Non-technical Summary
The name eomysticetids refers to the “dawn” of the baleen whale, suggesting the critical role in understanding the origin and early evolution of baleen whales. Eomysticetids represent an early diverging lineage showing the baleen-assisted feeding mode—an essential feature for baleen whales, including giant animals such as the blue or fin whales. This paper describes a new eomysticetid species—Echericetus novellus—from the Oligocene of Mexico, about 28 million years old. Our discovery of a new species of Oligocene eomysticetids from Mexico shows a much broader global distribution and higher diversity than previously recognized. The existence of Echericetus novellus from Mexico indicates that eomysticetids also inhabited warmer waters in the Northern Hemisphere instead of primarily higher latitudinal regions. Last, our discovery of a new but extinct eomysticetid from the Oligocene of Mexico provides new insights into the distribution pattern and habitat use of Eomysticetidae, which helps to decipher the ultimate demise of this once-successful baleen whale lineage.
Introduction
The Oligocene, ranging approximately from 34 to 23 Ma, witnessed an unparalleled period for the early evolution and diversification of Mysticeti, the largest mammals ever to have evolved (Marx and Fordyce, Reference Marx and Fordyce2015; Tsai and Kohno, Reference Tsai and Kohno2016). Groups such as eomysticetid are examples of great diversity and disparity, where developmental processes (Boessenecker and Fordyce, Reference Boessenecker and Fordyce2015b) and environmental factors (e.g., climate change; Marx et al., Reference Marx, Fitzgerald and Fordyce2019) likely drove their evolutionary pathways. Eomysticetid display a widespread geographic distribution, unique longirostrine skull morphology, and possible ecological role as a planktivore (Sanders and Barnes, Reference Sanders and Barnes2002b; Clementz et al., Reference Clementz, Fordyce, Peek and Fox2014; Boessenecker and Fordyce, Reference Boessenecker and Fordyce2015b, Reference Boessenecker and Fordyce2017b). More interestingly, eomysticetid represent a transitional step between the toothed and baleen-bearing mysticetes despite no fossil evidence of baleen in Oligocene mysticetes yet (Boessenecker and Fordyce, Reference Boessenecker and Fordyce2015b; Marx et al., Reference Marx, Collareta, Gioncada, Post, Lambert, Bonaccorsi, Urbina and Bianucci2017; Gatesy et al., Reference Gatesy, Ekdale, Deméré, Lanzetti, Randall, Berta, El Adli, Springer and McGowen2022). The eomysticetid fossil record is primarily in the South Pacific Ocean (New Zealand). However, the Northern Hemisphere has also produced abundant eomysticetid fossils (Sanders and Barnes, Reference Sanders and Barnes2002a, Reference Sanders and Barnesb; Peredo and Uhen, Reference Peredo and Uhen2016; Peredo et al., Reference Peredo, Pyenson, Marshall and Uhen2018; Hernández-Cisneros and Nava-Sanchez, Reference Hernández-Cisneros and Nava-Sánchez2022).
Here we describe a new eomysticetid genus and species (Mysticeti, Eomysticetidae) from the Oligocene of Mexico (La Paz, Baja California Sur; Fig. 1). The holotype of this new taxon, MRAHBCS Pal/V119, was excavated and collected in 1995 (Schwennicke et al., Reference Schwennicke, González-Barba and de Anda-Franco1996). Previously, this specimen was tentatively considered similar to “Mauicetus” lophocephalus (Barnes, Reference Barnes1998), now known as the eomysticetid Tokarahia lophocephalus (Boessenecker and Fordyce, Reference Boessenecker and Fordyce2015a). In addition to the anatomical description, we analyze its phylogenetic relationships within Cetacea and interpret eomysticetid morphological disparity and diversification.
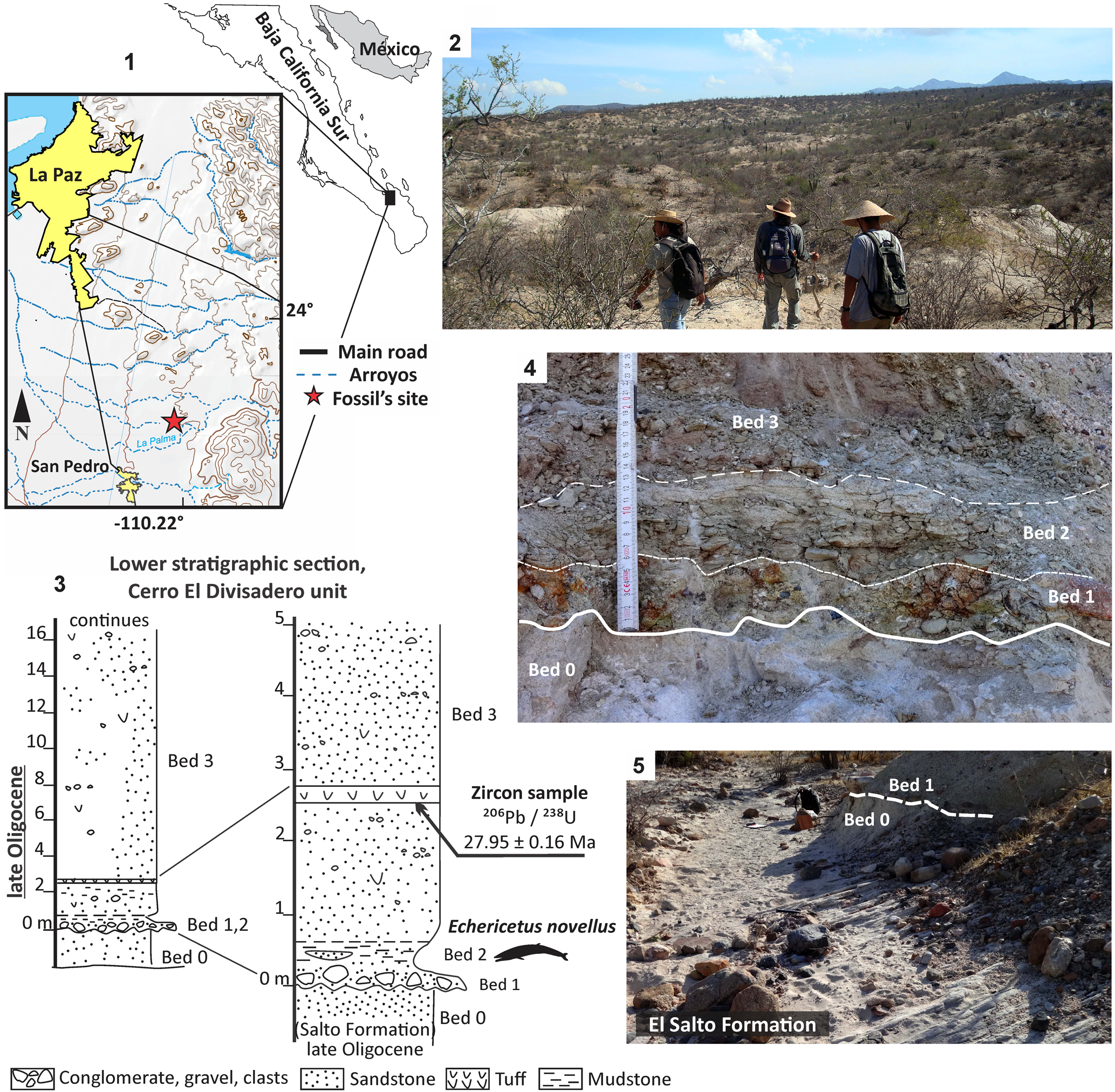
Figure 1. (1) Locality of Echericetus novellus n. gen. n. sp. (topographic map modified from INEGI, 2007, 2017). (2) Panoramic view at the Cerro El Divisadero locality. (3) Stratigraphic section. (4, 5) Details and contact of the lower strata with the Salto Formation (NAD 27 23°58'23"N, 110°13'42.4"W).
Geological setting
The specimen MRAHBCS Pal/V119 (Echericetus novellus n. gen. n. sp.), recovered in 1995, was found in a marine bed poorly exposed at the bottom of an ~5 m wide dry streambed. At this site, the fossil-bearing bed and the strata below were not exposed adequately (see Schwennicke et al., Reference Schwennicke, González-Barba and de Anda-Franco1996). However, during visits to the locality area in 2018 and 2021, we observed that these strata are exposed about 30 m to the northeast of the original fossil site. Beds dip ~3° southwest, which explains why these beds have not been exposed at the position where Echericetus novellus n. gen. n. sp. has been found. For the present paper, we gathered new stratigraphic field data and obtained a radiometric age from a tuff bed; these data allow a more detailed stratigraphic interpretation.
The lowermost unit, named bed 0 by Schwennicke et al. (Reference Schwennicke, González-Barba and de Anda-Franco1996), is a large-scale cross-bedded fine-grained arkosic sandstone, seemingly of eolian origin. About 1 m is exposed. We consider this bed as part of the Upper Oligocene–lowermost Miocene Salto Formation exposed north and east of La Paz and elsewhere in Baja California Sur along its eastern margin (McFall, Reference McFall1968; Hausback, Reference Hausback and Frizzel1984; Schwennicke et al., Reference Schwennicke, Plata-Hernández and Vázquez-Balderas2000, Reference Schwennicke, Alvarado Gastelum and Pérez-Venzor2006; Umhoefer et al., Reference Umhoefer, Dorsey, Willsey, Mayer and Renne2001; Plata-Hernández, Reference Plata-Hernández2002; Alvarado-Gastelum, Reference Alvarado-Gastelum2007; Drake et al., Reference Drake, Umhoefer, Griffiths, Vlad, Peters and McIntosh2017). The distance from the fossil site (Bed 2; see Fig. 1) to the southernmost exposures of the Salto Formation east of La Paz (called Cerro Chichonal sandstone by Aranda-Gómez and Pérez-Venzor, Reference Aranda-Gómez and Pérez-Venzor1988) is 18 km. The Salto Formation accumulated in terrestrial, coastal areas in alluvial fan and eolian settings. The unit is contemporaneous with the marine San Juan Member of the El Cien Formation (Fischer et al., Reference Fischer, Galli-Ollivier, Gidde and Schwennicke1995; Alvarado-Gastelum, Reference Alvarado-Gastelum2007) exposed in San Juan de la Costa (late Early Oligocene, but also see Hernández-Cisneros and Nava-Sánchez, Reference Hernández-Cisneros and Nava-Sánchez2022, p. 3, fig. 3).
The Salto Formation is discordantly overlain at the fossil site by the marine beds, which contained the mysticete described herein (bed 2; see Fig. 1). The lowermost layer on the erosional surface is a sandy conglomerate (bed 1) that grades laterally into conglomeratic sandstone. The clasts are mainly rounded pebbles, cobbles, and a few boulders of varying composition, including volcanic, plutonic, and metamorphic rocks. The sandy matrix contains some phosphatic grains and exhibits internal lamination (Schwennicke et al., Reference Schwennicke, González-Barba and de Anda-Franco1996). This bed represents a gravelly beach deposit and marks the beginning of a marine transgression (Schwennicke et al., Reference Schwennicke, González-Barba and de Anda-Franco1996). Some cobbles exhibit imbrication, indicating breaking waves from the southwest, suggesting a northwest–southeast-oriented shoreline. The basal conglomeratic texture of bed 1 grades upward into sandstone, mudstone, and greenish tuffaceous mudstone with sandstone lenses (bed 2). These sediments exhibit mainly parallel lamination in addition to some lenticular and flaser bedding. Bioturbation is nearly absent. In addition, at the top of bed 2, we found a lenticular reworked tuff up to 15 cm in thickness that was not observed by Schwennicke et al. (Reference Schwennicke, González-Barba and de Anda-Franco1996). This tuff has been used for radiometric dating, as explained in the following. Bed 2 reflects a lagoonal depositional environment influenced by volcanic activity (Schwennicke et al., Reference Schwennicke, González-Barba and de Anda-Franco1996). Field observations in 1995 revealed that besides the recovered specimen MRAHBCS Pal/V119, there were two more poorly preserved mysticete fossils in bed 2 (one of them is the specimen MRAHBCS Pal/V141, an eomysticetid fragmented skull and vertebrae). All these fossils showed the same orientation (east–west direction), perhaps due to wave action or currents, and it seems likely that these fossilized mysticetes indicate a stranding event in the coastal lagoon environment (Schwennicke et al., Reference Schwennicke, González-Barba and de Anda-Franco1996). Bed 2 grades upward into gray, slightly tuffaceous volcaniclastic sandstone of bed 3 (about 30 m thick). Texturally, bed 3 varies from regularly sorted, silty fine-grained sandstone to poorly sorted, fine- to coarse-grained sandstone. The silty sandstone is found predominantly in the lowermost portion of bed 3. Some volcanic granules and pebbles are scattered in the rock or form thin conglomeratic lenses. Whereas the bottom 2 m of bed 3 commonly is finely laminated, the middle and upper parts of bed 3 also exhibits cross-bedding and cross-lamination. Volcanic influence during deposition of bed 3 is also revealed by a 20 cm thick bed of reworked fine-grained tuff not recognized by Schwennicke et al. (Reference Schwennicke, González-Barba and de Anda-Franco1996). Bed 3 reflects a distal fluvial depositional setting and grades upward into coarser muddy sandstone and pebbly sandstone (bed 4; Schwennicke et al., Reference Schwennicke, González-Barba and de Anda-Franco1996, p. 4, fig. 3), all it of volcaniclastic composition (Fig. 1).
