Introduction
Epidemiological studies show that lower cognition in childhood is associated with the manifestation of schizophrenia (Jones et al. Reference Jones, Rodgers, Murray and Marmot1994; Cannon et al. Reference Cannon, Jones and Murray2002b ; Zammit et al. Reference Zammit, Allebeck, David, Dalman, Hemmingsson, Lundberg and Lewis2004; Koenen et al. Reference Koenen, Moffitt, Roberts, Martin, Kubzansky, Harrington, Poulton and Caspi2009; Welham et al. Reference Welham, Isohanni, Jones and McGrath2009; Reichenberg et al. Reference Reichenberg, Caspi, Harrington, Houts, Keefe, Murray, Poulton and Moffitt2010; Khandaker et al. Reference Khandaker, Barnett, White and Jones2011). This is in line with clinical case–control studies for first-episode psychosis as well as clinical high risk for psychosis (Fusar-Poli et al. Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung, Howes, Stieglitz, Vita, McGuire and Borgwardt2012; Giuliano et al. Reference Giuliano, Li, Mesholam-Gately, Sorenson, Woodberry and Seidman2012). Lower childhood cognitive function is also observed in people with self-reported psychotic experiences (Horwood et al. Reference Horwood, Salvi, Thomas, Duffy, Gunnell, Hollis, Lewis, Menezes, Thompson, Wolke, Zammit and Harrison2008; Barnett et al. Reference Barnett, McDougall, Xu, Croudace, Richards and Jones2012; Gur et al. Reference Gur, Calkins, Satterthwaite, Ruparel, Bilker, Moore, Savitt, Hakonarson and Gur2014; Khandaker et al. Reference Khandaker, Stochl, Zammit, Lewis and Jones2014, Nishida et al. Reference Nishida, Xu, Croudace, Jones, Barnett and Richards2014), suggesting that investigating the broader psychosis spectrum may help to understand the pathophysiology of schizophrenia. However, cognitive impairment in childhood also occurs with other psychiatric disorders such as major depression and self-report affective symptoms, suggesting common risk pathways (van Os et al. Reference van Os, Jones, Lewis, Wadsworth and Murray1997; Zammit et al. Reference Zammit, Allebeck, David, Dalman, Hemmingsson, Lundberg and Lewis2004; Hatch et al. Reference Hatch, Jones, Kuh, Hardy, Wadsworth and Richards2007; Koenen et al. Reference Koenen, Moffitt, Roberts, Martin, Kubzansky, Harrington, Poulton and Caspi2009; Meier et al. Reference Meier, Caspi, Reichenberg, Keefe, Fisher, Harrington, Houts, Poulton and Moffitt2014; Rock et al. Reference Rock, Roiser, Riedel and Blackwell2014; Trivedi & Greer, Reference Trivedi and Greer2014).
Some longitudinal epidemiological studies have suggested that a developmental deficit in childhood (Reichenberg et al. Reference Reichenberg, Caspi, Harrington, Houts, Keefe, Murray, Poulton and Moffitt2010) or a developmental lag (delay of typical development) through adolescence (Fuller et al. Reference Fuller, Nopoulos, Arndt, O'Leary, Ho and Andreasen2002; MacCabe et al. Reference MacCabe, Wicks, Lofving, David, Berndtsson, Gustafsson, Allebeck and Dalman2013) in verbal cognition differentiates those with schizophrenia from healthy participants, whereas verbal developmental lag does not appear to differentiate affective disorders (Cannon et al. Reference Cannon, Moffitt, Caspi, Murray, Harrington and Poulton2006; Reichenberg et al. Reference Reichenberg, Caspi, Harrington, Houts, Keefe, Murray, Poulton and Moffitt2010). The former is also observed in children with dyslexia who are likely to have psychotic experiences in adolescence (Khandaker et al. Reference Khandaker, Stochl, Zammit, Lewis and Jones2014). Although still controversial (Carrion et al. Reference Carrion, Goldberg, McLaughlin, Auther, Correll and Cornblatt2011; Lin et al. Reference Lin, Yung, Nelson, Brewer, Riley, Simmons, Pantelis and Wood2013), clinical high-risk studies for psychosis showed that lower scores in verbal memory and verbal fluency tests could predict the later onset of psychosis (Giuliano et al. Reference Giuliano, Li, Mesholam-Gately, Sorenson, Woodberry and Seidman2012), suggesting that developmental deficits and/or lag in verbal cognition play a key role in the emergence of psychotic symptoms. In contrast, epidemiological studies showed that people with affective symptoms have no specific developmental deficit and lag pattern in childhood (Reichenberg et al. Reference Reichenberg, Caspi, Harrington, Houts, Keefe, Murray, Poulton and Moffitt2010) and adulthood (Meier et al. Reference Meier, Caspi, Reichenberg, Keefe, Fisher, Harrington, Houts, Poulton and Moffitt2014) in verbal and non-verbal cognition. Examining deficits and adolescent developmental lag in verbal and non-verbal cognition, and within-individual cognitive discrepancy between the two cognitive domains between those with and without psychological symptoms in later life would test the specificity of the neurocognitive (and thus neurodevelopmental) model of psychosis and depression spectra (Fig. 1) (van Os, Reference van Os2013). However, to the best of our knowledge, there has been no prospective cohort study that has examined whether relative verbal cognitive developmental lag in the general population is associated with adult psychotic experiences, and, conversely, whether non-verbal lag is associated with adult affective symptoms.

