INTRODUCTION
Helicobacter pylori is a common pathogen of the gastric mucosa and a major cause of peptic ulcer disease and is associated with chronic gastritis, mucosa-associated lymphoid tissue lymphoma, and adenocarcinoma of the stomach [Reference Kusters, van Vliet and Kuipers1]. Infection with this bacterium has been found in persons residing in developed countries (6–40% seroprevalence) and those in developing nations (50–90% seroprevalence) [Reference Mandeville2–Reference Graham4]. A limited number of antimicrobial agents have activity against H. pylori and treatment requires two or three agents usually administered with a proton pump inhibitor (PPI) for a 7- to 14-day course [Reference Luther5].
The proportion of persons who become reinfected after successful eradication of the organism ranges widely. In developing countries, annual reinfection rates vary widely from <10% [Reference Aydin6, Reference Louw7] to >50% [Reference Hoffenberg8, Reference RamirezRamos9] and are lower in developed countries, usually <10% [Reference AbuMahfouz10–Reference Berstad13]. However, few studies have looked at risk factors for reinfection [Reference Knippig14, Reference Rowland15].
Alaska Native (AN) persons have a 75–80% seroprevalence for antibodies to H. pylori [Reference Parkinson16, Reference Zhu17]. Seroprevalence of antibody to H. pylori was 32% in children aged 0–4 years and increased to 78% in 10- to 14-year-olds, remaining at that level within all older age groups [Reference Parkinson16]. We prospectively followed American Indian/Alaska Native (AI/AN) and Alaska non-Native (NN) patients diagnosed with H. pylori to determine the reinfection rates over a 2-year period and risk factors for reinfection after successful eradication of H. pylori. Objectives of our study were to determine: (1) post-eradication reinfection rates in the following three groups: AI/AN living in the largest metropolitan city (Anchorage, group 1), AI/AN living in rural, isolated communities (group 2), and NN living in Anchorage (group 3); (2) risk factors associated with reinfection; and (3) the prevalence of H. pylori infection in household members of the study participants.
METHODS
From 1 September 1998 to 30 March 2005, patients scheduled for oesophagogastroduodenoscopy (OGD) were recruited at the Alaska Native Medical Center, two private practice settings in Anchorage, one large hospital and one Gastroenterology clinic, and at the following three rural hospitals: the Yukon Kuskokwim Regional hospital in Bethel, the Kanakanak hospital in Dillingham (Bristol Bay) and the Norton Sound hospital in Nome (Norton Sound). Study participants had to be aged ⩾18 years and a resident of the region where their endoscopy was performed. The population of Anchorage, the largest urban metropolitan area in Alaska, is about 300 000 persons (http://quickfacts.census.gov/qfd/states/02/0203000.html). In contrast, the combined population of the three rural regions participating in the study (the Yukon Kuskokwin Delta, population 25 000; Bristol Bay, population 6000; and Norton Sound, population 9700) is 40 700. Patients were excluded from the study if they had a history of gastric cancer, gastric resection, were pregnant or had undergone cancer chemotherapy or immunosuppressive therapy within the previous year.
Patients were enrolled at the initial visit if they were found to have a positive 13C-urea breath test (13C-UBT, Meretek Diagnostics Inc., USA) for H. pylori; however, only those enrollees who were treated and documented as being cured (H. pylori-negative) by 13C-UBT were entered into the 2-year long-term reinfection study. Information regarding results for H. pylori from histology, culture, and CLOtest® on biopsy tissue (Ballard Medical Products, USA) was collected on each participant. Upon enrolment, a medical record review was conducted to determine if there was a history of: peptic ulcer disease, gastric surgery, gastritis, previous treatment for H. pylori, or evidence of medication prescribed for acid suppression treatment as previously reported [Reference McMahon18]. Patients who tested positive for H. pylori were treated with an antibiotic regimen selected by the patient's provider, as previously reported [Reference McMahon19]. Compliance with taking medicines was monitored with twice-weekly phone calls from a study nurse and treatment completion date was recorded. Patients who were taking a histamine 2 (H2) blocker or PPI were asked to stop taking these medications for at least 3 days prior to all follow-up 13C-UBT testing. Patients who tested 13C-urea breath test-positive for H. pylori at 8 weeks were offered a second treatment regimen at the provider's discretion. Patients who tested 13C-UBT-negative for H. pylori 8 weeks after the treatment start date continued in the long-term follow-up portion of the study and were subsequently tested for H. pylori recurrence by 13C-UBT at 4, 6, 12, and 24 months after treatment. At each follow-up visit, patients were interviewed to determine recent history of antimicrobial use, gastrointestinal symptoms, and risk factors for reinfection. At the end of the follow-up period, or at the time a patient became reinfected, current household members of the study participant were invited to be tested for H. pylori by 13C-UBT. Follow-up OGD for those who became reinfected was not part of the study protocol. Patients who became reinfected and household members who were found to be positive for infection with H. pylori were referred back to their providers after study completion.