The sedimentology and stratigraphy of the succession at the fossil site suggest that beds 1 and 2 reflect a short-lived marine transgression in the area. To obtain more information related to the age of this event (and the age of the specimen of Echericetus novellus n. gen. n. sp. at the bottom of bed 2), we searched the marine sediments of beds 2 and 3 for micro- and nannofossils, but unfortunately none could be found. However, we dated the already mentioned reworked tuff at the top of bed 2. The U–Pb zircon age of this tuff is 27.95 ± 0.16 Ma (latest Rupelian, late Early Oligocene; Cohen et al., Reference Cohen, Harper and Gibbard2023). This age is similar to the age of the middle San Juan Member of the marine El Cien Formation at San Juan de la Costa (Schöllhorn et al., Reference Schöllhorn, Houben, Gertsch, Adatte, Alexey, de Kaenel, Spangenberg, Janssen, Schwennicke and Föllmi2020). We therefore conclude that the marine beds 1 and 2 in the study area are contemporaneous with the middle part of the San Juan Member and may represent a marginal facies of this unit. The fluvial deposits of beds 3 and 4 (Schwennicke et al., Reference Schwennicke, González-Barba and de Anda-Franco1996, p. 4, fig. 3) are therefore interpreted as basal strata of the volcaniclastic arc-derived Comondú Group (Hausback, Reference Hausback and Frizzel1984; Drake et al., Reference Drake, Umhoefer, Griffiths, Vlad, Peters and McIntosh2017).
Material and methods
The specimen MRAHBCS Pal/V119 (previously known as MRAHBCS 002; Hernández-Cisneros and Nava-Sánchez, Reference Hernández-Cisneros and Nava-Sánchez2022) is stored in the paleontological collection of the Museo Regional de Antropología e Historia de Baja California Sur, Baja California Sur, México. The preparation of MRAHBCS Pal/V119 included mechanical and chemical (bathed in 10% acetic acid) methods. Polyvinyl acetate was used to glue the fragmented pieces. After the preparation, photos were taken with a Nikon D3400 camera with an 18 mm lens. Anatomical terms follow Mead and Fordyce (Reference Mead and Fordyce2009). Data and remarks matrix for phylogenetic analysis and measurements can be found in the Supplemental files.
Phylogenetic analysis
The phylogenetic analysis was performed on the basis of the original data matrix from Marx and Fordyce (Reference Marx and Fordyce2015), including recent updates from Hernández-Cisneros and Nava-Sánchez (Reference Hernández-Cisneros and Nava-Sánchez2022) and Hernández-Cisneros (Reference Hernández-Cisneros2022). We reviewed coded characters for Maiabalaena and Sitsqwayk, considering the observations from Boessenecker et al. (Reference Boessenecker, Beatty and Geisler2023) that suggest their inclusion in Eomysticetidae. The following characters were changed in both taxa: character (c.) 26 coded from the state (s.) “?” to s. “1” (teeth in adult individuals absent or vestigial; see discussion on baleen origins; Ekdale and Deméré, Reference Ekdale and Deméré2022; Gatesy et al., Reference Gatesy, Ekdale, Deméré, Lanzetti, Randall, Berta, El Adli, Springer and McGowen2022); c. 83 from s. “2” to s. “1” (the temporal fossa form a large parasagittal oval); c. 100 from s. “0” to s. “2” (almost 90° twisting of the zygomatic process of squamosal). For Maiabalaena, c. 11 was changed from s. “?” to s. “1” (antorbital process present and defined by a steep face separating the posterolateral corner of the maxilla from its more anterior rostral portion); c. 103 from s. “0” to s. “1” (absent supramastoid crest of the zygomatic process of squamosal). For Sitsqwayk, c. 24 was changed from s. “?” to s. “1” (a straight facial portion of the rostrum in lateral view). In addition, the following characters in Tlaxcallicetus guaycurae Hernández-Cisneros, Reference Hernández-Cisneros2018 were changed: c. 83 from s. “2” to s. “?”; c. 99 from s. “0” to s. “?”; c. 117 from s. “2” to s. “1”; c. 119 from s. “1” to s. “0”; c. 132 from s. “1” to s. “0”; c. 136 from s. “0” to s. “1”; c. 160 from s. “0” to s. “?”; c. 161 from s. “0” to s. “?”; c. 187 from s. “0” to s. “0&1.” The present matrix of 272 characters has 112 terminal taxa (see the Supplemental files). During the analysis, we inactivated unpublished taxa (not ready for re-examination) and poorly preserved species (e.g., Willungacetus). Thus, 106 taxa were treated under the parsimony analysis using T.N.T. v.1.5 software (Goloboff and Catalano, Reference Goloboff and Catalano2016). Characters were analyzed under equal and implied weights (under the standard value k = 3 and the calculated k = 28.18360 using the script “setk”; Goloboff, Reference Goloboff1993; Goloboff et al., Reference Goloboff, Carpenter, Arias and Miranda-Esquivel2008, Reference Goloboff, Torres and Arias2018), ordered and using a backbone constraint tree—force / (5 ((16 51) (33 (48 ((19 21) ((26 73) (24 (22 25)))))))). Settings include 10,000 random stepwise-addition replicates per tree, bisection reconnection (TBR) branch swapping with 10 trees per replicate and enforce constraints. The multiple most parsimonious trees were summarized under strict consensus. Support values were obtained by symmetric resampling based on 2,000 replicates and reported as GC frequency differences (Goloboff et al., Reference Goloboff, Farris, Källersjö, Oxelman and Szumik2003). Under equal weights, a second analysis was run due to overflow replications using saved trees in the memory from the first result. Only the consensus tree resulting from the implied weights (k = 28.18360) is explained regarding the specimen MRAHBCS Pal/V119.
Repository and institutional abbreviation
MRAHBCS, Museo Regional de Antropología e Historia de Baja California Sur—Instituto Nacional de Antropología e Historia.
Systematic paleontology
Cetacea Brisson, Reference Brisson1762
Mysticeti Gray, Reference Gray, Richardson and Gray1864
Eomysticetidae Sanders and Barnes, Reference Sanders and Barnes2002b
Echericetus new genus
Type species
Echericetus novellus n. sp., by monotypy.
Diagnosis
As the monotypic species.
Occurrence
Oligocene (slightly older than 27.95 Ma, latest Rupelian), North Pacific.
Etymology
Echeri means land, earth, or ground in Purepecha, a local language in Mexico; cetus indicates large sea creature in Greek.
Remarks
Echericetus represents a new genus of the Eomysticetidae in the North Pacific. The other definite eomysticetid genus is Yamatocetus from the Oligocene of Japan, on the other side of the North Pacific. Interestingly, a recent phylogenetic analysis (Boessenecker et al., Reference Boessenecker, Beatty and Geisler2023) recognized the eomysticetid affinity of Sitsqwayk and Maiabalaena, and if this taxonomic interpretation proves to be correct, the eomysticetid diversity in the North Pacific is much higher than previously known. In addition, Hernández-Cisneros and Nava-Sanchez (Reference Hernández-Cisneros and Nava-Sánchez2022) identified the existence of Eomysticetus from the Oligocene of Mexico, indicating the occurrences in both the Pacific and Atlantic oceans of this eomysticetid genus.
Echericetus novellus new species
Figures 2–16
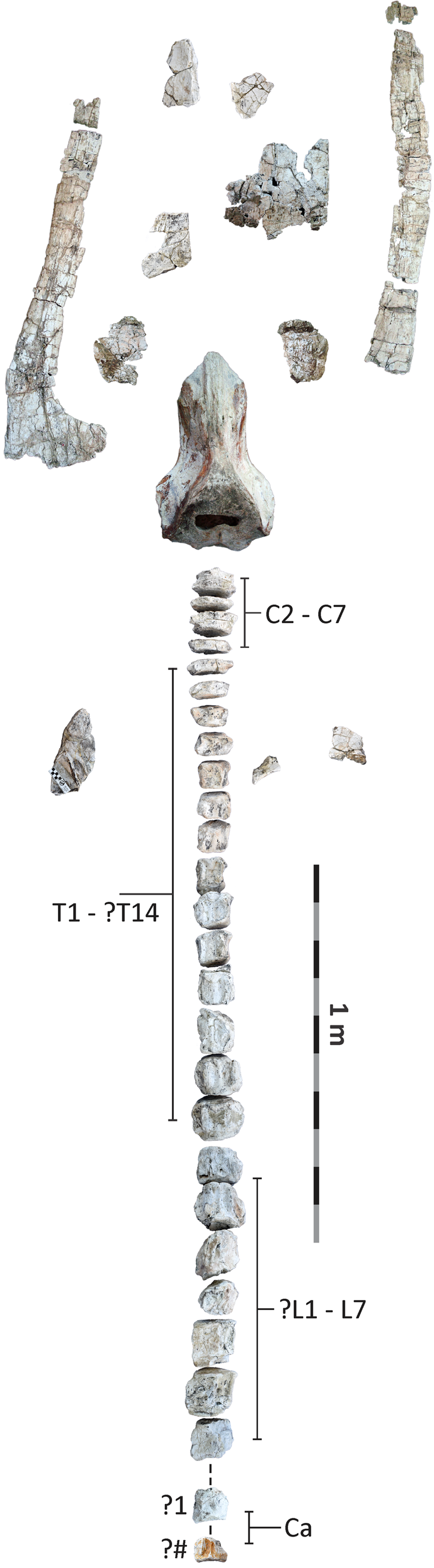
Figure 2. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS Pal/V119; cervical vertebrae (C2–C7), thoracic vertebrae (T1–?T14), lumbar vertebrae (?L1–L7), and caudal vertebrae (Ca).
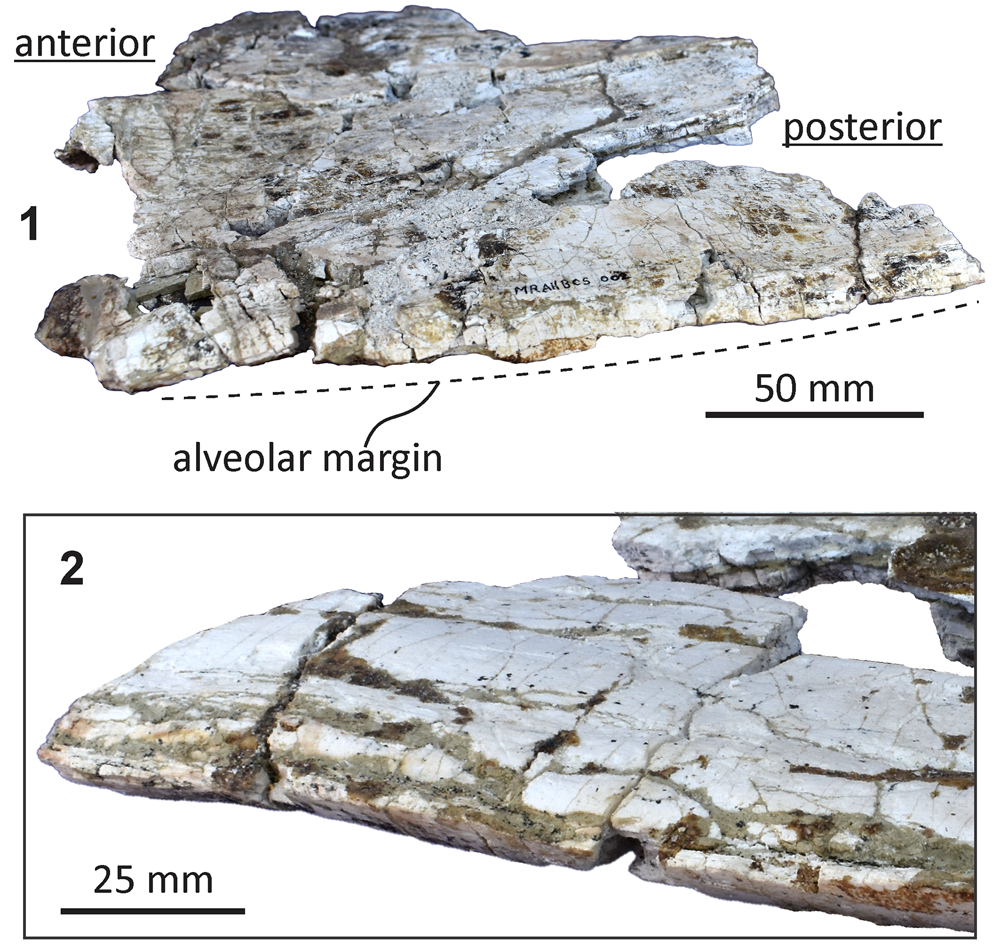
Figure 3. Echericetus novellus, holotype, MRAHBCS PAL/V118. (1, 2) Fragment of the right maxilla: (1) dorsal view; (2) ventral view.