Fig. 1. Example of cognitive development in the two domains. Cognitive development using Z-scores of the two domains in this study is illustrated. Gap a indicates cognitive discrepancy between the two domains at age 8 years. Gap b indicates relative cognitive lag during adolescence, hypothesizing no difference in Z-scores through development within an individual (dashed line). Black line and square dots represent verbal cognition, and grey line and circle dots represent non-verbal cognition.
The Medical Research Council (MRC) National Survey of Health and Development (NSHD) is one of the longest continuously followed prospective birth cohort studies in the world, beginning in 1946 in England, Scotland and Wales (Wadsworth et al. Reference Wadsworth, Kuh, Richards and Hardy2006). Studies based on this cohort have reported cognitive delay in clinical cases of schizophrenia (Jones et al. Reference Jones, Rodgers, Murray and Marmot1994) and depression (van Os et al. Reference van Os, Jones, Lewis, Wadsworth and Murray1997), as well as broader spectra of psychotic experiences (Barnett et al. Reference Barnett, McDougall, Xu, Croudace, Richards and Jones2012; Nishida et al. Reference Nishida, Xu, Croudace, Jones, Barnett and Richards2014) and affective symptoms (Hatch et al. Reference Hatch, Jones, Kuh, Hardy, Wadsworth and Richards2007). Although symptoms in self-report questionnaires might have a different distribution to those from clinical interviews, the former are likely to be more representative at the general population level with broader disease spectra. In the present study, we extended this by examining psychosis and depression spectra in relation to deficits and adolescent developmental lag in verbal and non-verbal cognition. We hypothesized that a specific developmental deficit and lag in verbal cognition is associated with psychotic experiences in adulthood, but not with affective symptoms.
Method
Participants
The NSHD is one of the longest large prospective cohort studies, originally consisting of 5362 randomly selected children from all single births within marriage during 1 week in March 1946 in England, Scotland and Wales. Of the original sample, 3820 participants had complete cognitive data at ages 8 and 15 years. When study members were aged 53 years, they reported psychotic experiences and depressive symptoms in self-reported questionnaires. Of 3035 participants who were interviewed at that age, 2923 and 2920 participants provided information on psychotic experiences and affective symptoms (see below). Reasons for non-response at age 53 years were 469 deaths, 640 permanent refusals, 580 non-residents and 638 failures to contact. However, the responding participants were broadly representative of the national population of similar age (Wadsworth et al. Reference Wadsworth, Butterworth, Hardy, Kuh, Richards, Langenberg, Hilder and Connor2003). Finally, one or more adult psychiatric outcomes were available for 2384 study members with complete cognitive data. Those with complete data were more likely to be female, had heavier birth weight, and higher cognition at ages 8 and 15 years than those with missing data (p < 0.05).
Ethical approval for this study was obtained from the North Thames Multicentre Research Ethics Committee. All participants gave written informed consent.
Measures
Psychological symptoms in adulthood
Psychotic experiences were self-reported over the previous 12 months at age 53 years using the five-item version of the Psychosis Screening Questionnaire (PSQ) (Bebbington & Nayani, Reference Bebbington and Nayani1995; Johns et al. Reference Johns, Cannon, Singleton, Murray, Farrell, Brugha, Bebbington, Jenkins and Meltzer2004) including three delusional (thought interference, persecution, and strange experiences), one hallucinatory and one hypomanic questions. All items were answered as either ‘no’, ‘unsure’ or ‘yes’. In the original PSQ each of these questions has at least one follow-up question to elicit further detail; however, due to time constraints these were not asked in the NSHD. We therefore defined ‘yes’ for each leading question as the existence of a symptom and the other responses as negative. As in a previous study using this cohort (Nishida et al. Reference Nishida, Xu, Croudace, Jones, Barnett and Richards2014), more than half of the respondents gave a positive response to the hypomania item ‘Have there been times when you felt very happy without a break for days on end?’. Further, 643 respondents (22.0%) rated themselves as ‘unsure’ on this item. In addition, there has been a biological and cognitive debate over whether mania and hypomania should be included within the psychosis or affective spectrum (Trotta et al. Reference Trotta, Murray and MacCabe2015; Bora, Reference Bora2016). Therefore, consistent with the previous study, we did not use this item in the analysis.