The following risk factor variables were collected from each participant and entered into the univariate analysis: age, sex, household crowding, presence of a child aged <5 years in the household, employment, education level, alcohol consumption, tobacco use, private well water, consuming of water from lake, spring or river, travel within Alaska, pet ownership, diagnosis of an ulcer, previous H. pylori treatment, presence of moderate to severe gastritis and pre-mastication of food. For risk factors, we used the result collected at the time of enrolment with the exception of private well water, and consumption of lake, spring or river water. For these, if participant answered ‘yes’ at any point after the 2-month follow-up they were considered as having that risk factor. For risk factors where we used the result in the enrolment interview, we conducted sensitivity analyses using the information collected in the visit just prior to study endpoint. Results were unchanged and not reported. The following risk factor variables were entered into the multivariate model: household crowding, employment status, education level, tobacco use, consumption of lake spring, or river water, travelling within Alaska, pet ownership, diagnosis of an ulcer, previous H. pylori treatment, and moderate to severe gastritis.
The Institutional Review Boards of the Centers for Disease Control and Prevention, Indian Health Service, Alaska Area Tribal Health Consortium, and the Western IRB approved the study. In addition, the study was approved by the Southcentral Foundation, The Norton Sound Health Corporation, Yukon Kuskokwim Health Corporation and the Bristol Bay Area Health Corporation. Written informed consent was obtained from all participants.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Laboratory testing
Gastric biopsies were processed and inoculated to solid media as described previously [Reference McMahon18]. One biopsy, usually from the antrum, was taken for the CLOtest® (an agar medium containing urea and a pH sensitive indicator) for the detection of urease. Gastric biopsy tissue obtained at the time of OGD were stained with Diff-Quik® (Mercedes Medical, USA) stain, for identification of H. pylori and with haematoxylin and eosin stain for histological evaluation.
Statistical methods
Reinfection rates were calculated using a Kaplan–Meier estimate. Statistical tests of risks factors were conducted using a Cox proportional hazards model. Participants were censored at the visit of reinfection or their final visit if they were not reinfected. Univariate tests were run, and those risk factors with a univariate P value <0·25 were considered in multivariate models as well as age and sex which have previously been associated with H. pylori reinfection. Because of lower power associated with the sample size of patients with H. pylori reinfections, the multivariate model was built using purposeful forward selection [20]. Variables were considered confounders and remained in the model if their exclusion changed the value of the coefficient(s) of interest by >15% [20]. Two additional risk factors were examined separately because they were not available and/or relevant for all participants. We tested household members of persons reinfected and those not reinfected on their last study visit for their H. pylori infection status using the 13C-UBT test. All household members were invited to participate. Fifty-four percent (123/229) of the long-term participants had household members who were tested for H. pylori; a total of 324 household members were tested. In a retrospective questionnaire conducted after the end of the study, we ascertained whether participants in the rural arm lived in households with in-home running water; all participants living in the urban centre of Anchorage, Alaska had access to running water and were eliminated from this sub-analysis. Presence of running water in the household, and household members who were H. pylori positive, were tested on a univariate basis using methods described previously. Statistical analysis was performed by using StatXact 9 (Cytel Software Corp., USA) and SAS software v. 9·3 (SAS Institute Inc., USA). P values are two-sided and confidence limits and P values are exact when appropriate.