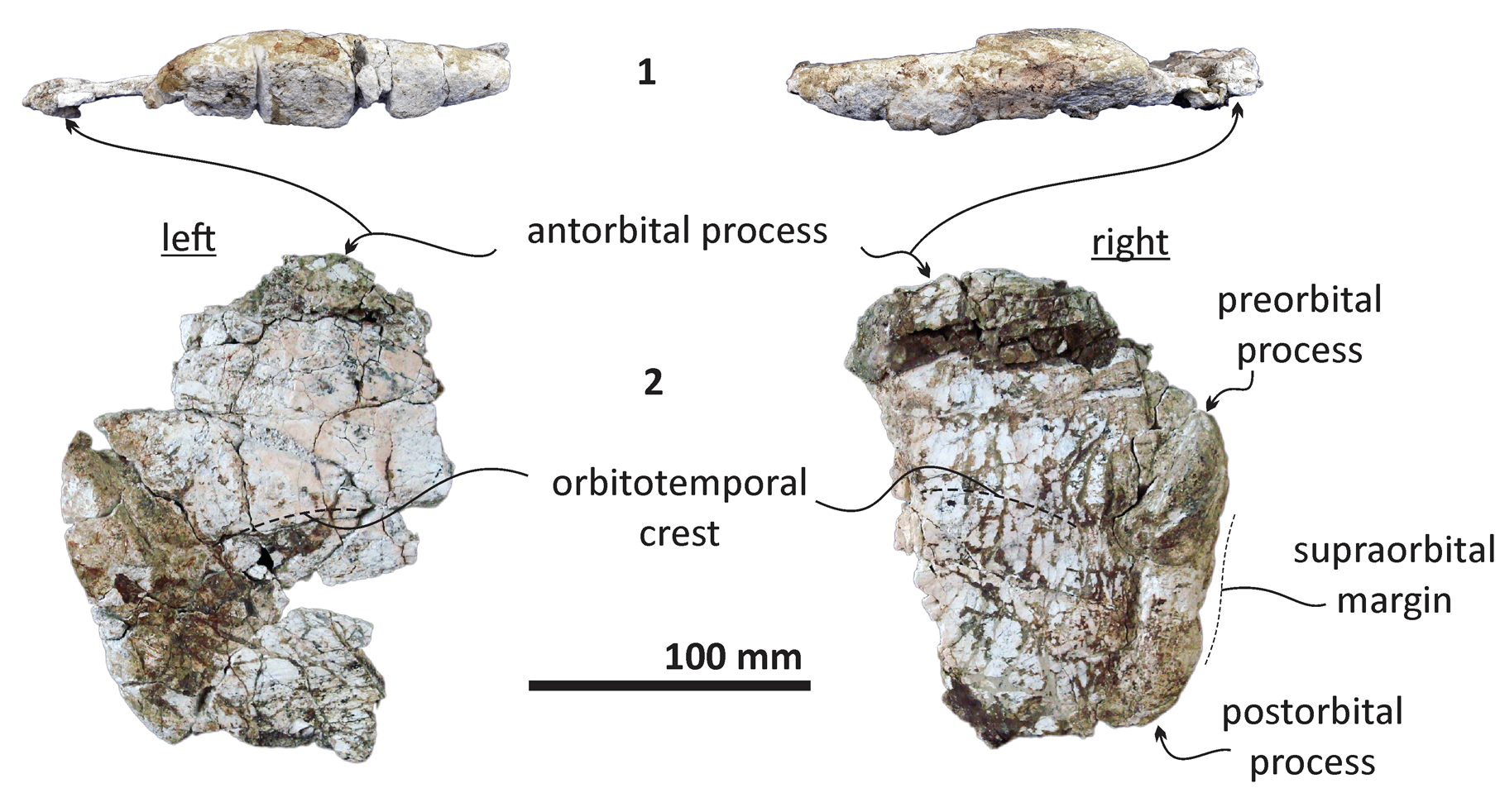
Figure 4. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS Pal/V119. (1, 2) Supraorbital processes: (1) lateral view; (2) dorsal view.
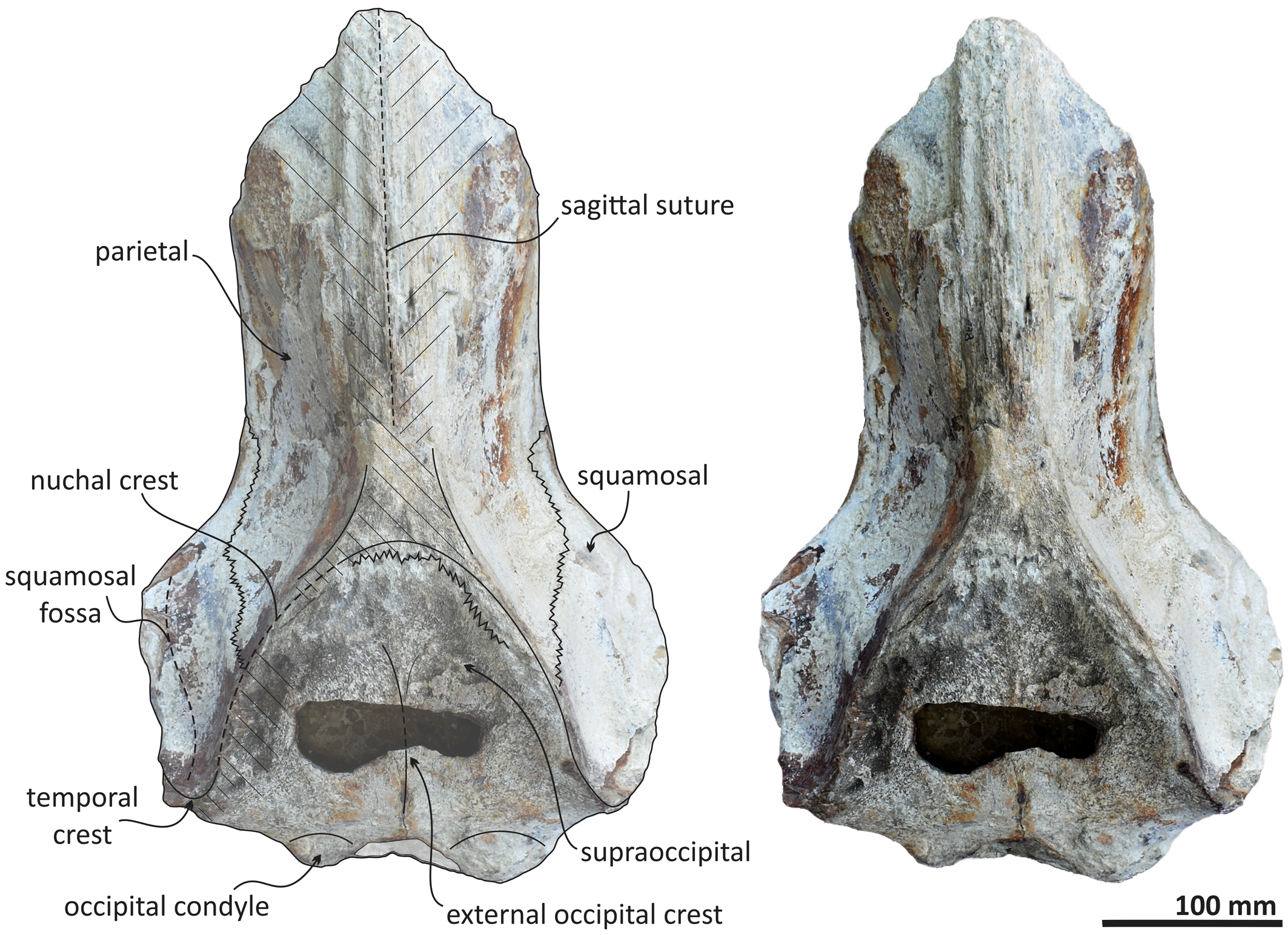
Figure 5. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS Pal/V119, cranium, dorsal view. Crosslines denote broken or eroded surface.
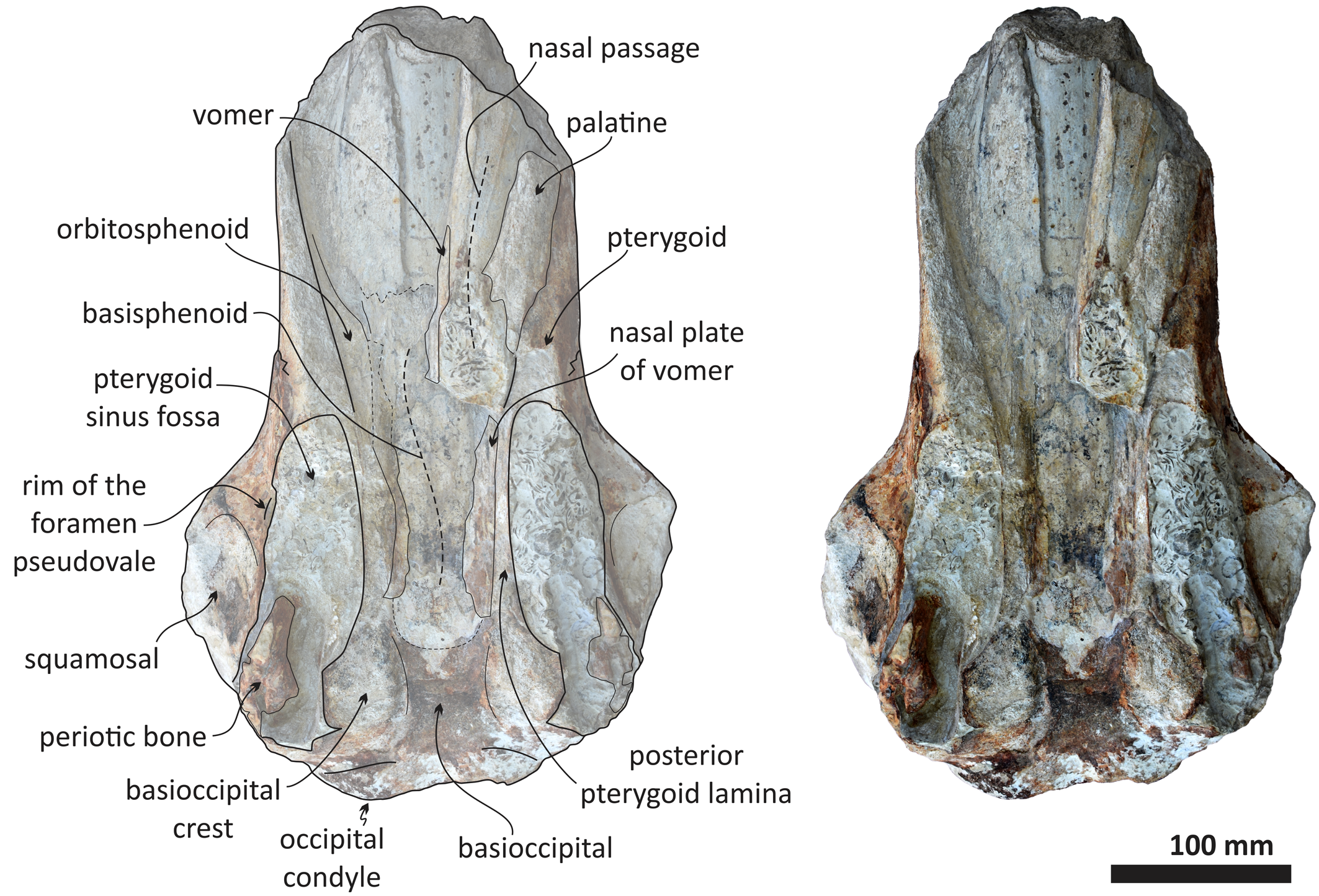
Figure 6. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS Pal/V119, cranium, ventral view.
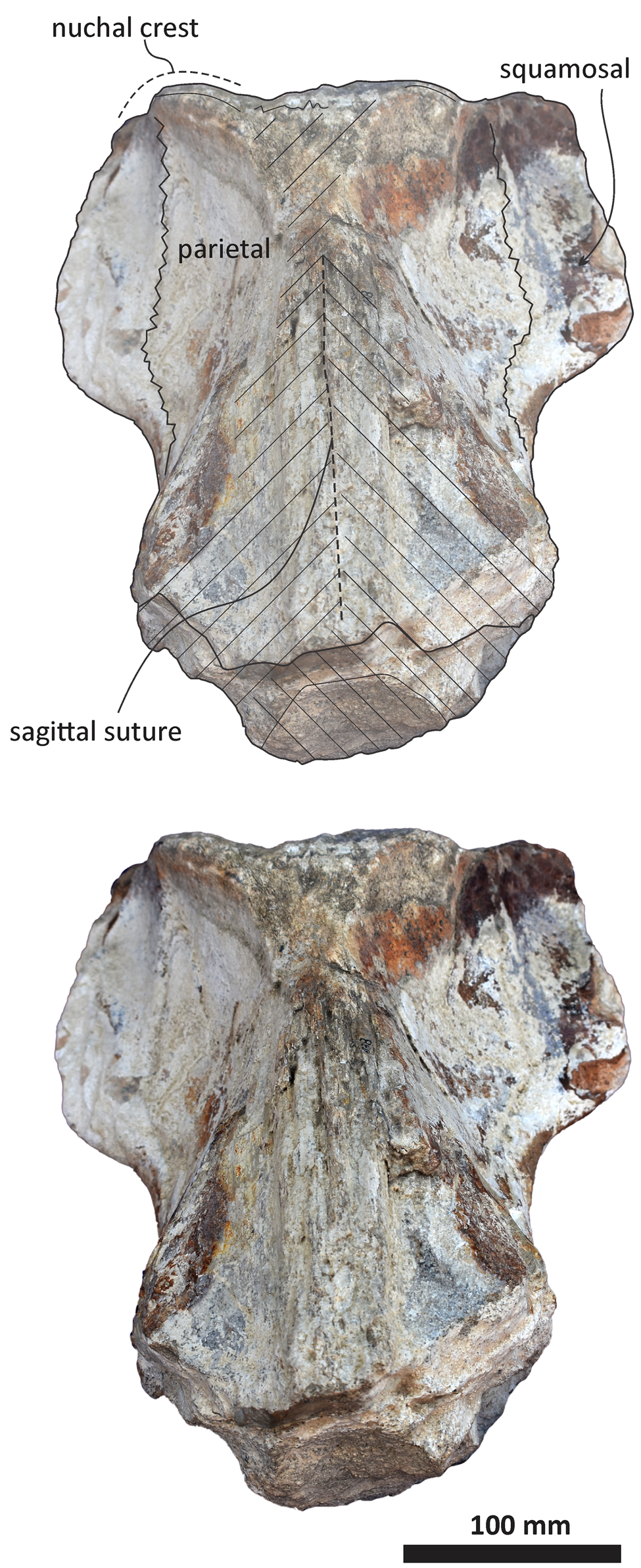
Figure 7. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS PAL/V118, cranium, anterior view. Crosslines denote broken or eroded surface.
