the experimental treatments tested in the current study include the dynamics of heavy metals in the soil solution, the fungal species identity, variations in cellular and molecular mechanisms for tolerance to different heavy metals, and differences in metal translocation to plant structures which are discussed below (Morán-Diez et al., Reference Morán-Diez, Rubio, Domínguez, Hermosa, Monte and Nicolás2012; Haydee and Dalma, Reference Haydee, Dalma, Rahman and Asiri2017; Azouzi et al., Reference Azouzi, Charef, Ayed and Khadhar2019; Priyadarshini et al., Reference Priyadarshini, Priyadarshini, Cousins and Pradhan2021).
Dynamics of heavy metals in soil
The mobility of heavy metals is influenced by various factors in the soil-water system, which in turn affect their toxicity and bioaccumulation in plants. These effects are particularly notable when there are changes in the pH, ionic composition, or redox conditions. The pH affects the solubility of heavy metals, with decreased solubility at higher pH levels (Antoniadis et al., Reference Antoniadis, Levizou, Shaheen, Ok, Sebastian, Baum, Prasad, Wenzel and Rinklebe2017; Priyadarshini et al., Reference Priyadarshini, Priyadarshini, Cousins and Pradhan2021). In the current study, it is likely that pH (7.7 ± 0.4) did not significantly affect the solubility of the metals studied. However, the low organic matter content of the soil (1.73 ± 0.1%), which plays a role in metal absorption and complexation, could be involved (Sheoran, Sheoran, and Poonia, Reference Sheoran, Sheoran and Poonia2016; Azouzi et al., Reference Azouzi, Charef, Ayed and Khadhar2019). Dissolved organic matter can reduce the adsorption of heavy metals onto solid soil components, either by competing for free metal ions through the formation of soluble organometallic complexes or by adsorbing to the soil instead of metals, thereby reducing the capacity of the soil to retain metal ions (Haydee and Dalma, Reference Haydee, Dalma, Rahman and Asiri2017). On the other hand, the cation exchange capacity in the studied soil was determined to be 27.86 cmol kg−1, indicating it is a loamy clay soil. Soils with high clay content might retain higher concentrations of cations than sandy soils (Antoniadis et al., Reference Antoniadis, Levizou, Shaheen, Ok, Sebastian, Baum, Prasad, Wenzel and Rinklebe2017).
Effect of Trichoderma spp. on the accumulation of heavy metals
All the Trichoderma spp. used in this study reduced the concentration of heavy metals in bean plant structures, but the response in some cases was heavy metal specific and showed variation among plant structures. In general, T. harziarium was the fungal species that showed the highest reduction of concentration of Pb, Cr, Cu for leaves, pods, and grains, whereas the treatments that combined two Trichoderma spp. showed a higher reduction of Cd in the bean plants evaluated. These results indicated differences in the specificity of heavy metals among Trichoderma spp. Specificity and maximum tolerance limit for metals vary greatly at the genus and species levels (Priyadarshini et al., Reference Priyadarshini, Priyadarshini, Cousins and Pradhan2021; Devi et al., Reference Devi, Behera, Raza, Mangal, Altaf, Kumar, Kumar, Tiwari, Lal and Singh2022). These differences can occur even among strains isolated from the same locality because of their different strategies to tolerate metals (Jacob, Bardhan, and Raj Mohan, Reference Jacob, Bardhan and Raj Mohan2013). For example, Cadavid-Velásquez et al. (Reference Cadavid-Velásquez, del Socorro, Pérez-Vásquez and Marrugo-Negrete2019) found that the fungus Polyporus spp. had a higher concentration of Cu (27.54 mg kg−1) compared to other studied fungi belonging to 18 genera. Rhizophagus intraradices show a significant decrease in Cd content (Li et al., Reference Li, Luo, Zhang, Zhao, Li, Cai, Wong and Mo2016; Parmar et al., Reference Parmar, Daki, Bhattacharya, Shrivastav, Shah, Rodriguez-Couto and Thapar2022). Penicillium chrysogenum and Trichoderma viride have been reported to tolerate Cr up to a concentration of 600 mg l−1, compared to A. flavus and other strains of Fusarium sp. and Penicillium sp., which can tolerate up to 400 and 100 mg l−1 of Cr, respectively (Iram et al., Reference Iram, Parveen, Usman, Nasir, Akhtar, Arouj and Ahmad2012). In this sense, some Trichoderma spp. are known as Pb- and Cd-tolerant fungi species (Ting and Choong, Reference Ting and Choong2009; Sun et al., Reference Sun, Sun, Chao, Wang, Pan, Yang, Cui, Lou and Zhuge2020) with a capacity for Cu removal of up to 84.6% at pH levels ranging from 5 to 8 (Kumar and Dwivedi, Reference Kumar and Dwivedi2021), and have the ability to absorb high concentrations of Cr at specific pH (Narolkar and Mishra, Reference Narolkar and Mishra2022).