RESULTS
During the study period, 582 patients were enrolled in the study and 571 had an OGD completed (Fig. 1). A total of 362 persons tested positive for H. pylori by 13C-UBT and were eligible for antimicrobial treatment. Treatment recommendations and choice of antimicrobial regimen were at the discretion of clinical providers; 333 (92%) H. pylori-positive persons were treated with an antimicrobial regimen; 236 persons were eligible for long-term follow-up, 200 after their first treatment for H. pylori, 30 after their second treatment, and an additional six persons after their third treatment (Fig. 1), and 229 (97%) participated in long-term follow-up for H. pylori reinfection. Seven persons moved from the study area or were lost to follow-up after completion of the 2-month visit. There were 98 participants in group 1 (the urban AI/AN arm), 69 persons in group 2 (the rural AI/AN arm) and 62 persons in group 3 (the urban NN Alaskan arm) of the study. Characteristics of study participants according to study arm are shown in Table 1. The median age of all participants was 51 years and 55% of participants were female. Among participants who tested positive for H. pylori by 13C-UBT, 85% (272/322), 90% (306/341), and 84% (242/289), were positive by CLOtest®, culture and histology, respectively.

Fig. 1. Flow diagram of participation in the Alaska reinfection study in three different populations. AN, Alaska Native; NN, Alaska non-Native.
Table 1. Characteristics of patients at enrolment who entered into long-term follow-up (n = 229) for H. pylori reinfection
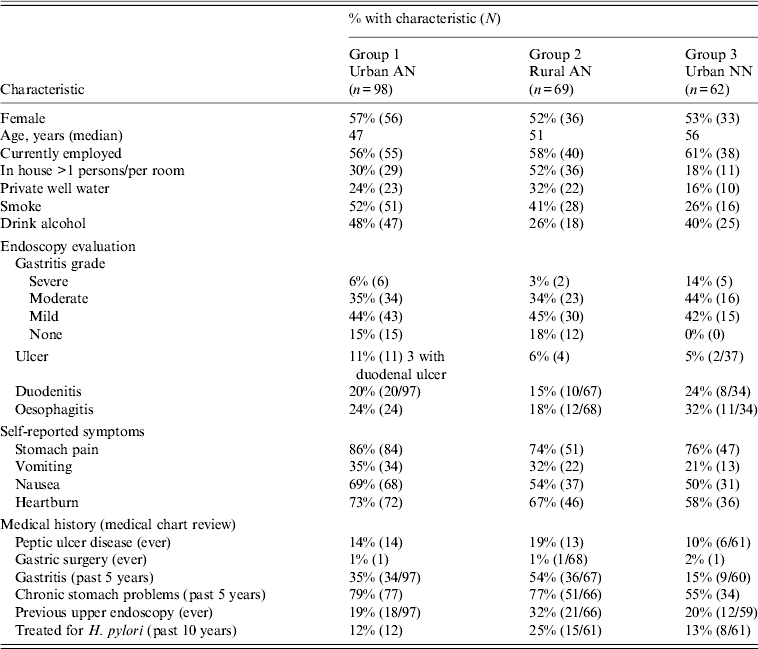
AN, Alaska Native; NN, Alaska non-Native.
Values given are % (n).
Participants in long-term follow-up
The analyses that follow were restricted to the 229 patients who had ⩾1 visit after the 2-month test of cure. Of those in long-term follow-up, 216 (91%) completed the 2-year follow-up or were reinfected. Three patients were lost to follow-up after the 4-month follow-up visit, three patients after the 6-month visit, and seven patients after the 1-year visit. Demographic characteristics, OGD evaluation, self-reported symptoms and medical history at the time of enrolment for the 229 participants involved in long-term follow-up are shown in Table 1. Most participants at enrollment reported stomach pain (79%), nausea (59%) or heartburn (67%) and 30% complained of vomiting prior to treatment.