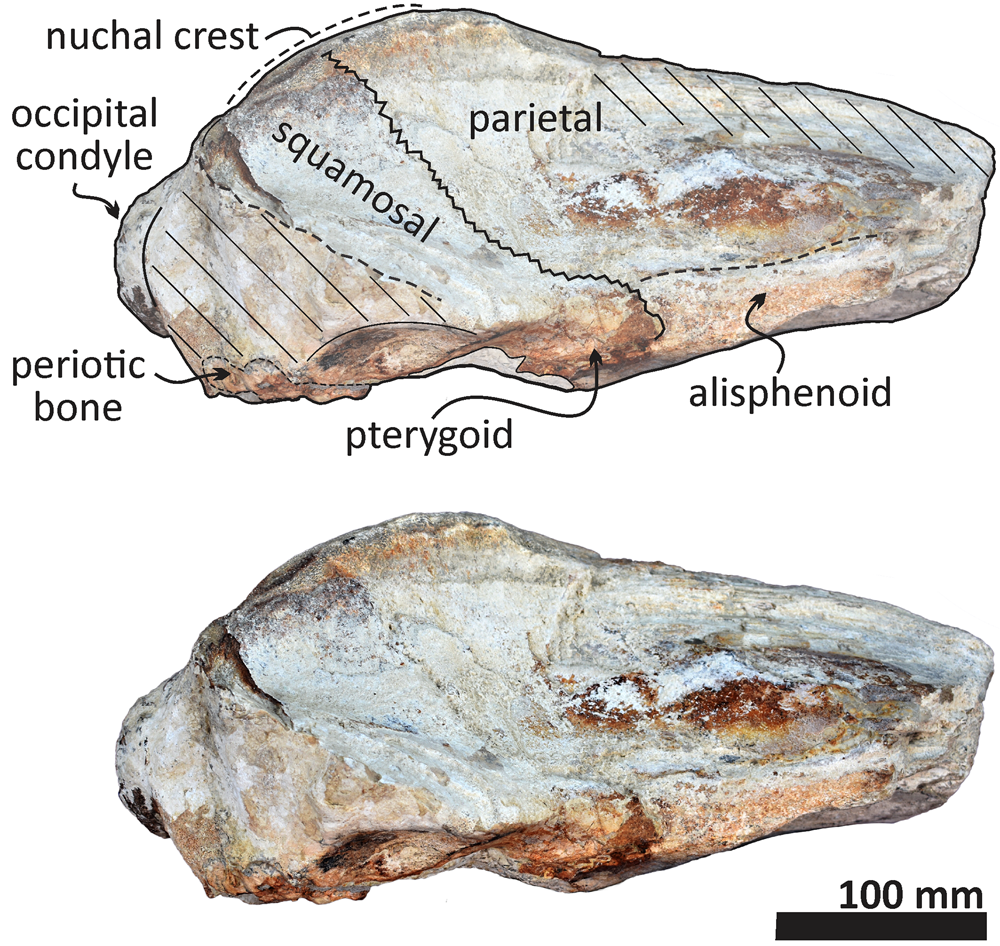
Figure 8. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS PAL/V118, cranium, lateral view. Crosslines denote broken or eroded surface.
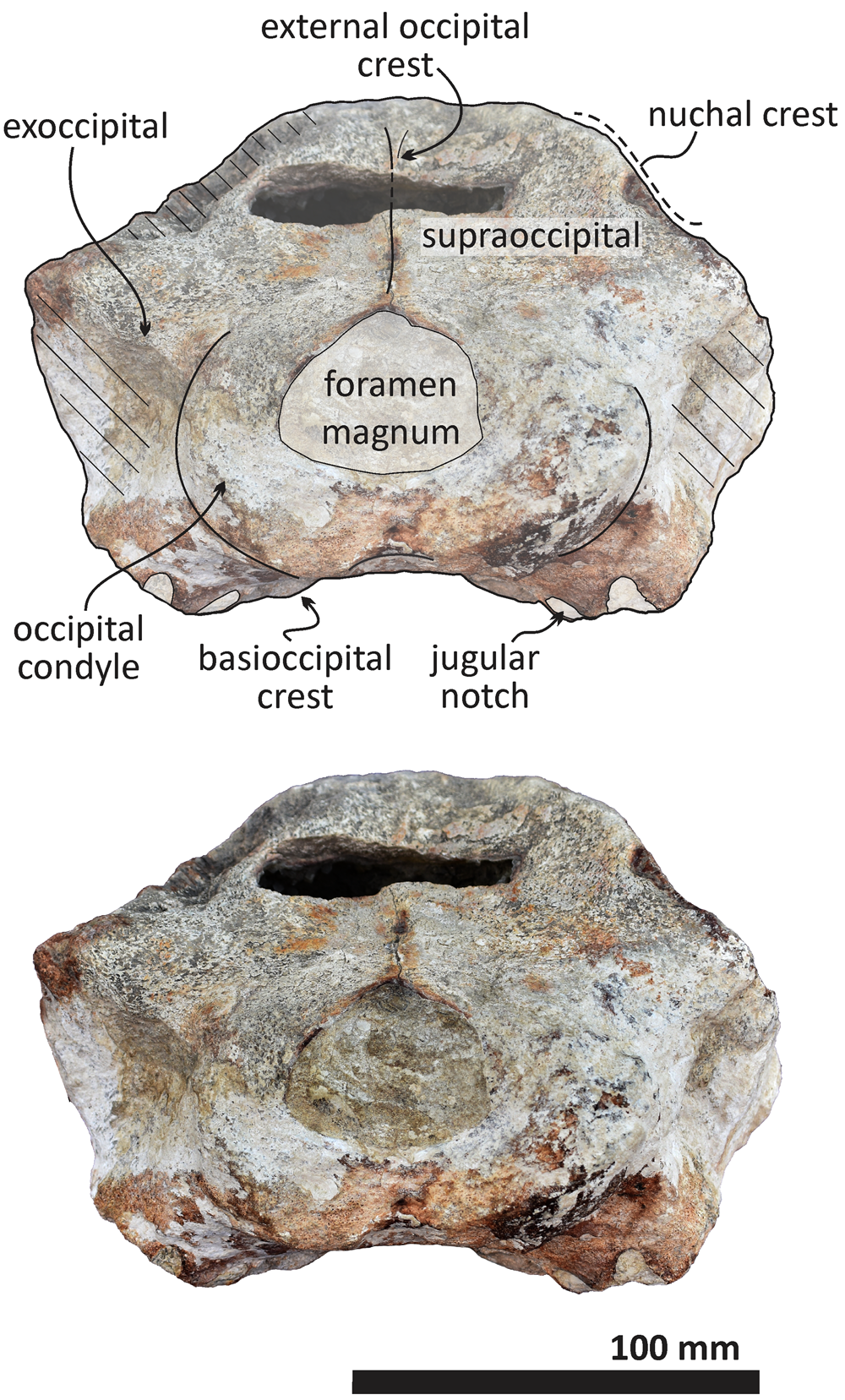
Figure 9. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS PAL/V118, cranium, posterior view. Crosslines denote broken or eroded surface.
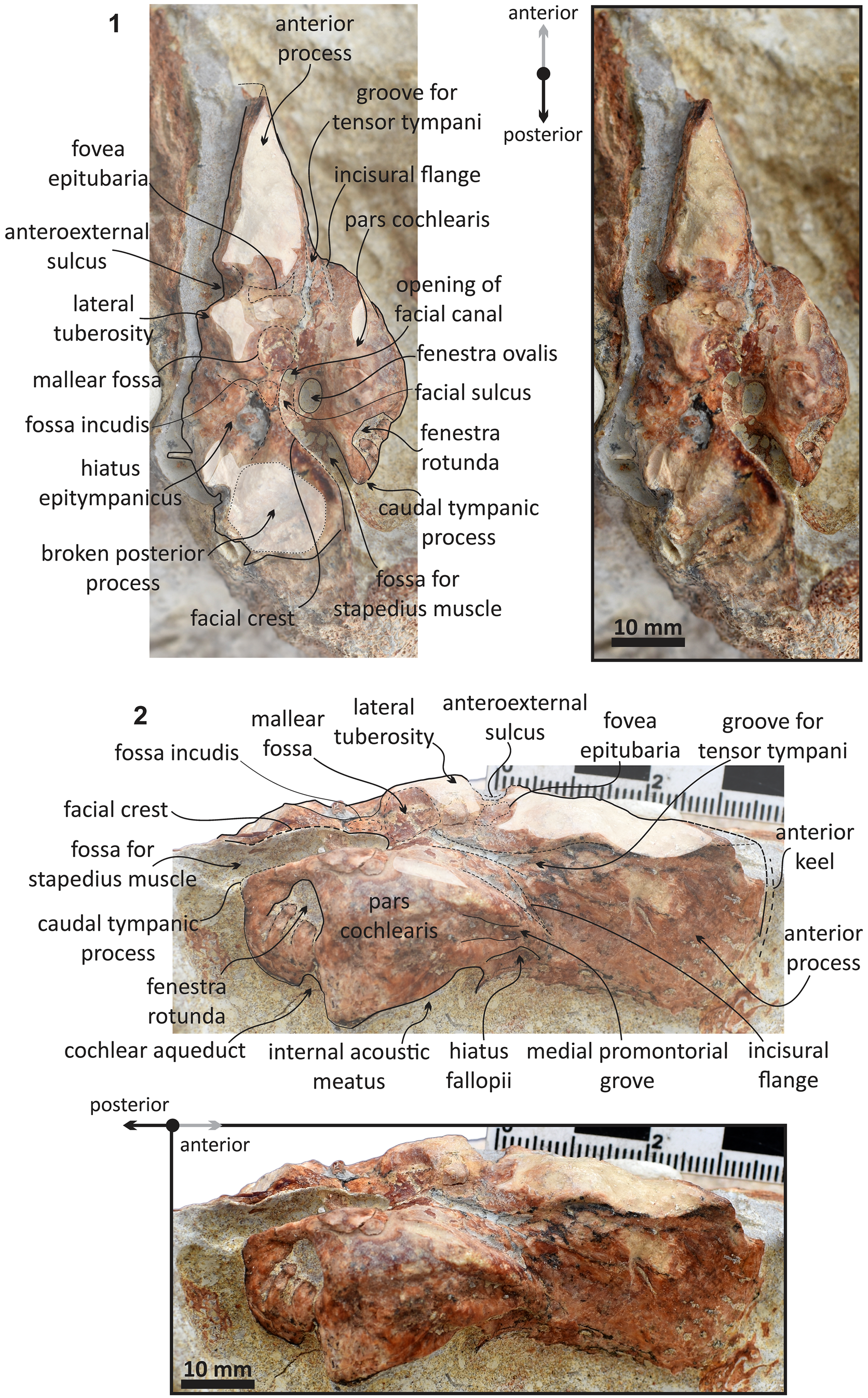
Figure 10. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS Pal/V119. (1, 2) Right periotic: (1) ventral view; (2) medial view.