Self-reported affective symptoms were measured at age 53 years using the 28-item version of the General Health Questionnaire (GHQ-28) (Goldberg & Hillier, Reference Goldberg and Hillier1979). Each item was scored using a four-level Likert scale for total symptom score, and was recoded into 0-0-1-1 for deriving a diagnostic risk threshold (‘caseness’). This threshold was defined as a score of 5 or more on this summed recoded score (range 0–28), which was validated using the Clinical Interview Schedule (Goldberg & Hillier, Reference Goldberg and Hillier1979). The GHQ-28 consists of four components (somatic symptoms, anxiety and insomnia, social dysfunction, and severe depression) (Goldberg & Hillier, Reference Goldberg and Hillier1979). To test possible differential effects of these types of affective symptoms, we set thresholds for each subscale at the top 10% score, which matched the prevalence of GHQ-28 caseness and that of least one subscale.
Cognition in childhood and adolescence
Cognition at age 8 years was measured at school using three verbal tests (reading comprehension, word reading, and vocabulary) and one non-verbal test (picture intelligence, which consisted of three tasks: odd-one out, sequence detection and abstract reasoning) devised by the National Foundation for Educational Research (Pigeon, Reference Pigeon and Douglas1964). Cognition at age 15 years was measured at school using three verbal tests (Watts Vernon sentence completion, mathematics, and the verbal section of the Alice Heim Group Ability Test), and one non-verbal test (reasoning) using the non-verbal section of the Alice Heim Group Ability Test (Heim, Reference Heim1970). The non-verbal tests at both ages had some similarity with the matrix reasoning and picture concepts subtests of the Wechsler Intelligence Scale for Children (Kaplan & Saccuzzo, Reference Kaplan and Saccuzzo2013). Non-verbal cognition also involves processing speed and is thought to be less dependent on educational and cultural learning than verbal cognition (Kaplan & Saccuzzo, Reference Kaplan and Saccuzzo2013). As verbal and non-verbal cognition was measured by different tests at ages 8 and 15 years, we calculated standardized verbal and non-verbal scores at both ages by summing each standardized score. We also calculated a relative developmental lag score for verbal and non-verbal cognition as the relevant Z-score at age 15 years minus corresponding Z-score at 8 years; thus a negative value represents relative decrease in scores from age 8 to 15 years, i.e. increasing developmental lag with respect to peers (Fig. 1). The ages 8 and 15 years were, respectively, the earliest and latest that cognition was tested during development. Thus this interval maximized the potential for capturing any effect of cognitive lag.
Potential confounding variables
Based on previous studies with this cohort the following potential confounders were selected: sex, birth weight, birth order, mother's education, and social class of origin (at age 11 years or, if this was unknown, at age 4 or 15 years).
Mother's education was dichotomized into primary only or above, and family social class was classified into three categories (professional or intermediate, skilled non-manual, and skilled manual or unskilled).
Statistical analysis
We initially used χ2 tests and t tests, as appropriate, to test for differences between the independent variables in those with and without a positive response on the adult mental health outcomes [each individual PSQ item (all coded as positive or negative) or GHQ-28 ‘caseness’]. Multivariable logistic regression was then used to test associations between childhood and adolescent cognition and the outcomes, using verbal and non-verbal cognition at age 8 years and verbal and non-verbal developmental lag from age 8 to 15 years as independent variables. Outcomes were coded as separate for each PSQ item and for GHQ-28 caseness. We also tested whether cognitive characteristics were associated with the number of positive PSQ responses using multivariate regression analysis. As several participants who reported the existence of psychotic experiences also had affective symptoms (53.9%), we also grouped into psychotic experience only (PE only), affective symptoms only (AFF only), and both, with each of these groups compared with those with neither psychotic experience nor affective symptoms (no symptom) as the reference category. In addition, we compared the PE-only group with the AFF-only group to directly investigate differences in cognitive profiles. In this second analysis, psychotic experience presence was defined as one or more psychotic experience items of strange experience and hallucination, because previous studies showed a relatively low degree of positive responses that could represent more definitive characteristics of a psychosis spectrum (Johns et al. Reference Johns, Cannon, Singleton, Murray, Farrell, Brugha, Bebbington, Jenkins and Meltzer2004; Nishida et al. Reference Nishida, Xu, Croudace, Jones, Barnett and Richards2014). For all multivariable models, we mutually adjusted verbal and non-verbal cognition. In addition, to test for possible synergy between verbal and non-verbal cognitive domains increasing the severity of outcomes, we tested interactions between verbal and non-verbal cognition at age 8 years and lag from age 8 to 15 years. Since verbal and non-verbal cognition are positively correlated (age 8 years: r = 0.57, p < 0.001; age 15 years: r = 0.61, p < 0.001), we tested multicollinearity using variance inflation factors (VIF) for mutual adjustment of cognitive variables. No collinearity was defined by less than 10 of each VIF value for the independent variable.