Long-term follow-up and reinfection rate
During the 2-year follow-up period, a total of 36 persons were reinfected with H. pylori: 14 at 4 months, seven at 6 months, five at 12 months, 10 at 24 months for an overall reinfection rate of 16·1% (Table 2). Cumulative reinfection rates at 2 years were highest (22%) among rural AN persons (group 2) compared to urban AN persons (group 1) and urban NN persons (group 3) who had reinfection rates of 14·5% and 12%, respectively. In order to eliminate any cases that could have occurred due to recrudescence, we removed the 14 patients who had positive 13C-UBTs at 4 months after treatment and determined that the cumulative reinfection rate for all arms would be 3·3% (95% CI 1·6–6·8)at 6 months, 5·7% (95% CI 3·3–9·8) at 1 year, and 10·7% (95% CI 7·1–15·7) at 2 years. In a post-hoc analysis of data within group 2, the reinfection rate in the Yukon Kuskokwim Delta region at 2 years was 29·9% (95% CI 18·2–46·4) which trended higher than in the Norton Sound and Bristol Bay regions combined (10·7%, 95% CI 3·6–29·6, P = 0·06) and was higher than in group 1 (P = 0·03). We detected no difference in the prevalence of symptoms (heartburn, nausea, stomach pain, vomiting) between reinfected patients and non-reinfected patients on their last visit. Of the 36 participants with reinfections, 15 (42%) reported no change in their symptoms at the final reinfection visit, six (17%) reported a worsening of symptoms, 13 (36%) reported improvement of their symptoms, and two (5%) persons reported new symptoms.
Table 2. Two-year cumulative reinfection rate among three groups, Alaska
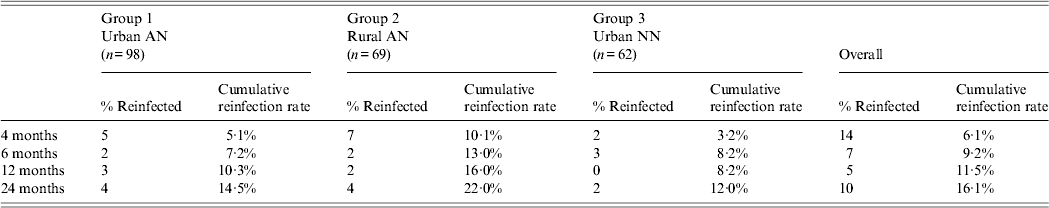
AN, Alaska Native; NN, Alaska non-Native.
Risk factors for reinfection
For all three arms combined, we examined a variety of risk factors for reinfection with H. pylori. On univariate analysis, we found that risk of H. pylori reinfection was associated with living in a crowded house [defined as ⩾1 person per room, risk ratio (RR) 2·3 95% CI 1·2–4·4], not having graduated from high school (RR 0·4 95% CI 0·2–0·8), an ulcer diagnosis (either at enrolment or having a history of ulcers, RR 2·4, 95% CI 1·2–4·8) and consumption of lake, spring or river water (RR 2·0, 95% CI 1·0–3·9, Table 3). On multivariate analysis, for all three arms combined, we found that risk of reinfection was associated with diagnosis of ulcer (RR 2·3, 95% CI 1·2–4·5) and graduating from high school (RR 0·4, 95% CI 0·2–0·9). The multivariate results were similar for ulcer and high school in a model that additionally included age and sex. Trends for reinfection rates showed that among those who had not graduated from high school, reinfection rates were higher (compared to persons who did graduate) in each of the three study arms (group 1:33% vs. 12%; group 2:31% vs. 17%; group 3: 27% vs. 8%); however, these trends were not statistically significantly different. Living in a crowded house was also statistically significant (P = RR 2·0, 95% CI 1·0–4·0) in a two-variable model with ulcer diagnosis, but the P value dropped to 0·11 in a three-variable model which included ulcer diagnosis and graduating high school.