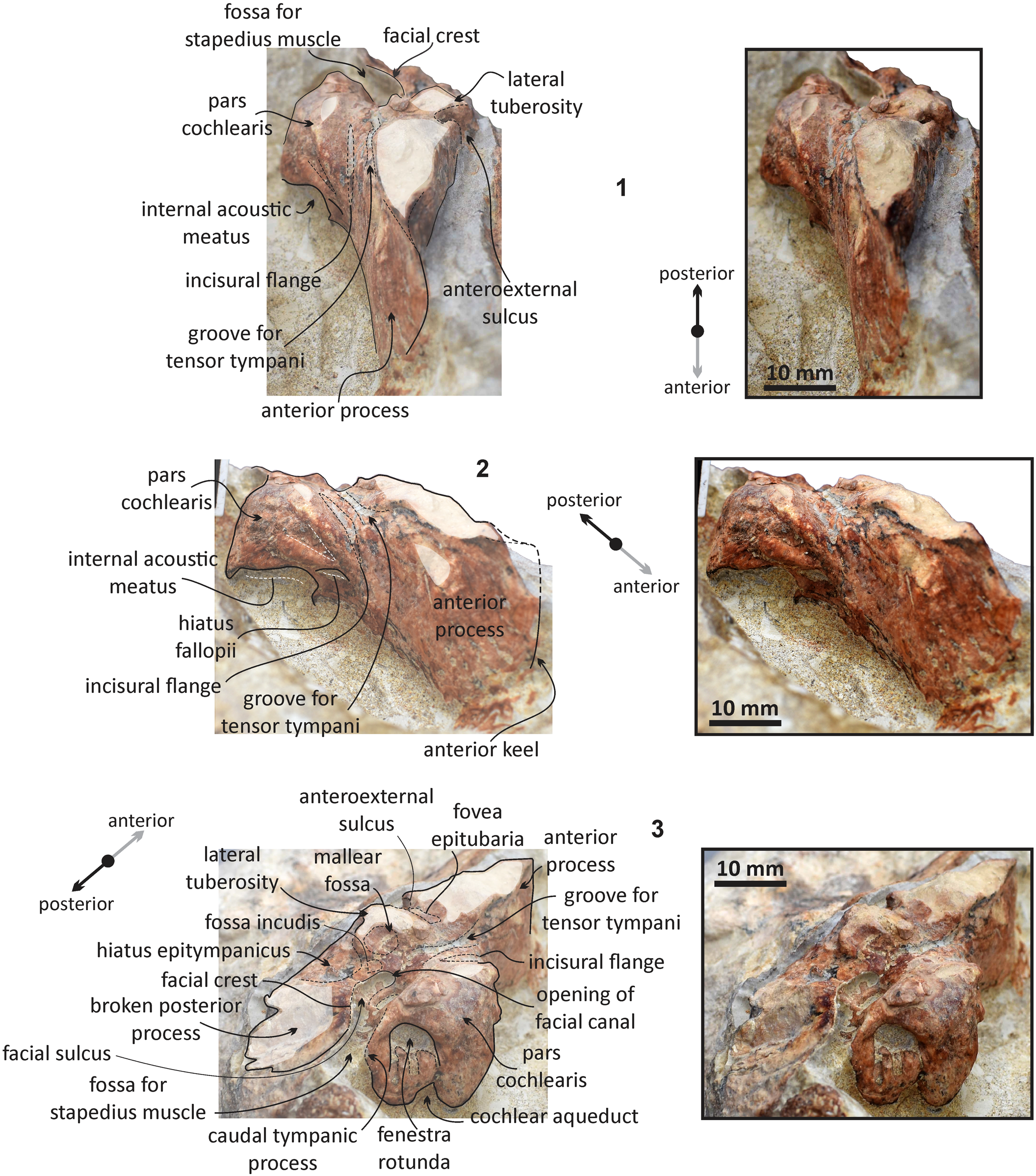
Figure 11. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS Pal/V119. (1–3) Right periotic: (1) anterior view; (2) anteromedial view; (3) posteromedial view.
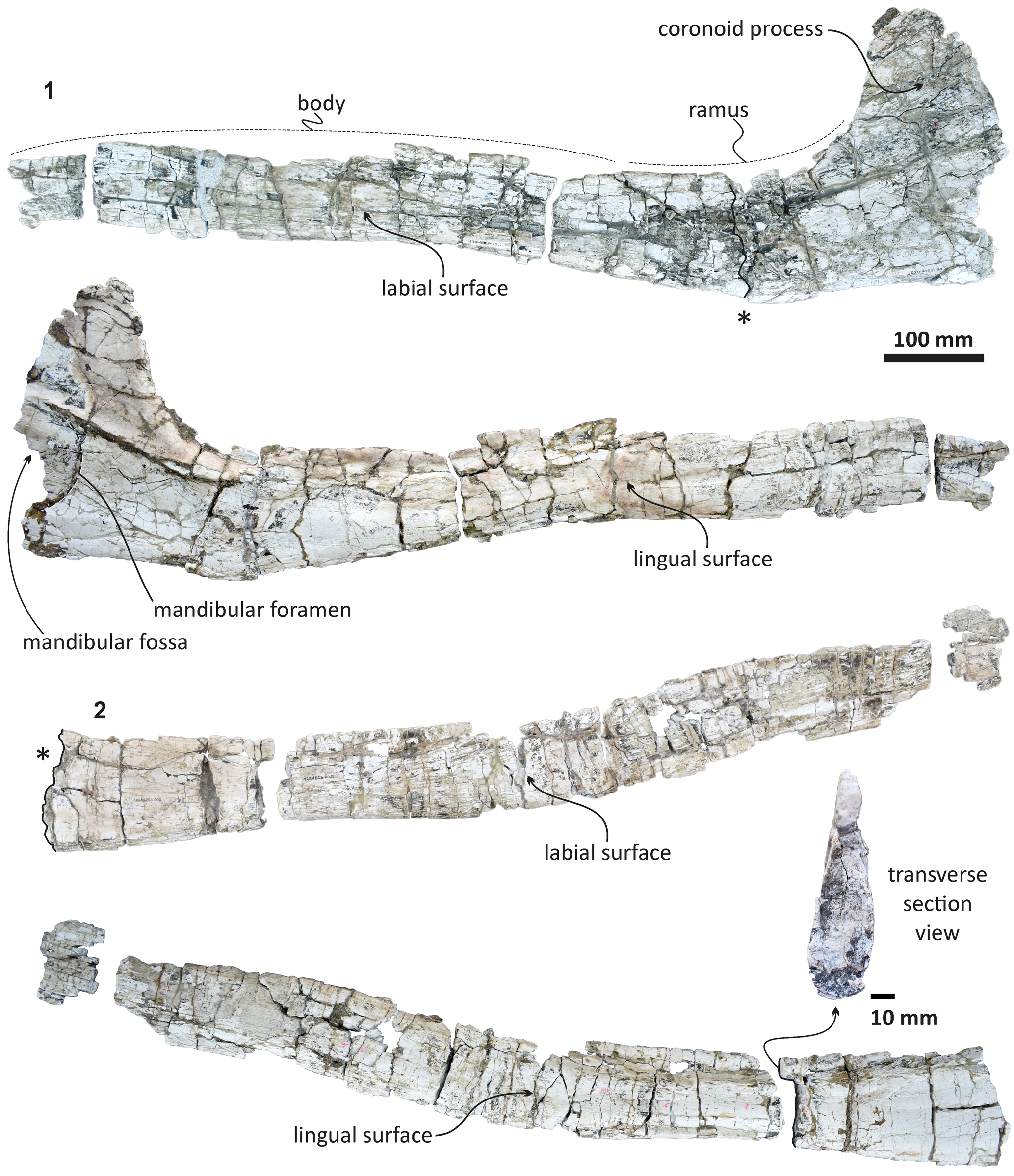
Figure 12. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS Pal/V119. (1) Left mandible. (2) Right mandible. The asterisks mark the equivalent section level at the right mandible.
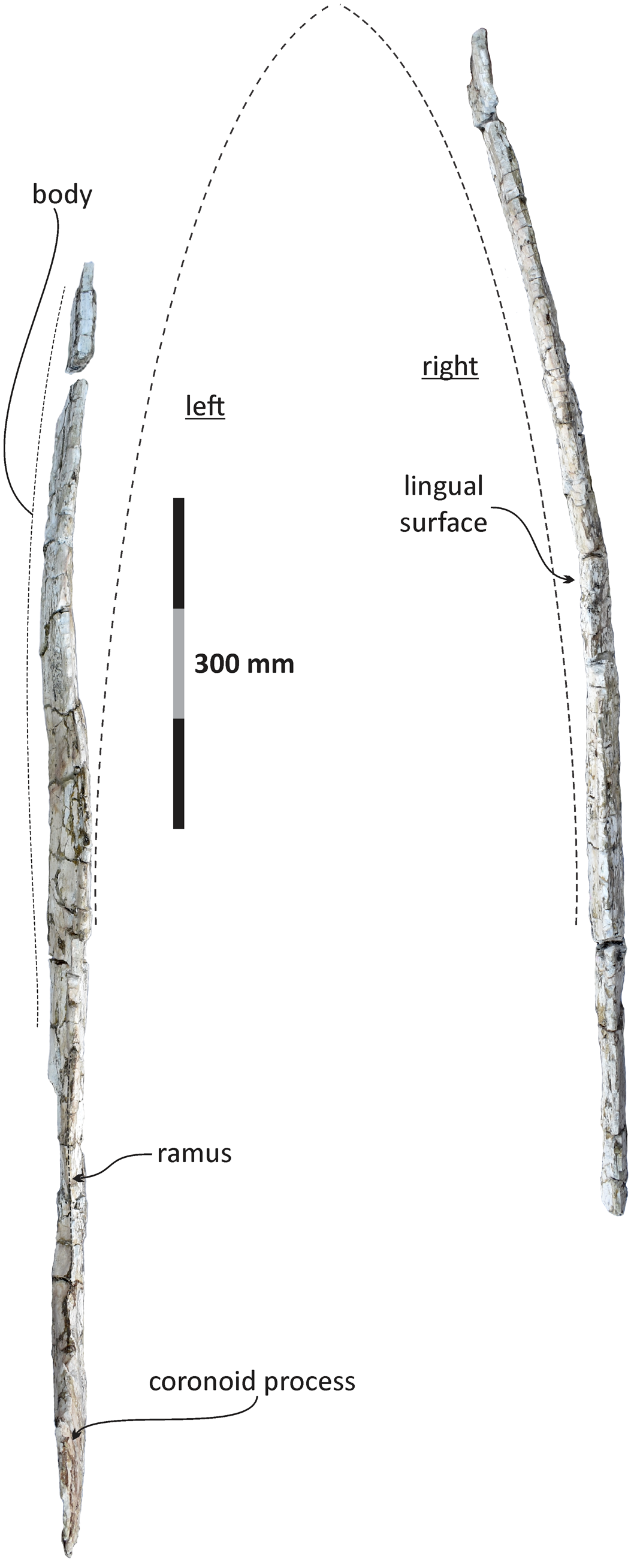
Figure 13. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS PAL/V118, mandibles, dorsal view. The dashed line represents the reconstructed outline.
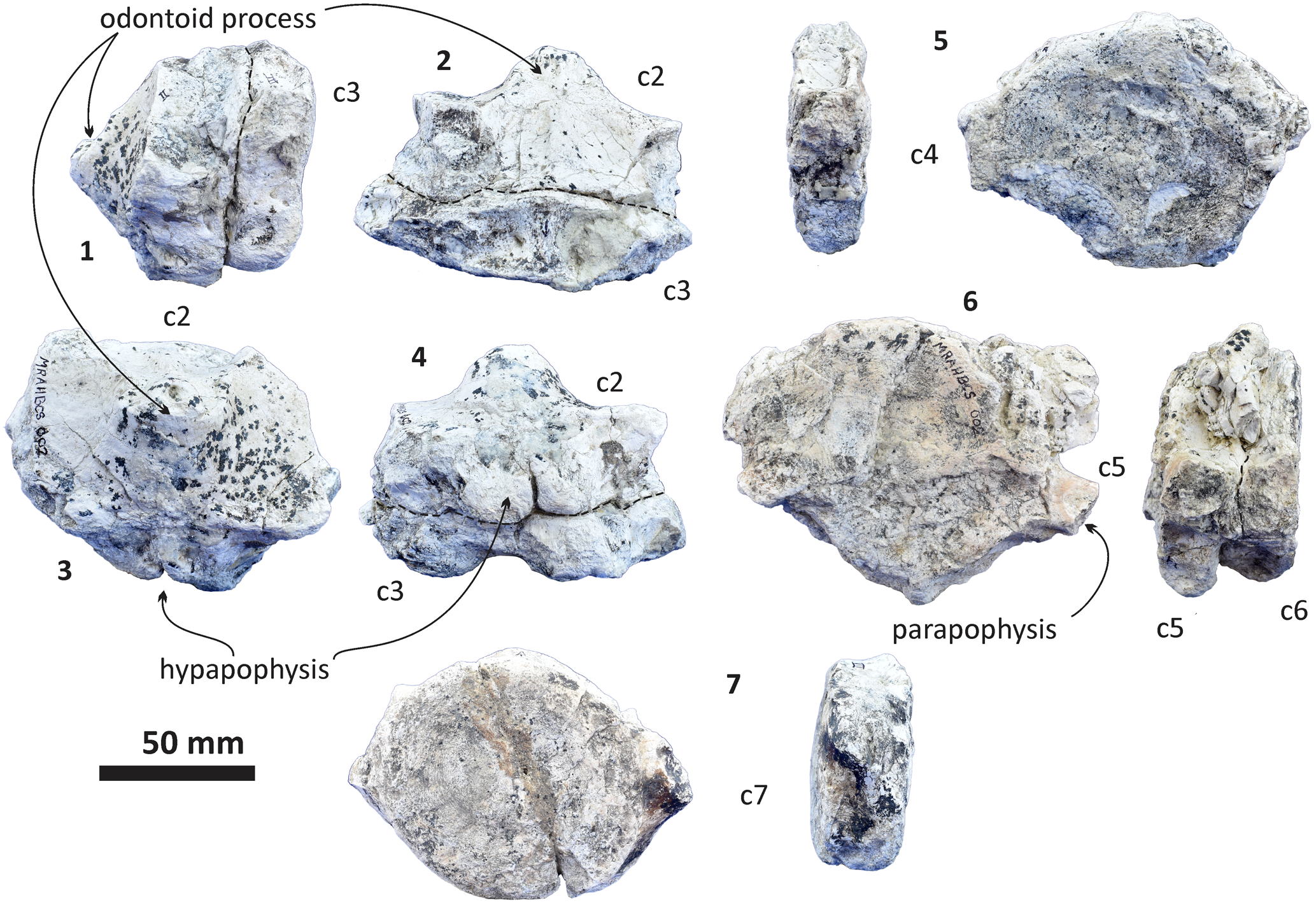
Figure 14. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS Pal/V119, cervical vertebrae. (1–4) Axis (fused with the c3): (1) lateral view; (2) dorsal view; (3) anterior view; (4) ventral view. (5) Lateral and anterior views of the fourth cervical vertebra. (6) Fused cervical vertebra (c5–6), anterior and lateral views. (7) Anterior and lateral views of the seventh cervical vertebrae.