Statistical significance was set at 0.05, and all analyses were conducted using SPSS Statistics 22 (IBM Corp., USA).
Results
Overall, the no-symptom group had stable Z-transformed cognitive scores from age 8 to 15 years in verbal and non-verbal domains (Table 1).
Table 1. Cognitive characteristics in childhood and adolescence on each psychotic experience and affective symptoms

Data are given as mean (standard deviation) unless otherwise indicated.
PSQ, Psychosis Screening Questionnaire; GHQ-28, 28-item version of the General Health Questionnaire.
Value significantly different from the no or unsure participants: * p < 0.05, ** p < 0.01, *** p < 0.001 (by χ2 test for sex, and t test for the other variables).
a Differences in existence of GHQ-28 subscale cases are shown in online Supplementary Table S1.
b Differences in Z-scores from age 8 to 15 years for verbal and non-verbal domains (score at age 15 years – 8 years). A positive score represents an increase of developmental lag from the mean.
Associations with adult psychotic experiences
The rates of positive respondents for each psychotic experience items varied between 3.7% and 21.4%, ‘hallucination’ was the lowest and ‘persecution’ the highest (Table 1). In comparing those with and without each psychotic experience, those with lower cognition in at least one domain were more likely to have psychotic experiences for all PSQ items at age 53 years except ‘persecution’. These associations remained significant after adjusting for confounding variables (online Supplementary Table S2).
After mutual adjustment for verbal and non-verbal cognitive variables (maximum VIF = 2.2), lower verbal cognition at age 8 years was associated with higher likelihood of each PSQ symptom except for ‘persecution’, whereas no significant association between non-verbal cognition and any of these psychotic experiences was evident (Table 2). In addition, greater verbal developmental lag from age 8 to 15 years was associated with higher likelihood of the psychotic experiences ‘strange experience’ and ‘hallucination’, whereas, again, there was no association between non-verbal lag and any of these psychotic experiences. In sum, those with relatively lower verbal cognition in childhood and adolescent verbal developmental lag, which resulted in wider cognitive discrepancy with relatively lower verbal cognition during adolescence, were more likely to have psychotic experiences (Fig. 2a ).

Fig. 2. Cognitive development patterns by existence of psychotic experiences (PE) and affective symptoms (AFF). (a, b) Characteristics of cognitive scores in those with PE (and AFF) are illustrated using adjusted Z-transformed scores for sex and mother's education, and for those without PE (and AFF) as zero. (c–e) Characteristics of three different subgroups (PE only, AFF only, and both symptoms) are also illustrated using adjusted Z-transformed scores, with the no-symptom group as zero.
Table 2. Association of cognitive characteristics in childhood and adolescence with each psychotic experience and affective symptoms a
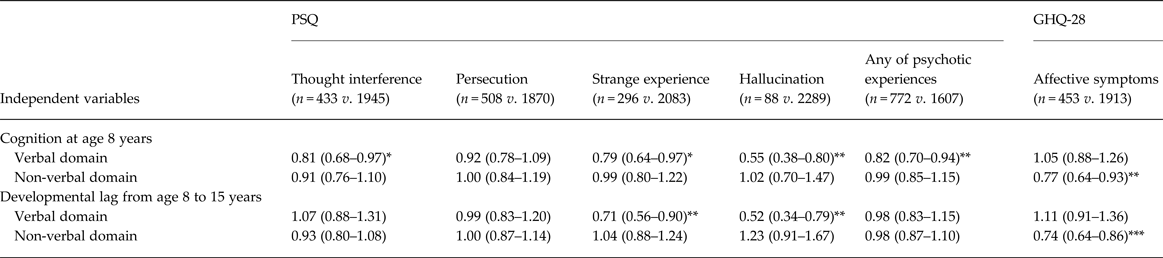
Data are given as odds ratio (95% confidence interval).