Table 3. Univariate risk factors associated with H. pylori reinfection in Alaskans enrolled in 2-year follow-up after successful treatment for H. pylori
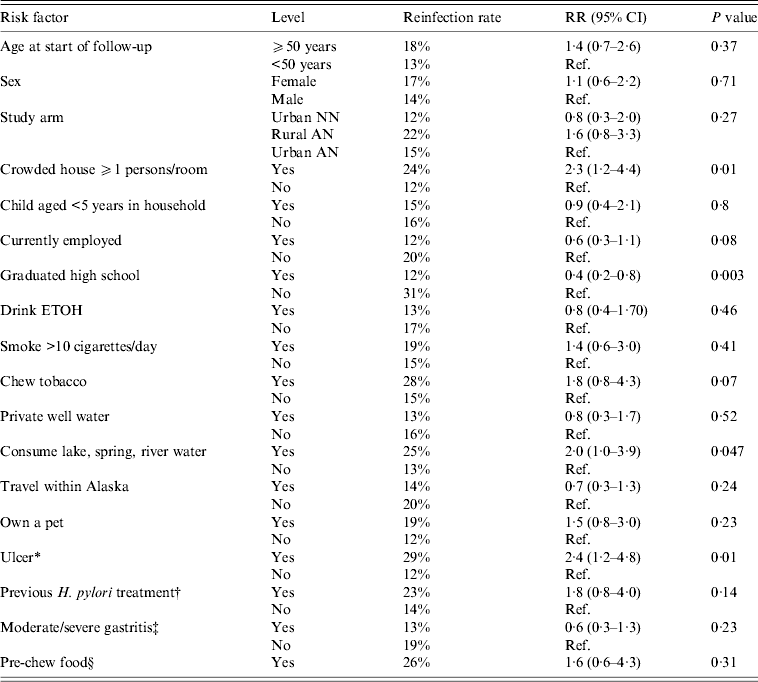
RR, Risk ratio; CI, confidence interval; AN, Alaska Native; NN, Alaska non-Native.
* History of gastric or duodenal ulcer or ulcer diagnosed at enrolment.
† Previously treated for H. pylori in the 10 years prior to enrolment.
‡ At enrolment visit.
§ ‘Do you pre-chew food for someone else?’
Additional risk factors
Household member prevalence and reinfection in study participants
Three hundred and twenty-four (70%) of 460 eligible household members of study participants (from all three groups) participated at study endpoint (visit of reinfection or 2-year final visit). Spouses or partners comprised 25% (n = 81) of these household members; children and grandchildren accounted for 67% (n = 217). Overall, 182 (56%) household members were positive for H. pylori by 13C-UBT test. The percentage of household members that were H. pylori-positive differed by group: group 1 (urban AI/AN) 46% (48/105), group 2 (rural AI/AN) 78% (119/153) and group 3 (urban NN) 23% (15/66), P < 0·0001. Among all three groups participating in the study, 59 household members of these participants were tested from households where the study participant was reinfected and 265 members from households where the study participant was not reinfected. After controlling for study arm, household member positivity was associated with H. pylori reinfection (P = 0·04). The reinfection rate was 7·0% in study participants with no household members positive for H. pylori, 9·3% when some household members were positive and 27·3% in study participants where every household member tested positive for H. pylori. This association was primarily accounted for by the results within group 2 (P = 0·04 within this group) vs. group 1 (P = 0·51 within this group) and group 3 (P = 0·69 within this group) (Fig. 2).