Figure 15. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS Pal/V119. (1, 2) Thoracic, lumbar, and caudal vertebrae series: (1) lateral views; (2) anterior views.
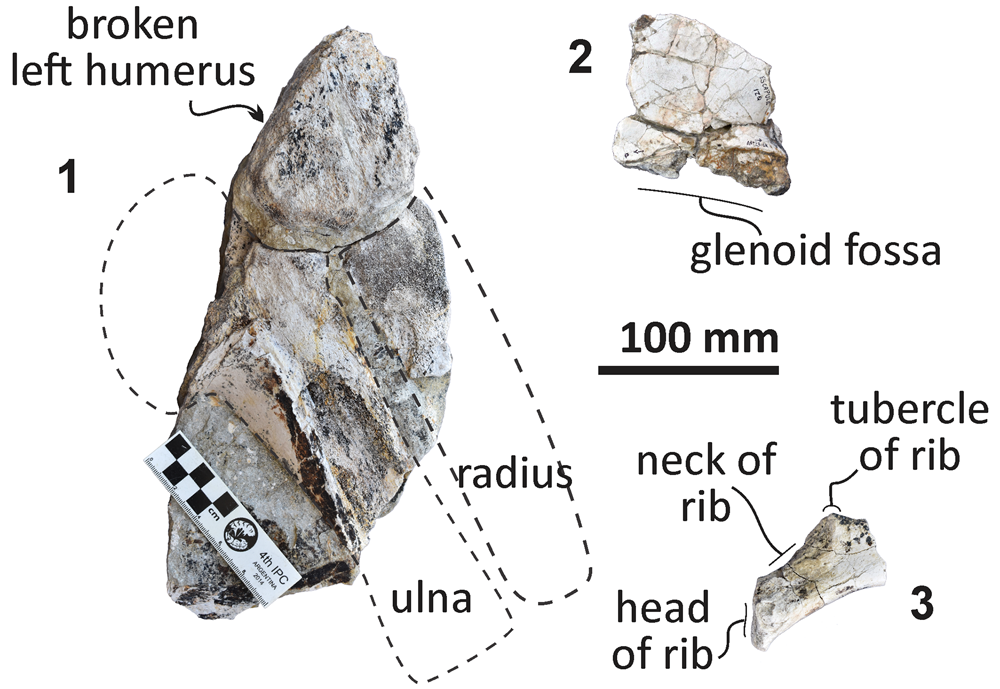
Figure 16. Echericetus novellus n. gen. n. sp., holotype, MRAHBCS PAL/V118. (1) Left humerus, ulna, and radius in medial view. (2) Fragment of right scapula, lateral view. (3) Fragment of the right fifth or six rib.
Holotype
MRAHBCS Pal/V119 includes a partial skull (fragmentary rostrum, cranium with periotics, mandibles, and several bone fragments) and postcranial elements (31 vertebrae, ribs, and forelimb). It is curated in the paleontological collection of the MRAHBCS.
Diagnosis
Echericetus novellus n. gen. n. sp. displays the combination of eomysticetid diagnostic features (Sanders and Barnes, Reference Sanders and Barnes2002a, Reference Sanders and Barnesb; Boessenecker and Fordyce, Reference Boessenecker and Fordyce2015a): the elongate intertemporal region; large and oval temporal fossa; occipital shield relatively posterior to the postorbital process of frontal; broad and high coronoid process; transversely compressed blade-like anterior process of the periotic. Further, Echericetus novellus differs from known eomysticetids in having dorsoventrally thickened orbital rim with rounded surface; reduced and inconspicuous postorbital process; preorbital process thicker than the postorbital process; wide and long intertemporal region; supraoccipital with anteroposteriorly convex surface; elongated and elliptical pterygoid sinus fossa; squared-like basioccipital crest; rectangular anterior process of the periotic in medial view; S-shaped mandible in lateral view and the axis vertebra with bilobed hypapophysis. Echericetus novellus also differs from Sitsqwayk cornishorum Peredo and Uhen, Reference Peredo and Uhen2016 and Maiabalaena nesbittae Peredo et al., Reference Peredo, Pyenson, Marshall and Uhen2018 in lack of a prominent postorbital process, relatively thin orbital rim, concave dorsal surface of the supraoccipital, and straight nuchal crest; it differs from Mauicetus parki (Benham, Reference Benham1937), Horopeta umarere Tsai and Fordyce, Reference Tsai and Fordyce2015b, Whakakai waipata Tsai and Fordyce, Reference Tsai and Fordyce2016, Tlaxcallicetus, and Toipahautea waitaki Tsai and Fordyce, Reference Tsai and Fordyce2018 in the lack of a massive body of the periotic; transversally thick or very thin, dorsally prominent anterior process. Echericetus novellus differs from the crown Mysticeti (Balaenidae, Balaenopteridae, Cetotheriidae, Eschrichtiidae) in having archaic features, such as a poorly telescoped skull, prominent plate-like coronoid process, the presence of the fovea epitubaria, and the acoustic meatus as a single aperture.
Occurrence
Marine marginal facies, San Juan Member, El Cien Formation, ~20 km southeast of La Paz, Baja California Sur, Mexico, (NAD 27 23.973618°N, 110.228921°W), 1.2 km southwest of Sierra El Novillo, 500 m northwest of Rancho La Palma, and 500 m northeast of Cerro El Divisadero. Based on the lithology and the U–Pb zircon dating from the horizon slightly above MRAHBCS Pal/V119, the geological age for Echericetus novellus n. gen. n. sp. (bed 2; Fig. 1) is slightly older than 27.95 Ma (latest Rupelian).
Description
Overall, the bioerosion of MRAHBCS Pal/V119 is minor, having relatively good preservation; some vertebrae show a degree of pre-burial degradation. The rostrum, mandibles, supraorbital processes of the frontal, and vertebrae display considerable distortion by the diagenetic processes. The bony fractures and loss of bones may be from weathering after exposure. The skull contains invertebrate shells (bivalves) in the pterygoid fossa and gypsum crystals inside the braincase cavity, which formed likely to the current desert climate.
Ontogenetic age and body size.—Echericetus novellus n. gen. n. sp. includes both mature and immature physical features (Walsh and Berta, Reference Walsh and Berta2011; Boessenecker and Fordyce, Reference Boessenecker and Fordyce2015a): open skull sutures that are not tightly fused (immature), well-fused vertebral epiphyses (mature), and closed occipital synchondroses (mature). Thus, the holotype MRAHBCS Pal/V119 likely represents a subadult. Judging from the preserved skull and mandibles, the total body length of Echericetus novellus may be comparable to Tokarahia, around 5 to 6 m long.
Skull.—Echericetus novellus n. gen. n. sp. preserves only fragments of the maxillae (Fig. 2), showing a flattened maxilla without alveoli (at least on the lateral borders posterior to the rostrum tip); no baleen is preserved. The frontal fragments indicate an exposed skull vertex, and the supraorbital processes have been preserved (Figs. 4, 5); the frontoparietal suture is not visible. The antorbital process is over the anterior end of the supraorbital process. The orbit is shorter than <25% of the bizygomatic width, and the orbital rim is dorsoventrally thick (26.4–31 mm, maximum) with a rounded lateral surface. The preorbital region is dorsoventrally flat; the postorbital process is not prominent, with a rounded tip oriented posteriorly. The postorbital process is dorsoventrally thinner (11.7 mm) than the preorbital process (16.5 mm). Above the supraorbital process, the orbitotemporal crest is indistinct with a swollen surface, which is oriented subparallel to the posterior border and indicates the limit of the insertion area for the temporalis muscle.
The anterior and lateral profiles of the cranium indicate an ascending angle >10° toward the posterior edge of the skull, showing the broken frontal at a level above the lateral border of the rostrum. Dorsally, the temporal fossa and the intertemporal region are anteroposteriorly longer than the transverse width. The parietals are posterior to the postorbital process, and part of the dorsal surface in the parietals has been lost (Figs. 5, 7, 8). The sagittal suture anterior to the supraoccipital apex is present. In lateral view, parietals are anteroposteriorly longer than high and are in contact with the supraoccipital, resulting in the partial nuchal crest. Ventrally, the parietal contacts and fuses with the alisphenoid and orbitosphenoid. The suture of the parietal–squamosal joint is smooth and orients obliquely without tubercles or foramen.
As in other stem mysticetes, the apex of the supraoccipital is located posterior to the postorbital process and directly posterior to the exposed parietals. In Echericetus novellus n. gen. n. sp., the anteriormost point of the supraoccipital is within the temporal fossa and likely posterior to the zygomatic process tip. The preserved supraoccipital indicates a broadly rounded anterior margin. The nuchal crest is convex in dorsal view. The supraoccipital outline is triangular in dorsal view. The posterior apex of the nuchal crest is anterior to the occipital condyles and aligned with the medial half of the temporal fossa. The nuchal crest seems not to overhang the squamosal fossa. Dorsally, the supraoccipital lacks paired tubercles, and both lateral margins of the nuchal crest in dorsal view are slightly convex. The external occipital crest is present mainly over the posterior half of the supraoccipital and ends slightly anterior to the foramen magnum. In posterior view, the exoccipital is partially preserved and well fused with the supraoccipital. The condyles are present but eroded; the foramen magnum is circular. Ventrally, the exoccipital contributes to the narrow jugular notch and is fused with the basioccipital. In ventral view, the basioccipital and basisphenoid (basioccipital/basisphenoid synchondrosis) are well fused (Fig. 6), and the incomplete nasal plate of the vomer overlies the basisphenoid and contacts the basioccipital. The basioccipital crests are broad, bulbous (slightly eroded), and subparallel to the sagittal plane, with a square-like outline and a straight lateral border.
The preserved portion of the squamosal is lateral to the parietal and supraoccipital. The dorsomedial surface of the squamosal is slightly convex and smooth without elevation, and the more lateral portion indicates a relatively narrow squamosal fossa. Ventrally, the medial portion of the incomplete squamosal belongs to the lateral part of the pterygoid sinus fossa and includes the ventral opening of the foramen ovale. The pterygoid is exposed ventrally and is not well preserved. The pterygoid, squamosal, and basioccipital mark the outline of a long and elliptic pterygoid sinus fossa anterior to the foramen ovale. Most of the sphenoid bones are poorly preserved. The alisphenoid is broadly exposed laterally and contacts anteriorly with the eroded orbitosphenoid and dorsally with the parietal; the exposed and remaining alisphenoid is anteroposteriorly elongated and rectangular in lateral view. The basisphenoid is visible in the palatine region and well fused posteriorly with the basioccipital. Fragments of the vomer (including nasal plates and crest) cover part of the basisphenoid.
Periotic.—MRAHBCS Pal/V119 preserves two periotics, but only the right one was prepared to show the morphological features (Figs. 11, 12). The anterior, medial, and posterior views of the periotic are observable. Still, the dorsal surface with the suprameatal area, superior process, and vestibular and cochlear aqueducts are not visible (covered by the matrix). In medial view, the anterior process is rectangular and separated from the pars cochlearis; the incisural flange is present. The keel is mainly vertical, the anterodorsal angle is not exposed, and the anteroventral angle shows a blunt tip at the level of the ventral edge of the pars cochlearis. In ventral view, the anterior process is compressed transversely with a blade-like shape and lacks secondary tuberosity, and the anterior bullar facet forms an elongated triangle on the ventral surface of the anterior process. The fovea epitubaria is an eroded small area, indicating an accessory ossicle for the contact between the anterior process and bulla. The anterior process length is smaller than the anteroposterior length of the pars cochlearis. The lateral side remains embedded, but the anteroexternal sulcus is present and observable from the ventral rim of the artery channel. In medial view, a minor excavation above the fovea epitubaria marks the surface for the tensor tympani.
The pars cochlearis lacks a cranial elongation and shows a rounded surface at the anteromedial corner with a short ridge below the shallow promontorial groove in ventral view. Anterior and separated from the internal auditory meatus, a small fissure marks the opening of the hiatus fallopii. The posterior region of the pars cochlearis displays a small caudal tympanic process, which is triangular and well separated from the facial crest (crista parotica). In the rounded posteromedial corner, the fenestra rotunda is a circular opening with three small furrows in the dorsal edge separated from the rounded cochlear aqueduct. In ventral view, the small and round lateral tuberosity is anterolateral to the well-excavated mallear fossa; the body of the periotic is not prominent, and the area of the hiatus epitympanicus can be observed posterior to the mallear fossa. The fossa incudis is indistinct. The facial crest forms the lateral border of the opening of the facial canal, facial sulcus, and the fossa for the stapedius muscle. The medial surface close to the fenestra ovalis is not prominent. The posterior process is broken and oriented posterolaterally. The texture of the posterior process is dense and lacks a neck.