In general, four intracellular and extracellular strategies for metal tolerance are recognized by microorganisms: (1) biosorption (2) bioaccumulation and compartmentalization (3) metal chelation, and (4) efflux transport for metal exclusion (Priyadarshini et al., Reference Priyadarshini, Priyadarshini, Cousins and Pradhan2021). Among these, T. harzianum has proven to be tolerant to Cd by employing a superficial biosorption mechanism that does not involve metabolic processes. Uptake occurs through physical and chemical pathways or through metabolically mediated routes. This mechanism involves the binding of negatively charged groups in various biopolymers that make up the cell wall independent of metabolism (Ting and Choong, Reference Ting and Choong2009). Indeed, it has been observed that fungi capable of capturing higher concentrations of heavy metals develop abundant mycelial masses, particularly in the top 5 cm of soil, enabling extensive contact with the soil (Allen, Reference Allen1991). Hence, as the fungal cell wall plays a crucial role because it contains functional chemical groups such as chitin, proteins, phenolic polymers, melanins, and methionines which have an affinity for the uptake of heavy metals (Ayangbenro and Babaloba, Reference Ayangbenro and Babalola2017), even small differences in the chemical composition of cell wall among fungal species can result in differences of metal biosorption (Priyadarshini et al., Reference Priyadarshini, Priyadarshini, Cousins and Pradhan2021).
Most of the available information focuses on the individual properties of the fungus itself, and there is limited knowledge regarding the specific effects of inoculating microorganisms on the reduction of heavy metal bioaccumulation in plants. It has been observed that some heavy metals, such as Pb and Cd, are distributed unevenly in different parts of the plant (Lara-Viveros et al., Reference Lara-Viveros, Ventura-Maza, Muhammad, Rodríguez, Vargas and Landero-Valenzuela2015; Abdelgawad et al., Reference Abdelgawad, Zinta, Hamed, Selim, Beemster, Hozzein, Wadaan, Asard and Abuelsoud2020; Sassykova et al., Reference Sassykova, Aubakirov, Akhmetkaliyeva, Sassykova, Sendilvelan, Prabhahar, Prakash, Tashmukhambetova, Abildin and Zhussupova2020; Rasool et al., Reference Rasool, Ur-Rahman, Adnan Ramzani, Zubair, Khan, Lewińska, Turan, Karczewska, Khan, Farhad, Tauqeer and Iqbal2021). Specifically, some studies with grasses and mung beans have reported decreased translocation as the heavy metal moves away from the roots, leading to a higher concentration of Pb in leaves than in grains (Rasool et al., Reference Rasool, Ur-Rahman, Adnan Ramzani, Zubair, Khan, Lewińska, Turan, Karczewska, Khan, Farhad, Tauqeer and Iqbal2021). Nonetheless, previous studies on bean plants reported the opposite pattern for Pb (De Temmerman et al., Reference De Temmerman, Waegeneers, Ruttens and Vandermeiren2015; Govarthanan et al., Reference Govarthanan, Mythili, Selvankumar, Kamala-Kannan and Kim2018) and higher concentrations of Cd in leaves than in grains; however, the high concentration of Cd in bean leaves was related to atmospheric deposition rather than translocation from the soil (De Temmerman et al., Reference De Temmerman, Waegeneers, Ruttens and Vandermeiren2015). Similarly, the results of this study only indicated an uneven distribution of Pb in bean plants, with a higher concentration in the grains, followed by pods and leaves, suggesting that bean plants show a consistent pattern of Pb translocation from the soil that differs from the Pb translocation pattern of other studied plants. Nonetheless, besides the plant species effect and even cultivar effect, other factors such as solubility and the presence of chelating agents and transporters also play a significant role in the uptake of heavy metals (Sharma, Dietz, and Mimura, Reference Sharma, Dietz and Mimura2016). In addition, many plant species possess genes that encode proteins responsible for transporting metals to different plant structures (Tian et al., Reference Tian, He, Qin, Li, Meng, Huang and He2021). ATPase proteins also use energy derived from ATP hydrolysis to transport cations, such as Pb, throughout the plant (Williams and Mills, Reference Williams and Mills2005; Haque et al., Reference Haque, Gohari, El-Shehawi, Dutta, Elseehy and Kabir2022). Therefore, further research is needed to elucidate the specific mechanisms involved in heavy metal translocation to plant tissues.