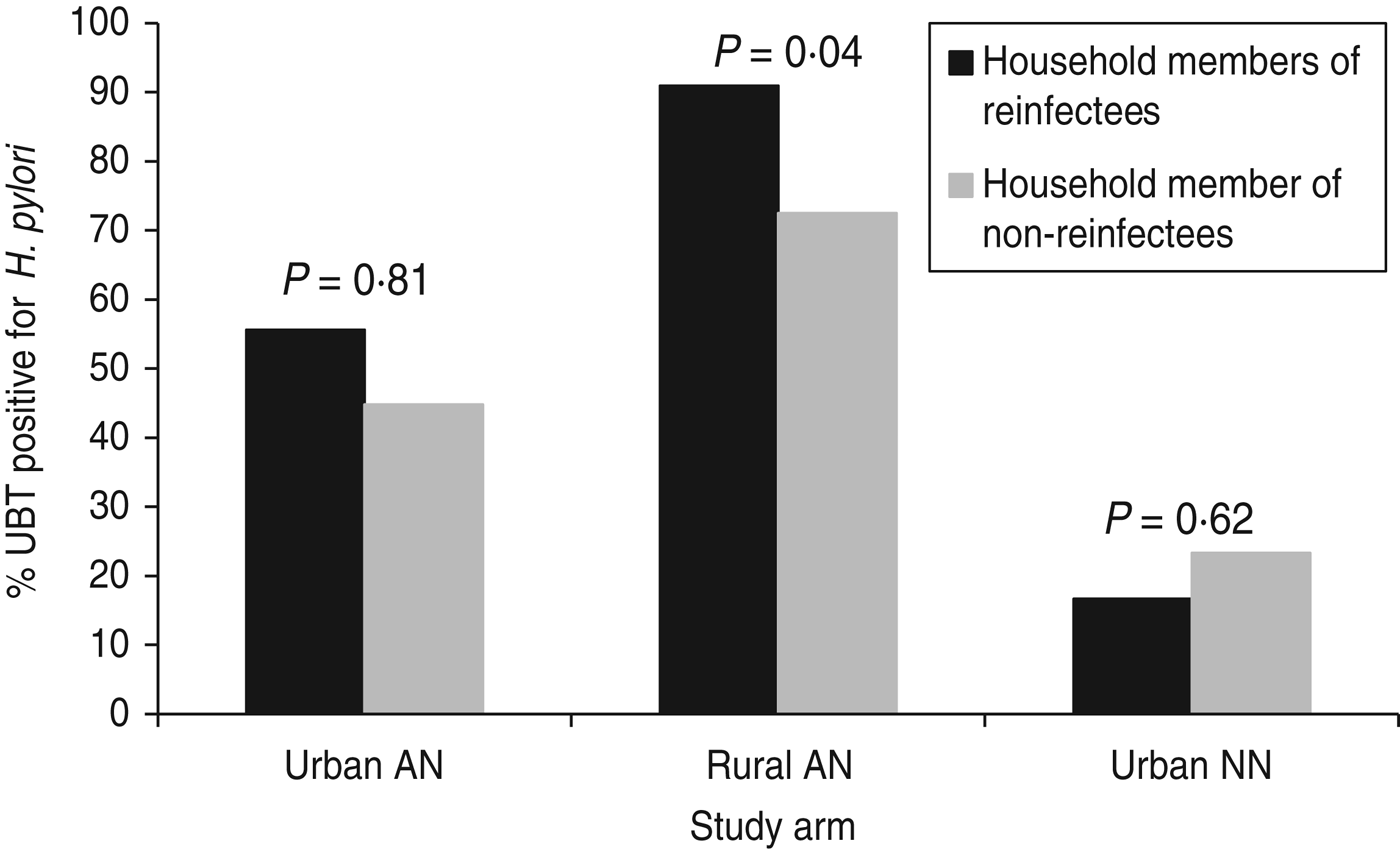
Fig. 2. H. pylori positivity of household members of study participants by group, Alaska.
H. pylori reinfection and water service
In a retrospective oral questionnaire, participants in the rural arm were asked about their access to running water in their homes. Because all participants in urban Anchorage had access to running water, this sub-analysis was restricted to the rural Alaska arm. Only 58/69 participants were available to participate, and 76% (44/58) of them had access to running water in their home. Among persons without running water in their home, 43% were reinfected with H. pylori compared to 20% in persons with access to running water (RR 2·3, 95% CI 0·8–6·6).
DISCUSSION
This is the first study examining the rate of H. pylori reinfection in AN and NN populations in Alaska. We found a high overall cumulative reinfection rate (16·1%) and elevated rates in all three groups during the first 2 years of follow-up after successful treatment of H. pylori compared to other studies in the USA and Europe. The highest cumulative 2-year rate of reinfection (22%) was found in AN persons residing in rural settings (group 2). In one region, where 20% of villages lack running water and flush toilets, 29·9% of participants were reinfected at 2 years. Risk factors for reinfection in study participants were: peptic ulcer disease (past or present), low education level, and having H. pylori-positive household members.