PSQ, Psychosis Screening Questionnaire; GHQ-28, 28-item version of the General Health Questionnaire.
a This model was adjusted by the confounding variables of sex, birth weight, birth order, mother's education, and childhood social class, and the independent variables of cognition at age 8 years and developmental lag from age 8 to 15 years in verbal and non-verbal domains. Unadjusted and other adjusted models, and models for GHQ-28 subscale cases are shown in online Supplementary Table S2.
Significant coefficient: * p < 0.05, ** p < 0.01, *** p < 0.001.
Associations with adult affective symptoms
For case-level affective symptoms, 19.1% (453/2376) participants were identified as cases. Frequencies of case-level somatic symptoms, anxiety and insomnia, social dysfunction and severe depression were 180 (7.6%), 214 (9.0%), 219 (9.2%) and 182 (7.7%), respectively (online Supplementary Table S1). There were 450 (19.0%) study members with case-level symptoms for at least one subscale. Those with case-level symptoms had lower non-verbal cognition at age 15 years, and greater developmental lag in the non-verbal domain (Table 1). For verbal cognition, however, there was no association between those with and without affective symptoms.
After mutually adjusting for cognitive variables, lower non-verbal cognition at age 8 years and greater non-verbal developmental lag were associated with higher risk of GHQ-28 caseness (Table 2). There was no association between verbal cognitive scores and GHQ-28 caseness. These trends were similar for GHQ-28 subscore cases (online Supplementary Table S2). Multivariate regression analysis also showed that lower verbal cognition at age 8 years was associated with the number of positive responses in the PSQ at age 53 years (B = −0.090, s.e. = 0.033, p = 0.007). In sum, those with relatively lower non-verbal cognition in childhood and adolescent non-verbal developmental lag, which resulted in wider cognitive discrepancy with relatively lower non-verbal cognitions, were more likely to have affective symptoms (Fig. 2b ).
Associations with adult psychotic experiences and affective symptoms
Sample sizes for the grouped outcomes were 194 for PE only (8.2%), 315 for AFF only (13.3%), 138 for both (5.8%) and 1714 for the no-symptom group (72.6%). In logistic regression models with the no-symptom group as the reference, the AFF-only group showed the characteristics of the cognitive discrepancy; a smaller verbal developmental lag (i.e. relatively better development in verbal cognition), lower non-verbal cognition at age 8 years, and a greater non-verbal developmental lag (Table 3). These trends are illustrated in Fig. 2 c–e, particularly the non-verbal developmental lag in the AFF-only group and greater cognitive discrepancy (non-verbal < verbal), while the PE-only group had a developmental lag in verbal cognition and greater cognitive discrepancy (verbal < non-verbal).
Table 3. Cognitive characteristics in childhood and adolescence by existence of psychotic experience and/or affective symptoms a
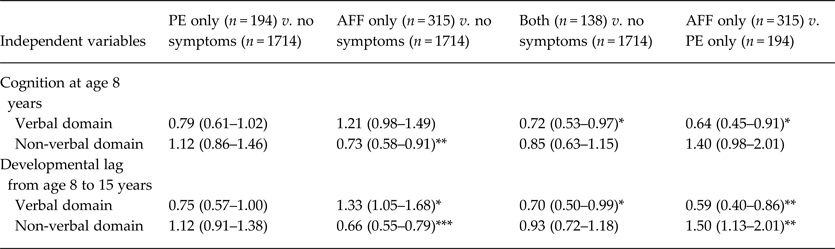
Data are given as odds ratio (95% confidence interval).
PE, Psychotic experience; AFF, affective symptoms.
a Participants were grouped into PE-only (defined by the existence of one or more items of strange experience and hallucination), AFF-only, both, and no-symptom groups. This model was adjusted by the confounding variables of sex, birth weight, birth order, mother's education and childhood social class, and the independent variables of cognition at age 8 years and developmental lag from age 8 to 15 years in verbal and non-verbal domains. Unadjusted and other adjusted models are shown in online Supplementary Table S3.
Significant coefficient: * p < 0.05, ** p < 0.01, *** p < 0.001.