Mandible.—The mandibles are partially preserved; the anterior tip, the articular surface, and the angular process are missing. The diagenetic processes likely transformed the mandibular morphology slightly; we prudently interpreted the morphology and reconstructed the outline in dorsal view (Figs. 12, 13). The mandible is not straight in lateral view, distinct to Eomysticetus, Yamatocetus, Tokarahia, and Waharoa. The overall mandibular shape resembles that of Sitsqwayk cornishorum. Both mandibles are transversely compressed, and the left mandible displays more alteration, including a completely flat coronoid process and a distorted mandibular body. The preserved dorsal surface of the mandibles and the preserved maxilla indicate that Echericetus novellus n. gen. n. sp. lacks both functional teeth and alveolar grooves. Despite the absence of the mandibular tip, we suggest that Echericetus novellus has an unfused mandibular symphysis. The mandible preserves a flattened medial surface in the central part relative to the lateral surface. The ramus shows a flat dorsomedial surface, and the mandibular body of the right mandible displays a central constriction, like in aetiocetids, Sitsqwayk, and Maiabalaena. In dorsal view, the right mandible is bowed laterally, but mainly at the anterior portion. The medial wall of the broad mandibular foramen is broken, but the preserved mandibular foramen shows a rounded opening and at the base of the coronoid process. The medial projection on the dorsal margin and the satellite process are absent. The mandibular fossa is incomplete. The coronoid process is a transversely thin plate and lacks a posteromedial ridge and postcoronoid elevation. The insertion area for the mylohyoid muscle is absent.
Vertebrae.—MRAHBCS Pal/V119 includes 31 partially preserved vertebrae (Figs. 14, 15). The identification and probable arrangement of the vertebrae are based on the criteria and comments by Watson and Fordyce (Reference Watson and Fordyce1993; Balaenoptera acutorostrata), Okazaki (Reference Okazaki2012; Yamatocetus canaliculatus), Martínez-Cáceres et al. (Reference Martínez-Cáceres, Lambert and de Muizon2017; Cynthiacetus peruvianus), and de Muizon et al. (Reference de Muizon, Bianucci, Martínez-Cáceres and Lambert2019; Mystacodon selenensis). In general, the body of the vertebrae (anterior epiphysis, centrum, and posterior epiphysis) is relatively complete, although the diagenesis and erosion are evident on the MRAHBCS Pal/V119 vertebrae set. Only six of seven cervical vertebrae are preserved (from the axis to the seventh cervical vertebrae). The cervical vertebrae are unfused and display a rounded body. Still, some seem fused due to the diagenetic process. The axis has the odontoid process and a bi-lobed hypapophysis. Note that the atlas and axis have an exceptionally distinct morphology (Martínez-Cáceres et al., Reference Martínez-Cáceres, Lambert and de Muizon2017). The other cervical vertebrae display a relatively anteroposteriorly flat body, and the characteristic parapophysis is absent in the thoracic, lumbar, and caudal vertebrae (Watson and Fordyce, Reference Watson and Fordyce1993; Martínez-Cáceres et al., Reference Martínez-Cáceres, Lambert and de Muizon2017). In Echericetus novellus n. gen. n. sp., many features are lost in the cervical vertebrae due to erosion and diagenetic alterations. However, the vertebrae order was based on the preserved trace of the parapophysis and the vertebral body shape comparable to the Yamatocetus canaliculatus vertebrae set (Okazaki, Reference Okazaki2012). Similar criteria were followed to order the thoracic vertebrae despite the poor preservation. Thus, the traces of the pedicle position, the presence of the fovea for the capitulum of the rib, the absence of the transverse process on the lateral wall of the vertebrae body in the first twelve thoracic vertebrae, and the thickness of the transverse process in the thoracic vertebrae 13 and ?14 (different from the flat and wide transverse process in lumbar vertebrae) allow the identification (Okazaki, Reference Okazaki2012; Martínez-Cáceres et al., Reference Martínez-Cáceres, Lambert and de Muizon2017; de Muizon et al., Reference de Muizon, Bianucci, Martínez-Cáceres and Lambert2019). The lumbar and caudal vertebrae are more eroded and diagenetically altered; the order remains uncertain. In the case of the documented lumbar vertebrae, we suggest that they are likely the first seven vertebrae, considering the vertebrae body shape and traces of the transverse process base (Okazaki, Reference Okazaki2012; Martínez-Cáceres et al., Reference Martínez-Cáceres, Lambert and de Muizon2017; de Muizon et al., Reference de Muizon, Bianucci, Martínez-Cáceres and Lambert2019). The lateral processes of the lumbar vertebrae show a subhorizontal orientation. The caudal vertebrae were interpreted on the basis of the preserved morphology, but again, the order remains uncertain. No detailed field notes for MRAHBCS Pal/V119 were recorded during the collecting process.
Forelimb.—MRAHBCS Pal/V119 includes the distal portion of the right scapula (Fig. 16), showing the glenoid fossa for articulating the humerus. The articulating surfaces of the left humerus, ulna, and radius are preserved. The olecranon process is broken, but the ulnar facet is shorter than the radial facet.
Etymology
The specific name novellus (Latin for youth, new) alludes to the Sierra El Novillo, a mountain complex east of the locality where the holotype was found south of La Paz, Baja California Sur, Mexico.
Remarks
Both Sitsqwayk cornishorum and Maiabalaena nesbittae, also recovered in the northeastern Pacific, likely belong to eomysticetids (Boessenecker et al., Reference Boessenecker, Beatty and Geisler2023; this study). In addition, a recent paper identified the existence of Eomysticetus sp. from the Oligocene of Mexico. If all taxonomic identifications are correct, our Echericetus novellus n. gen. n. sp. and the previously known eomysticetid diversity in the northeastern Pacific become comparable to the South Pacific assemblage.
Phylogenetic analysis
Our consensus trees (Fig. 17), with the addition of Echericetus novellus n. gen. n. sp., is broadly similar to previous phylogenetic analyses (Marx and Fordyce, Reference Marx and Fordyce2015; Fordyce and Marx, Reference Fordyce and Marx2018; Peredo and Pyenson, Reference Peredo and Pyenson2018). Noteworthy, the recent inclusion of new stem mysticetes has provoked a substantial change in the phylogenetic relationships (see Fordyce and Marx, Reference Fordyce and Marx2018; Schipps et al., Reference Schipps, Peredo and Pyenson2019; Boessenecker et al., Reference Boessenecker, Beatty and Geisler2023; also see phylogenies based on problematic Kekenodon, Corrie and Fordyce, Reference Corrie and Fordyce2022). In our analyses, we test the phylogenetic relationship of Echericetus novellus n. gen. n. sp. regarding Eomysticetidae under three different criteria related to the concavity value of k, default value 3 and the calculated value 28.18360, and equal weights criteria (k “determines how strongly homoplasious characters are down-weighted”; Goloboff, Reference Goloboff1993; Goloboff et al., Reference Goloboff, Carpenter, Arias and Miranda-Esquivel2008, Reference Goloboff, Torres and Arias2018). Only the consensus tree under the value k = 28.18360 is described (the preferred tree; Fig. 17.1), and the other trees are used to show alternative topologies (Fig. 17). A broad review or discussion on Mysticeti phylogeny is beyond the scope of this study. Similarly, our preferred tree (Fig. 17.1) also supports the recently suggested inclusion of Sitsqwayk and Maiabalaena in Eomysticetidae (Boessenecker et al., Reference Boessenecker, Beatty and Geisler2023). In a broad sense, Coronodonidae, Llanocetidae, and Mammalodontidae have been clustered with Odontoceti (Fig. 17), suggesting that the relationships among early neocete evolution remain problematic (also see Corrie and Fordyce, Reference Corrie and Fordyce2022 for similar results). Problematic taxa such as Coronodonidae (Boessenecker et al., Reference Boessenecker, Beatty and Geisler2023), Mystacodon and Llanocetus (Llanocetidae, sensu Fordyce and Marx, Reference Fordyce and Marx2018), Tlaxcallicetus and Mauicetus (crownward mysticetes) show various phylogenetic positions, depending on the chosen criteria. Thus, such contradictions might result from missing data (morphology and taxa). Among Chaeomysticeti, eomysticetids are the most basal chaeomysticetes diverging after the toothed mysticetes. In addition, under implied weights (k = 28.18360; Fig. 17.1), the position of Maiabalaena + Sitsqwayk differs from previous results (Peredo et al., Reference Peredo, Pyenson, Marshall and Uhen2018) and conforms to the result of Boessenecker et al. (Reference Boessenecker, Beatty and Geisler2023): both Maiabalaena and Sitsqwayk are eomysticetids. Interestingly, our result also shows that Maiabalaena and Sitsqwayk may be diverging earlier than eomysticetids under equal weights (Fig. 17.2) or more derived than eomysticetids under implied weights (Fig. 17.3).
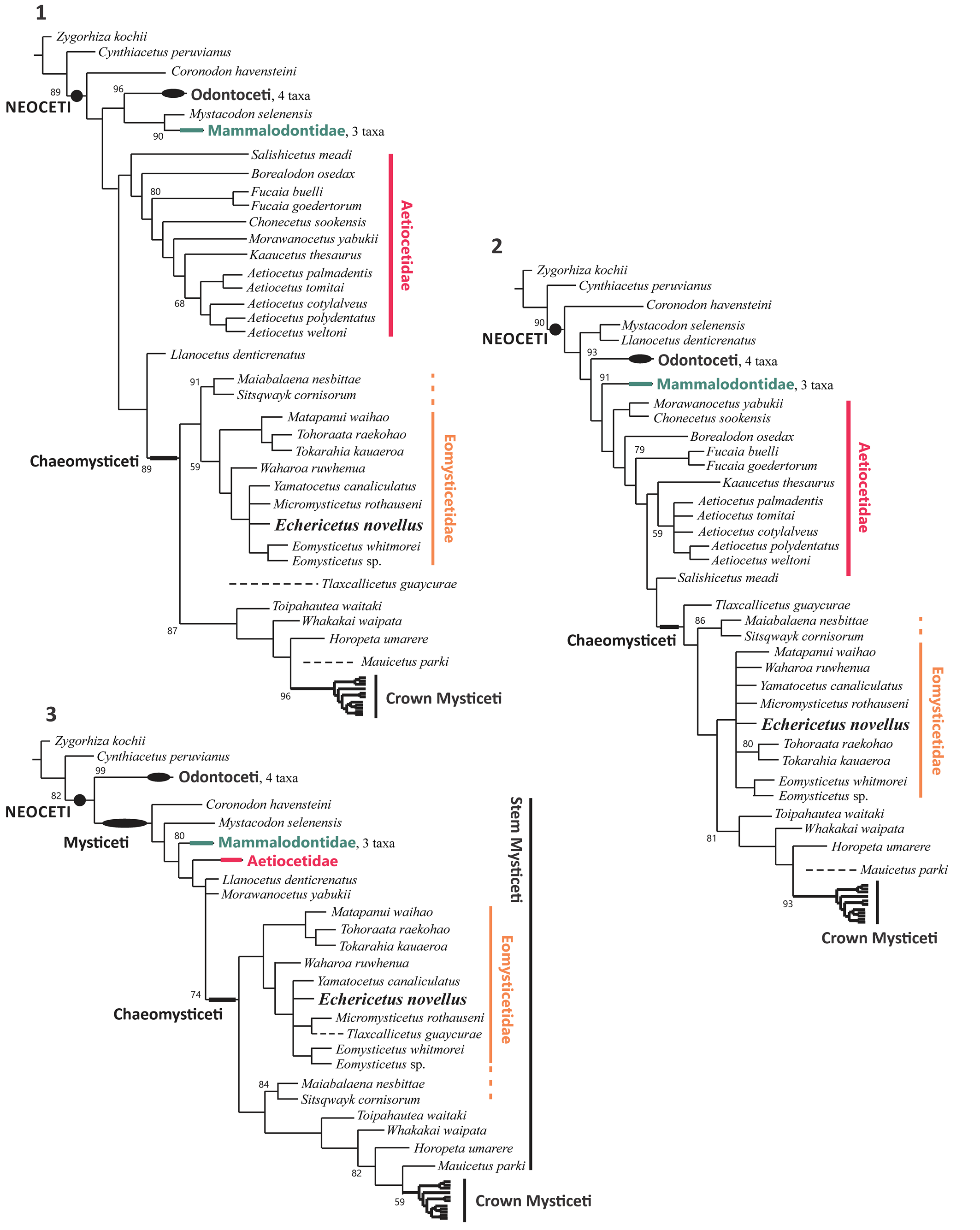
Figure 17. Phylogenetic position of Echericetus novellus n. gen. n. sp. under implied weights and equal weights. (1) Consensus tree from nine parsimonious trees: k = 28.18360; 30.77813 steps; CI = 0.254; RI = 0.752; RCI = 0.191008; tree total length 1,424. (2) Consensus tree from 20,160 parsimonious trees: equal weights; 1,434 steps; CI= 0.242; RI= 0.736; RCI = 0.178112; tree total length 1,494. (3) Consensus tree from nine parsimonious trees: k = 3; 129.26216 steps; CI = 0.248; RI = 0.743; RCI = 0.184264; tree total length 1462. Only the support branch > 50% is illustrated.