The proportion of persons successfully treated for H. pylori infection that later become reinfected varies widely throughout the world and, in general, parallels the prevalence of endemicity of this organism in the general population. In developed countries, the risk of reinfection is low, ranging from <1% to 6% [Reference Adachi11–Reference Knippig14, Reference Borody21, Reference Cutler and Prasad22], although reinfection rates as high as 73% have been reported [Reference Louw7–Reference RamirezRamos9, Reference Hildebrand23–Reference Morgan27]. The rates of reinfection found in this study are unusually high compared to the rest of the USA and are similar to those found in developing countries. Health disparities exist in rural Alaska which are rarely seen in other parts of the USA, such as lack of access to piped water in the home, lack of flush toilets, and crowded living conditions [Reference Hennessy28].
Distinguishing between reinfection and recrudescence can be a challenge in H. pylori infection; if antimicrobial therapy merely suppresses the organism rather than eradicating it, H. pylori could still be present early in the post -treatment period (within the first 2 or 4 months) which could represent recrudescence [Reference Niv29, Reference Xia30]. In order to account for recrudescence vs. reinfection, we did a separate analysis eliminating the 4-month follow-up results from the calculation for reinfection and determined that overall 11% were reinfected at 2 years. Follow-up OGD examinations could possibly help distinguish between the two by sequencing both the original isolate and the isolate which they later became infected with to determine if sequences were similar, suggesting recrudescence, or dissimilar, suggesting reinfection. However, persons can become reinfected with the same sequence-type isolate, for example from infected household members, and therefore seeing similar sequence types does not necessarily mean recrudescence has occurred. In addition, OGD is an invasive test and serial OGDs would have been difficult to justify.
We found that the presence of H. pylori by 13C-UBT in household members was associated with reinfection at 2 years; the strength of this association was greatest in group 2, AN participants living in rural Alaska. Other factors associated with reinfection included a history of peptic ulcer disease and lower education level (not graduating from high school). Very few studies have reported risk factors associated with reinfection of H. pylori after eradication; a recent study by Candelli et al. in Italy demonstrated an association between reinfection at 3 years and age and low family income in persons with type 1 diabetes [Reference Candelli31] and a study performed by Kim et al. in Korea showed an association between reinfection and male gender and low family income [Reference Kim32]. However, to date, few studies have demonstrated an association between reinfection in the index cases and H. pylori status in spouses, children or parents nor have they shown any association with household crowding. A recent multi-centre study in South America by Morgan et al. demonstrated that H. pylori recurrence 1 year post-eradication was associated with an increasing number of children in the household [Reference Morgan27]. The general lack of association seen in previous studies could be due to the small sample sizes in these reports.
We were unable to find any studies demonstrating the presence of peptic ulcer or low education level as risk factors for reinfection after eradication of H. pylori infection. However, this may be due to the fact that education level and peptic ulcer are rarely looked for as risk factors [Reference Rowland15, Reference Rollan26, Reference Gisbert33]. In our study, we did not collect information on income level, but lower education level may be a marker for lower socioeconomic status, a documented risk factor for reinfection [Reference Rowland15, Reference Kim32]. Our finding of peptic ulcer as a risk factor for reinfection is important because if H. pylori is not eradicated or recurs in a person with a history of peptic ulcer disease, the risk of peptic ulcer reoccurrence is >50% [Reference Louw7, Reference Borody34]. By contrast, a long-term follow-up study in Spain of 1000 patients with bleeding ulcers due to H. pylori (who were cured) found that only one person during the subsequent 12 months had a bleeding ulcer due to H. pylori infection, suggesting that acid suppression therapy could be stopped after successful treatment. [Reference Gisbert35]
During our study, information from a state-wide database on domestic water service in Alaskan communities became available and we determined that, among all participants in the rural arm of the study (group 2), 59% (41/69) lived in villages where >90% of homes had access to running water. Of the 41 participants living in villages with in-home water service, 12% (5/41) were reinfected with H. pylori compared to 35% (10/28) for persons living in villages where <90% of homes were serviced with water (P = 0·02, relative rate = 2·9). Homes without piped water service must haul treated water from a central watering point in the village and frequently use raw water sources from rivers, lakes or rooftops as a matter of convenience. Due to these findings, we developed a retrospective oral questionnaire asking group 2 study participants about access to running water in their homes. We found a trend towards a higher proportion of persons reinfected in homes without access to running water compared to persons in homes with running water that was not statistically significant; ~20% of communities in rural Alaska do not have running water available to their residents. H. pylori has been isolated from drinking water sources [Reference Moreno and Ferrús36] and studies from Peru have found that different drinking water sources were associated with H. pylori infection in children [Reference Klein37, Reference Hulten38]. More work investigating whether drinking water can be a source of H. pylori infection is warranted.