Interactions between verbal and non-verbal cognition on adult psychological symptoms
When we added interactions between verbal and non-verbal cognitive domains at age 8 years and developmental lags from age 8 to 15 years to the mutually adjusted models, we found a significant interaction between verbal and non-verbal cognition at age 8 years on ‘thought interference’ at age 53 years [odds ratio (OR) = 0.87, 95% confidence interval (CI) 0.78–0.99, p = 0.029), with the association between lower verbal cognition at age 8 years and PE similar to the above results. Thus, greater cognitive discrepancy (verbal < non-verbal) in addition to lower verbal cognition at age 8 years was additively associated with adult ‘thought interference’.
Compared with the ‘no’-symptom group, the interaction between verbal and non-verbal cognitive lags from age 8 to 15 years was also significant in the ‘both’-symptom group (OR = 0.68, 95% CI 0.52–0.90, p = 0.007), with a similar effect to the above results of lower verbal cognition at age 8 years and greater verbal cognitive lag. Thus, greater discrepancy between verbal and non-verbal cognitive lags from age 8 to 15 years (verbal < non-verbal) was associated with the simultaneous existence of psychotic and affective symptoms at age 53 years.
Discussion
A population-representative prospective birth cohort study revealed a developmental verbal–non-verbal differentiation in regard to the presence of adult psychotic experiences and affective symptoms. Low verbal cognition at age 8 years and relative developmental lag in this domain between age 8 and 15 years were associated with psychotic experiences at age 53 years, independently of non-verbal cognition and confounding factors. In contrast, lower non-verbal cognition at age 8 years and relative developmental lag in this between age 8 and 15 years were associated with risk of common mental disorder in adulthood, as assessed by the GHQ-28 caseness threshold, independently of verbal cognition and the other covariates. Participants with case-level affective symptoms but no psychotic experience additionally had higher verbal cognition at age 8 years, and rather better verbal development between the ages of 8 and 15 years. To the best of our knowledge, this is the first investigation of its kind in a population-representative prospective birth cohort study.
Strengths of this study include a large population-based prospective birth cohort, the availability of cognition objectively assessed in childhood and adolescence, and the availability of a wide range of potential confounders. In addition, since psychotic experiences are less common in midlife than in adolescence or early adulthood (van Os et al. Reference van Os, Linscott, Myin-Germeys, Delespaul and Krabbendam2009), these symptoms at age 53 years may be associated with more distinct cognitive profiles than those reported at a younger age (Nishida et al. Reference Nishida, Xu, Croudace, Jones, Barnett and Richards2014). The childhood and adolescent cognitive characteristics in those with adult psychotic or depressive symptoms are in line with previous clinical studies in patients and high-risk individuals, suggesting that the characteristics of cognitive development are common within each spectrum. Although some responses using self-report questionnaires are state-dependent, our results could deserve further exploration of the relationship between cognitive profiles and psychological outcomes.
However, several limitations should also be considered. Although demographic characteristics in the NSHD were representative of the population in the survey at age 53 years (Wadsworth et al. Reference Wadsworth, Butterworth, Hardy, Kuh, Richards, Langenberg, Hilder and Connor2003), those lost to follow-up were more likely to be male, and had lower birth weight and lower cognition scores at ages 8 and 15 years. Lower childhood cognition and adult psychological symptoms are associated with attrition in cohort studies. Similar to the results from clinical studies of schizophrenia and major affective symptoms, people with psychotic experiences had higher all-cause mortality in a prospective study (Sharifi et al. Reference Sharifi, Eaton, Wu, Roth, Burchett and Mojtabai2015). As clinical studies have repeatedly shown these characteristics to be risk factors for schizophrenia, higher attrition for participants with cognitive discrepancy may have led to underestimation of effect size in the relationship between greater verbal developmental lag and psychotic experiences. Attrition of those with low childhood cognitive function may similarly have caused underestimation of effect sizes. However, we have no reason to believe that this would have altered the pattern of associations observed. Second, due to time constraints we did not ask the supplementary questions to each of the five leading PSQ questions, which resulted in some detail about psychotic features being lost. Third, self-report questionnaires may influence responses through misinterpretation or misreading of items, or by transient events. Although the present findings were consistent with clinical case–control studies of disease spectra, repeated measures and interview-based assessment may increase response validity. In addition, the PSQ scale was tested at an item