Health implications of heavy metal accumulation in soil and bean plants
In line with the World Health Organization (WHO, 1996), the maximum allowable limits for Cd, Pb, Cr, and Cu in agricultural soils are 0.8, 85, 10, and 36 mg kg−1, respectively. It is evident from the results of this study that the concentrations of Pb and Cd in soil exceeded these limits, indicating a significant health risk to the local population. A previous study at the Mezquital Valley reported maximum permissible limits for Pb and Cd in soil as 3.5 and 3.7 mg kg−1, respectively, considering factors such as human body weight, assimilation rate, and daily food consumption by the population in this region (Vázquez-Alarcón et al., Reference Vázquez-Alarcón, Cajuste, Carrillo, Zamudio and Álvarez2005). Based on this, the studied soil should not be used for agricultural purposes.
In the case of heavy metals accumulated in plant tissues, it is important to note that bean grain, which is consumed by humans, is the plant structure with the highest concentrations of some heavy metals, especially Pb. Notably, both the control group and the treatment with T. harzianum and T. asperellum showed the highest values of Pb concentration (113.58 and 76 mg kg−1, respectively), while the treatment with T. harzianum at 3 × 106 spores ml−1 had the lowest Pb concentration (38.5 mg kg−1). These findings are particularly important because of the potential health risks associated with Pb ingestion such as kidney failure, liver damage, mental retardation, hearing impairment, and pregnancy complications (Chen et al., Reference Chen, Wang, Yuan, Zhang, Xia, Chen, Dong and Lu2021; Bazié et al., Reference Bazié, Compaoré, Bandé, Kpoda, Méda, Kangambega, Ilboudo, Sandwidi, Nikiema, Yakoro, Bassolé, Hien and Kabré2022). According to the World Health Organization (WHO, 1996), the maximum allowable levels of Pb in plants, particularly in grains, are 2.0 and 0.2 mg kg−1, respectively. However, even the lowest Pb concentrations obtained in this study exceeded these limits by more than two orders of magnitude. This also applies for Cd concentration in bean grains (11.83–14.66 mg kg−1), since the maximum permissible limit for Cd in legumes is 0.1 mg kg−1 based on the Codex Alimentarius, and Cr with high concentrations found in grains (12.16–25.75 mg kg−1). Cr, in particular, is classified as a highly carcinogenic substance when it exceeds the recommended limit of 1.30 mg kg−1 by the WHO and the Food and Agriculture Organization of the United Nations (Paweena et al., Reference Paweena, Ramnaree, Piriyaporn, Sutha and Phitsanu2022). Finally, Cu is essential for plant growth, but excessive levels in plant tissues can deactivate enzymes involved in important metabolic processes, such as photosynthesis and cellular respiration (Schmitt et al., Reference Schmitt, Andriolo, Silva, Tiecher, Chassot, Tarouco, Lourenzi, Nicoloso, Marchezan, Casagrande, Drescher, Kreutz and Brunettom2022). According to the World Health Organization (WHO, 1996), the maximum permissible limit for Cu in plant tissues is 10 mg kg−1. In the human body, excess Cu can lead to liver damage or even death, with daily intake exceeding 0.9 mg. In this study, the control group exhibited a Cu content of 26.5 mg kg−1 in the grains, surpassing the permitted limit. However, with the treatment of T. viride, T. harzianum and T, asperellum at 3 × 106 spores ml−1, the Cu content in this structure was below permissible limits.
Conclusions
In light of the limited information available on the reduction of heavy metals through the inoculation of microorganisms in cultivated plants, the findings of the current study revealed that selected Trichoderma strains have the ability to decrease the concentrations of heavy metals in agriculturally important crops. The results demonstrated that the levels of Pb, Cd, Cu, and Cr in the edible part of the bean plants, namely the grain, were effectively reduced when the plants were inoculated with Trichoderma, although the concentrations of heavy metals were still above the permissible limits for human consumption. The observed high concentrations of heavy metals in the studied soil might explain these results, emphasizing the need for further research using not only new isolates but also different spore concentrations. The risk to human health is minimized by reducing the metal content of the cultivated species. Therefore, investigating the inoculation of Trichoderma in plant species with high consumption rates will provide a deeper understanding of the mechanisms involved in fungus-plant interactions, ultimately leading to improved efficiency.
Acknowledgements
Authors thank to the anonymous reviewers for improving the MS.