This study has a number of limitations. First, it is not a population-based study; we enrolled persons with gastrointestinal symptoms who were scheduled for OGD and therefore these results are most relevant to persons seeking care for gastrointestinal symptoms and may not be generalizable to the entire population. Second, risk factors for reinfection with H. pylori may differ by group; however, this study was not adequately powered to look at these differences within groups 1, 2 and 3. Third, we acknowledge that the 4-month cut point for distinguishing reinfection vs. recrudescence may not adequately distinguish between the two. Fourth, although patients were re-tested for H. pylori after having been off PPIs and H2 blockers for ⩾3 days, when we removed patients who had any PPI use within 30 days of any follow-up visit with a 13C-UBT test, our cumulative 2-year reinfection rate was 17·6% (95% CI 11·7–25·9). Similarly, when we removed patients with any H2 blocker use within 30 days of any follow-up visit with a 13C-UBT test, the cumulative 2-year reinfection rate was 18·0% (95% CI 12·1–26·3); these reinfection rates are similar to the overall 2 year reinfection rate of 16%.
Another important finding of our study is that the presence of symptoms of gastric distress had no bearing on whether a person was reinfected or not after eradication. Thus, relying on symptoms to judge the success of treatment is unreliable and a ‘test of cure’ is necessary such as 13C-UBT or stool antigen. The timing of the test-of-cure is important and consideration should be given to performing it later than 4–6 weeks post-treatment, since negative tests performed ⩽4 months after this date may not rule out recrudescence. This needs further study.
In conclusion, we found high rates of reinfection in three different groups in Alaska, similar to rates found in developing countries where H. pylori infection is endemic. This study highlights the importance of demonstrating that H. pylori infection is truly eradicated in persons who were born or live in endemic regions of the world, such as rural Alaska. Finally, studies employing longer periods of follow-up with careful examination of potential risk factors need to be performed to better define the long-term risk of reinfection after successful eradication of H. pylori infection after antimicrobial treatment.
ACKNOWLEDGEMENTS
The authors thank the follow physicians who contributed patients to this study: Steven Westby MD, Mia Lee MD, John Harvey MD, David Barrett MD, Elaine Callahan MD, Kevin Stange MD, Mark Thorndike MD, Mary Christian MD, Frances Wilson MD, David Powers MD, Richard McGrath MD, Patrick Martinez MD, Bill Eggiman MD, Joe Klejka MD, Michael Swenson MD, Richard Buchanan MD, Charles Shannon MD, Thomas Shreves MD, and James Stragland MD. We also thank the following nurses who helped recruit and follow patients: Helen Peters, Cindy Hamlin, Marilyn Getty, and Susan Seidel. We especially thank Kenneth Petersen MD who was involved with the design of this study.
This study was funded by the Centers for Disease Control and Prevention, a North American Research Centers for Health (NARCH) grant no.: 1 U26 94 00005, and a grant from the Alaska Science and Technology Foundation.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
In this study, antibiotic regimen for treatment was selected by the patient's provider. Providers for group 3 participants were offered free prev-pac kits for their use from TAP Pharmaceuticals. No funding was provided by TAP Pharmaceuticals and TAP Pharmaceuticals had no access to the data and did not review this manuscript prior to publication.
DECLARATION OF INTEREST
None.