level that may not have allowed sufficient validity, and also was not comparable with the way we used the GHQ-28 as a total score. However, a total score based on four items would not have similar psychometric properties to that based on a 28-item Likert scale. In any case, recent evidence suggests that positive self-reported psychotic experiences are multi-factorial (Therman & Ziermans, Reference Therman and Ziermans2016). Future research using threshold-level psychotic experiences is needed to confirm the present findings. Fourth, since detailed information for clinical treatment was not available, we were unable to consider whether participants with schizophrenia and major depression in remission were among those who were classed as negative for symptoms. However, this is unlikely to be a significant source of bias, and those with clinical symptoms are only a subgroup of those who self-report symptoms (Jones et al. Reference Jones, Rodgers, Murray and Marmot1994; van Os et al. Reference van Os, Jones, Lewis, Wadsworth and Murray1997). Although 30 clinical cases of schizophrenia were identified in this cohort, only 11 of these cases completed the GHQ-28 at the 53-year survey (Jones et al. Reference Jones, Rodgers, Murray and Marmot1994). Thus we did not use this classification to test cognitive discrepancy in the psychosis spectrum compared with participants with affective symptoms. Consistent with this, a previous study using this cohort reported that psychotropic medication at age 43 years had little effect on the GHQ-28 score at age 53 years (Colman et al. Reference Colman, Croudace, Wadsworth, Kuh and Jones2008). Fifth, although comparisons with short-form intelligence tests suggest that non-verbal tests at age 8 and 15 years broadly represent non-verbal cognition (Christensen et al. Reference Christensen, Girard and Bagby2007), processing speed as measured by, for example, the symbol coding and symbol search subtests was not assessed in this study. We previously showed a deficit of motor development in those who later developed schizophrenia and affective disorders (Jones et al. Reference Jones, Rodgers, Murray and Marmot1994; van Os et al. Reference van Os, Jones, Lewis, Wadsworth and Murray1997); however, another cohort study did not show a developmental deficit in relation to digit symbol substitution, although a developmental lag through adolescence occurred in people with schizophrenia (Reichenberg et al. Reference Reichenberg, Caspi, Harrington, Houts, Keefe, Murray, Poulton and Moffitt2010; Meier et al. Reference Meier, Caspi, Reichenberg, Keefe, Fisher, Harrington, Houts, Poulton and Moffitt2014). In addition, it is a possibility that the participants might use a verbal strategy when performing non-verbal cognitive tests in this study. Further studies are needed of non-verbal cognitive deficit and lag in relation to psychotic and depressive symptoms.
Although cognitive deficits in various domains are evident in patients with schizophrenia (Jones et al. Reference Jones, Rodgers, Murray and Marmot1994; Cannon et al. Reference Cannon, Jones and Murray2002b ; Fuller et al. Reference Fuller, Nopoulos, Arndt, O'Leary, Ho and Andreasen2002; Zammit et al. Reference Zammit, Allebeck, David, Dalman, Hemmingsson, Lundberg and Lewis2004; Koenen et al. Reference Koenen, Moffitt, Roberts, Martin, Kubzansky, Harrington, Poulton and Caspi2009; Welham et al. Reference Welham, Isohanni, Jones and McGrath2009; Reichenberg et al. Reference Reichenberg, Caspi, Harrington, Houts, Keefe, Murray, Poulton and Moffitt2010; Khandaker et al. Reference Khandaker, Barnett, White and Jones2011; Trotta et al. Reference Trotta, Murray and MacCabe2015; Bora, Reference Bora2016) and individuals at high risk of psychosis (Carrion et al. Reference Carrion, Goldberg, McLaughlin, Auther, Correll and Cornblatt2011; Fusar-Poli et al. Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung, Howes, Stieglitz, Vita, McGuire and Borgwardt2012; Giuliano et al. Reference Giuliano, Li, Mesholam-Gately, Sorenson, Woodberry and Seidman2012; Lin et al. Reference Lin, Yung, Nelson, Brewer, Riley, Simmons, Pantelis and Wood2013), our results suggest that lower verbal performance is a specific characteristic of the psychosis spectrum. This is in line with another cohort study showing an inverse association between psychotic experiences and intelligence quotient (IQ), especially in verbal IQ after adjusting for non-verbal IQ (Horwood et al. Reference Horwood, Salvi, Thomas, Duffy, Gunnell, Hollis, Lewis, Menezes, Thompson, Wolke, Zammit and Harrison2008). Neuroimaging studies have also shown that individuals with clinical high risk for psychosis had similar brain volume reduction and function in the inferior frontal gyrus, a region responsible for language processing, to patients with first-episode psychosis and chronic schizophrenia (Koike et al. Reference Koike, Takizawa, Nishimura, Takano, Takayanagi, Kinou, Araki, Harima, Fukuda, Okazaki and Kasai2011; Iwashiro et al. Reference Iwashiro, Suga, Takano, Inoue, Natsubori, Satomura, Koike, Yahata, Murakami, Katsura, Gonoi, Sasaki, Takao, Abe, Kasai and Yamasue2012; Koike et al. Reference Koike, Satomura, Kawasaki, Nishimura, Takano, Iwashiro, Kinoshita, Nagai, Natsubori, Tada, Ichikawa, Takizawa and Kasai2016).