On the basis of the consensus tree under implied weights (k = 28.18360; Fig. 17.1) from the nine most parsimonious trees, the synapomorphies support the inclusion of Maiabalaena and Sitsqwayk within Eomysticetidae: anteriormost point of palatine located anterior to the level of the antorbital notch (c. 21/s. 1; coded only in Yamatocetus); the temporal fossa forms a large parasagittal oval (c. 83/s. 1; such a character might be interpreted and coded differently due to disparity and ontogenetic variants; here all the taxa share this character); highly irregular outline of the frontoparietal suture (c. 86/s. 2; different in Maiabalaena); an extremely well-developed and robust zygomatic process of squamosal (c. 96/s. 1; present in almost all the taxa); anterior process transversely compressed and blade-like (c. 150/s. 1; unknown in Sitsqwayk); subcondylar furrow as a medially well-defined groove with the dorsal border being accentuated by a medially well-developed condyle (c. 228/s. 2; coded only in Maiabalaena + Sitsqwayk and different in Yamatocetus); the apices of neural spines of posterior thoracic and anterior lumbar vertebrae anteroposteriorly expanded and squared off (c. 247/s. 1; unknown in more than half of taxa). Our analysis under implied weights (k = 28.18360; Fig. 17.1) differs from the results of Boessenecker et al. (Reference Boessenecker, Beatty and Geisler2023) in that the branch of Maiabalaena + Sitsqwayk and eomysticetids has a branch support of less than 50%. However, our dataset differs from that of Boessenecker et al. (Reference Boessenecker, Beatty and Geisler2023), but both datasets consistently recover that Maiabalaena and Sitsqwayk are eomysticetids. New materials of Maiabalaena and Sitsqwayk should further reveal the eomysticetid affinity and resolve the relationships within Eomysticetidae.
Last, Echericetus novellus n. gen. n. sp. is also an eomysticetid, based on 86 characters (31.6% of the matrix). The relationships of Echericetus novellus with other eomysticetid remain unresolved. Here, Echericetus novellus forms a polytomy with Yamatocetus, Micromysticetus, and Eomysticetus (Fig. 17.1). The branch that indicates the relationship of Echericetus novellus with six eomysticetid genera has 59% support. The recovered synapomorphies for this branch are an extended rostral portion of maxilla anterior to antorbital notch with more than one and a half times the bizygomatic width (c. 1/s. 2); transverse width of maxilla at midpoint with more than twice the width of the premaxilla (c. 5/s. 2); apex of the zygomatic process of squamosal deflected anteroventrally (c. 102/s. 1); posteriormost point of exoccipital located more anteriorly than the posterior edge of the occipital condyle (c. 139/s. 0); medial lobe of tympanic bulla subequal in width to the lateral lobe or smaller (c. 202/s. 1); subcondylar furrow as a medially well-defined groove with the dorsal border being accentuated by a medially well-developed condyle (c. 228/s. 2); the height of transverse process of the atlas at the base equal to half the height of the articular surface or less (c. 239/s. 1). The autapomorphies of Echericetus novellus n. gen. n. sp. are postorbital process short and not markedly projecting in any direction (c. 48/s. 3); the orbital rim of supraorbital process of frontal thickened with a rounded lateral surface (c. 50/s. 2); preorbital region of frontal dorsoventrally flat (c. 61/s. 1); flat or convex anterior half of dorsal surface of supraoccipital (c. 115/s. 1); absent distinct ridge delimiting insertion surface of tensor tympani on the medial side of anterior process but with an insertion surface distinctly excavated (c. 160/s. 1); well developed and large hiatus fallopii located anterior to proximal opening of the facial canal (c. 178/s. 1).
Discussion
The discovery of Echericetus novellus n. gen. n. sp. from the Oligocene of Mexico (slightly older than 27.95 Ma, latest Rupelian) adds to the growing list of new eomysticetids in the North Pacific (e.g., Hernández-Cisneros and Nava-Sánchez, Reference Hernández-Cisneros and Nava-Sánchez2022). The eomysticetid fossil record represents one of the best-sampled stem mysticete clades, but large-scale questions remain, such as the origin, adaptive traits, and functional morphology (whether eomysticetids were skim feeders, similar to right baleen whales), and the causes that drove their extinction after the Oligocene–Miocene transition (Boessenecker and Fordyce, Reference Boessenecker and Fordyce2015b, Reference Boessenecker and Fordyce2017b). The oldest eomysticetid fossils are Micromysticetus rothauseni and Yamatocetus canaliculatus, close to 30 Ma (Marx and Fordyce, Reference Marx and Fordyce2015), whereas the youngest geological occurrence is the early Miocene Waharoa (~22.28 Ma, Aquitanian; Boessenecker and Fordyce, Reference Boessenecker and Fordyce2017b; also see Boessenecker, Reference Boessenecker2022), indicating that eomysticetids thrived at least for 8 to 11 Myr. Not only the longevity of eomysticetid lineage but also the high disparity and diversity, at least nine genera and 13 species (including Echericetus novellus n. gen. n. sp.; but also see Boessenecker and Fordyce, Reference Boessenecker and Fordyce2017a for a review of Eomysticetidae), suggest that eomysticetids represent an ecologically competitive and successful lineage. The wide distribution in the Pacific, Atlantic, and likely Mediterranean–Paratethys oceans indicates that eomysticetids likely responded to environmental pressures and relocated with food sources efficiently (Boessenecker and Fordyce, Reference Boessenecker and Fordyce2017b; Marx et al., Reference Marx, Fitzgerald and Fordyce2019; Hernández-Cisneros and Nava-Sánchez, Reference Hernández-Cisneros and Nava-Sánchez2022), but the sudden disappearance of eomysticetids remains uncertain and likely opens up the ecological niches for the success of the “modern” mysticetes.
The environmental change during the Oligocene–Miocene boundary restructured the coastlines, likely leading to the demise of coastal cetacean species (e.g., aetiocetids and mammalodontids). Still, pelagic cetaceans, such as eomysticetids, presumably were not highly affected (Marx et al., Reference Marx, Fitzgerald and Fordyce2019). As a possible transitional form between toothed and baleen-bearing whales (e.g., Clementz et al., Reference Clementz, Fordyce, Peek and Fox2014; Boessenecker and Fordyce, Reference Boessenecker and Fordyce2015b), eomysticetids were likely outcompeted by more derived modern-looking mysticetes (early balaenopterids and balaenids; see Tsai, Reference Tsai2023 for early crown mysticetes), with efficient strategies for filter feeding. Notably, the ontogeny of Waharoa ruwhenua Boessenecker and Fordyce, Reference Boessenecker and Fordyce2015b shows the peramorphic process of acceleration, an archaic equivalent to balaenopterids (Tsai and Fordyce, Reference Tsai and Fordyce2014). If the interpretation of this developmental factor is correct, eomysticetids should have represented a relatively diverse and high-disparity clade, similar to modern balaenopterids, making them less vulnerable to extinction. Following this argument, an alternative hypothesis that has yet to be considered is that eomysticetids did not go extinct per se but evolved into a modern-looking baleen whale lineage, analogous to archaeocete–neocete (e.g., de Muizon et al., Reference de Muizon, Bianucci, Martínez-Cáceres and Lambert2019) or dinosaur–bird transition (e.g., Brusatte et al., Reference Brusatte, Lloyd, Wang and Norell2014). Developmental processes often reveal unexpected deep homology. If our hypothesis turns out to be correct, eomysticetids may have been early crown mysticetes (also see Tsai, Reference Tsai2023 for the origin of crown Mysticeti). Future finds with more complete specimens, accurate geochronological correlation, disparity analyses, detailed examination of morphological characters (similar to new character identification and combination revealing the pygmy right whale to be a cetotheriid; Fordyce and Marx, Reference Fordyce and Marx2013), and ancestor–descendant tests (see Tsai and Fordyce, Reference Tsai and Fordyce2015a for using ontogenetic clades to bracket the ancestral species) should uncover the hidden baleen whale transitions.
How did Eomysticetidae rise, and what biological and physical factors led to their diversification? In contrast to the toothed mysticetes (e.g., aetiocetids), these questions are crucial to understanding the bloom and evolution of baleen whales because eomysticetids likely represent the first fully baleen-assisted lineage (with nonfunctional teeth; Boessenecker and Fordyce, Reference Boessenecker and Fordyce2015a). Eomysticetids were probably driven by food sources (e.g., zooplankton; Clementz et al., Reference Clementz, Fordyce, Peek and Fox2014; Marx et al., Reference Marx, Fitzgerald and Fordyce2019; Hernández-Cisneros and Nava-Sánchez, Reference Hernández-Cisneros and Nava-Sánchez2022) that seem related to phosphogenetic episodes during the Oligocene (Bisconti et al., Reference Bisconti, Pellegrino and Carnevale2023). Still, the relationship between the physical conditions and marine ecological systems that have facilitated eomysticetid diversification remains uncertain. Physical factors (e.g., origins and effects of the Antarctic Circumpolar Current) have been proposed as the critical drivers of the neocete evolution (e.g., Fordyce, Reference Fordyce1977, Reference Fordyce, Prothero, Ivany and Nesbitt2003; Steeman et al., Reference Steeman, Hebsgaard, Fordyce, Ho, Rabosky, Nielsen, Rahbek, Glenner, Sørensen and Willerslev2009). However, the impact of the Antarctic Circumpolar Current has been questioned due to the uncertain geochronologic timings, disparate phylogenetic interpretations, and lack of ecological approaches, resulting in the poor explanation of the Oligocene cetacean diversity (Rabosky, Reference Rabosky2014; Marx et al., Reference Marx, Lambert and Uhen2016; Pyenson, Reference Pyenson2017). By contrast, an integrative approach is growing to explain cetacean evolution regarding global factors such as specific geological events (e.g., massive phosphorite deposition; Bisconti et al., Reference Bisconti, Pellegrino and Carnevale2023). Further and detailed biogeographic modelings with more eomysticetids discoveries may help to facilitate paleobiological interpretations and which ancestry lineage evolved into eomysticetids at the Eocene–Oligocene boundary. Given the eomysticetid global distribution in the Oligocene, they may have displayed high dispersal ability, leading to the colonization of new geographic areas and diversification. Alternatively, the dispersal events may imply long-distance migratory behavior (Clementz et al., Reference Clementz, Fordyce, Peek and Fox2014; Boessenecker and Fordyce, Reference Boessenecker and Fordyce2015b) or indicate intense competition driving niche partitioning (Tsai and Ando, Reference Tsai and Ando2015) and dispersal.
Last, a comprehensive paleobiogeographic framework for the Eomysticetidae remains immature to answer the origin, subsequent dispersal, and speciation events. The richness of eomysticetid occurs primarily in the Southern Hemisphere (New Zealand; Boessenecker and Fordyce, Reference Boessenecker and Fordyce2017b), which could indicate that eomysticetids originated in the Southern Hemisphere, but contrast with the oldest record of eomysticetids in the North Hemisphere. Given the synapomorphies and phylogenetic positions of Sitsqwayk and Maiabalaena within eomysticetids (Fig. 17; also see Boessenecker et al., Reference Boessenecker, Beatty and Geisler2023), Sitsqwayk and Maiabalaena likely represent highly disparate and early- or late-diverging eomysticetids that inhabited the North Pacific. Such a scenario and taxonomic composition indicate a critical differentiation between the south and north cetacean faunas in the Pacific Ocean, indicating the potential of finding more archaic chaeomysticetes from the North Pacific than from the South Pacific. This pattern may suggest a translocating event from the Northern Hemisphere to the colonization of southern oceans by archaic eomysticetids.
Acknowledgments
We thank the Museo Regional de Antropología e Historia de Baja California Sur for granting access to MRAHBCS Pal/V119 (Echericetus novellus, dubbed as Mazapán); to J.A. Zuñiga de la Toba and Q. Muñoz Garayzar for access to the collection and support during the preparation of MRAHBCS Pal/V119; and to A. Calderon Vega for allowing us to continue this work. We thank A. Piñeda Geraldo, A. Rosales López, G. González Barba, and collaborators for collecting MRAHBCS Pal/V119 in 1995. We also thank the handling editor, C. Scott, and two anonymous reviewers for constructive suggestions. We are grateful to locals from Rancho La Palma for their help during field visits and to everyone who helped in various ways during this study. A.E.H.-C. was a PhD student at the Instituto Politécnico Nacional - Centro Interdisciplinario de Ciencias Marinas (CICIMAR-IPN), supported by the Consejo Nacional de Ciencia y Tecnología (CONACYT), scholarship 290143 (2017–2021). C.-H.T. was financially supported by the Taiwan Ministry of Science and Technology (now known as National Science and Technology Council; MOST 108-2621-B-002-006-MY3; 111-2621-B-002-006; 112-2621-B-002-005) and public donations to the Lab of Evolution and Diversity of Fossil Vertebrates at the National Taiwan University (NTU FD107028) led by C.-H.T. A.E.H.-C. is grateful to M.R. Cisneros Renteria, F. Hernández Valencia, and the family for their support and care during convalescence, making it possible to finish the present work.
Declaration of competing interests
The authors declare no competing interests.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.70rxwdc4s




