Of interest, a study using national register data showed that siblings of patients with schizophrenia were more likely to work in creative occupations (e.g. in those with scientific occupations) (Kyaga et al. Reference Kyaga, Lichtenstein, Boman, Hultman, Långström and Landén2011). This association was strengthened after controlling for overall cognition (Kyaga et al. Reference Kyaga, Lichtenstein, Boman, Hultman, Långström and Landén2011). Creativity is more linked to non-verbal cognitive ability than verbal cognition (Cerruti & Wilkey, Reference Cerruti, Wilkey and DellaPietra2011), suggesting that a discrepancy towards higher non-verbal cognition may be associated with advantage in creative occupations. On the other hand, divergent thinking, related to creativity, is associated with thought disorder in schizophrenia, which is assumed to be derived from verbal cognitive impairment (Crow, Reference Crow2008; Levy et al. Reference Levy, Coleman, Sung, Ji, Matthysse, Mendell and Titone2010).
In contrast, participants with case-level affective symptoms had lower non-verbal cognition at age 8 years and greater relative developmental non-verbal lag, independently of verbal cognition and the other covariates. Consistent with previous studies (Cannon et al. Reference Cannon, Caspi, Moffitt, Harrington, Taylor, Murray and Poulton2002a ; Koenen et al. Reference Koenen, Moffitt, Roberts, Martin, Kubzansky, Harrington, Poulton and Caspi2009; Reichenberg et al. Reference Reichenberg, Caspi, Harrington, Houts, Keefe, Murray, Poulton and Moffitt2010), verbal cognition in those with affective symptoms was not different from that in participants without case-level affective symptoms, even without control for potential confounders, and was relatively high in those with co-morbid psychotic experiences. A meta-analysis suggests that non-verbal executive function, such as visual planning, spatial working memory, visual attention and visual pattern recognition memory, is one of the most prominent cognitive impairments in major depression (Rock et al. Reference Rock, Roiser, Riedel and Blackwell2014; Trivedi & Greer, Reference Trivedi and Greer2014). In addition, these impairments are evident before clinical onset, and show little improvement during clinical course, suggesting a trait factor (Rock et al. Reference Rock, Roiser, Riedel and Blackwell2014; Trivedi & Greer, Reference Trivedi and Greer2014). A recent neuroimaging study showed that a greater verbal–non-verbal discrepancy towards lower non-verbal function was associated with cortical thinning in the rostral part of the prefrontal cortex (Margolis et al. Reference Margolis, Bansal, Hao, Algermissen, Erickson, Klahr, Naglieri and Peterson2013), which is one of the regions responsible for executive functions (Badre & D'Esposito, Reference Badre and D'Esposito2009) as well as major depression (Disner et al. Reference Disner, Beevers, Haigh and Beck2011).
In conclusion, we found that people with adult psychotic experiences and depressive symptoms showed differential verbal–non-verbal cognitive profiles in childhood and adolescence. Our results suggest that these characteristics of cognitive development are common within each disease spectrum. Future prospective studies are needed at different stages across the spectra to elucidate how cognitive profiles are associated with the emergence of psychological symptoms.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717000393
Acknowledgements
The authors are grateful to NSHD study members for their continuing support. S.K. was supported by grants from the Japan Society for the Promotion of Science (JSPS) (KAKENHI grants no. 25870143 and 26118703) and the Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation. M.R. is funded by the UK Medical Research Council (Unit Programme no. MC UU 12019/3). P.B.J. is supported by Wellcome Trust grants no. 095844/Z/11/Z and 088869/Z/09/Z; National Institute for Health Research grant no. RP-PG-0606-1335; and by the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (BRC) and the NIHR Collaboration for Leadership in Applied Health Research & Care (CLAHRC) East of England.
Declaration of Interest
P.B.J. has received research grant support from GlaxoSmithKline, a speaker's honorarium from Eli Lilly, and is a co-inventor of patent PCT/GB2005/003279 (methods for assessing psychotic disorders). J.B. is an employee of Cambridge Cognition Ltd, and is a co-inventor of patent PCT/GB2005/003279 (methods for assessing psychotic disorders). S.K. and M.R. report no competing interests.







