Many studies have shown highly significant associations between weight gain during the first months or years of life and later adiposity( Reference Druet, Stettler and Sharp 1 , Reference Baird, Fisher and Lucas 2 ). The first 4–9 months of life have been identified as a sensitive period for the influence of rapid growth on fat mass (FM) at later ages( Reference Singhal, Kennedy and Lanigan 3 – Reference Ekelund, Ong and Linne 5 ) and later risk of obesity( Reference Stettler, Zemel and Kumanyika 6 – Reference Taveras, Rifas-Shiman and Belfort 8 ). Alternatively, other studies have found excessive weight gain in the period 2–6 years( Reference Ekelund, Ong and Linne 5 , Reference Lagstrom, Hakanen and Niinikoski 9 ) and no specific period (from birth to 15 years) at all( Reference Victora, Sibbritt and Horta 10 ) to be of particular importance.
Nutrition in infancy is thought to be particularly influential on later risk of obesity, as growth patterns and changes in body composition in early infancy are closely linked with feeding patterns( Reference Singhal, Kennedy and Lanigan 3 , Reference Dewey 11 – Reference Gale, Logan and Santhakumaran 13 ). Breastfed infants show slower weight gains in the first year of life( Reference Dewey 11 , Reference Victora, Morris and Barros 12 ), and have lower FM at 12 months compared to formula-fed infants( Reference Gale, Logan and Santhakumaran 13 ). Three meta-analyses have shown breastfeeding to be associated with a small but consistently reduced risk of obesity determined by BMI( Reference Arenz, Ruckerl and Koletzko 14 – Reference Owen, Martin and Whincup 16 ), while breastfeeding does not seem to reduce mean BMI( Reference Owen, Martin and Whincup 17 ). However, less consistency has been found with regard to the relationship between infant feeding and FM and fat-free mass (FFM) at later ages. Three studies have found an inverse relationship between duration of breastfeeding and FM at 4 years( Reference Robinson, Marriott and Crozier 18 ), 9–10 years( Reference Toschke, Martin and von Kries 19 ) and 16 years of age( Reference Yin, Quinn and Dwyer 20 ); one study found an inverse effect in 9-year-old girls but not boys( Reference Gale, Javaid and Robinson 21 ); three studies found no differences at 2 years( Reference Butte, Wong and Hopkinson 22 ), 5 years( Reference Burdette, Whitaker and Hall 23 ) and 18 years of age( Reference Tulldahl, Pettersson and Andersson 24 ).
Supporting the theory that early overnutrition leads to increased risk of obesity at later ages, two randomised controlled trials demonstrated increased growth rates and higher FM at 5–8 years of age in a group of children born small for gestational age who had received nutrient-enriched formula v. control formula until 6–9 months of age( Reference Singhal, Kennedy and Lanigan 3 ). However, fewer studies have focused on the relation between the timing of the introduction of solids and later risk of obesity, and the findings that exist are inconsistent( Reference Moorcroft, Marshall and McCormick 25 , Reference Przyrembel 26 ). Early introduction to solids has been found to be associated with higher energy intake( Reference Grote, Schiess and Closa-Monasterolo 27 , Reference Ong, Emmett and Noble 28 ), and earlier introduction to unhealthy foods( Reference Tarrant, Younger and Sheridan-Pereira 29 ).
Breastfeeding, formula feeding and complementary feeding practices are three highly related components, and it can be difficult to distinguish which component is responsible for certain effects. Therefore, both breastfeeding and information on complementary feeding should be included in analyses of effects of early diet and body composition in later childhood( Reference Robinson, Marriott and Crozier 18 , Reference Burdette, Whitaker and Hall 23 ). Although large variations in body composition exist within a given BMI( Reference Wells 30 ), most studies of both early growth and early feeding have used BMI or BMI standard deviation scores as indirect measures of adiposity at later age stages( Reference Druet, Stettler and Sharp 1 , Reference Baird, Fisher and Lucas 2 , Reference Arenz, Ruckerl and Koletzko 14 – Reference Owen, Martin and Whincup 17 ). In the present study, however, we supplement anthropometric measurements and BMI with fat mass index (FMI) and fat-free mass index (FFMI) estimated from bioelectrical impedance analysis (BIA). In order to elucidate the mechanisms behind rapid growth and to improve prevention strategies of childhood obesity, it is important to identify the age window that is most impacted in this regard, and modifiable factors that influence growth in this period. The main aim of the present study was to undertake a detailed investigation of how weight gain in four periods, spanning birth to 3 years of age, influences body composition at 3 years of age. Our secondary aim was to examine the impact of breastfeeding and age of introduction to solids on FMI and FFMI.
Subjects and methods
Study design and participants
The data of the present study is drawn from the SKOT cohort (in Danish: Småbørns Kost og Trivsel), a prospective observational cohort study that monitored healthy Danish children at 9, 18 and 36 months of age. The present study was previously described in detail by Madsen et al. ( Reference Madsen, Schack-Nielsen and Larnkjaer 31 ). In brief, the criteria for the study included singleton term infants (at a gestational age of 37–42 weeks) without any diseases expected to affect growth or food intake. Recruitment took place from April 2007 to May 2008, with 2211 families randomly selected from the National Danish Civil Registry to be invited to participate in the present study. Out of these, 330 families accepted the invitation, and were enrolled in the study. The 36-month examinations took place from October 2009 to October 2010.
Anthropometry
Birth weight and weight at 5 months (measured by midwives and general medical practitioners, respectively) were obtained from health records kept by the parents of the study participants. All other anthropometric measurements, including weight, height and skin folds, were obtained from physical examinations of 9-month ( ± 2 weeks), 18-month ( ± 1 month) and 36-month ( ± 3 months) children at the Department of Nutrition, Exercise, and Sports, Copenhagen, Denmark. The procedures for the anthropometric measurement of 9- and 18-month children were described previously by Madsen et al. ( Reference Madsen, Larnkjaer and Molgaard 32 ). At 36 months, naked body weight was measured to the nearest 0·1 kg on an annually calibrated digital scale (Tanita WB-100MA; Tanita Corporation), and height was measured by a stationary digital stadiometer (235 Heightronic Digital Stadiometer; QuickMedical) to the nearest 0·01 cm. Triceps and subscapular skin folds were measured by a Harpenden skinfold calliper (Chasmors Limited) and recorded to the nearest 0·1 mm. Except for weight, all measurements were performed in triplicate, and averages were used in analysis. Following standardised procedures, four well-trained observers conducted the examinations. Weight and height/length were entered into the software WHO Anthro 2005( 33 ) to achieve sex-specific z-scores. The number of overweight and obese children at 3 years of age was determined according to the cut-off values of the International Obesity Task Force( Reference Cole, Bellizzi and Flegal 34 ) and according to WHO growth standards( 35 ).
Body composition assessment at 3 years of age
Body composition at 36 months was measured using BIA, described in detail by Ejlerskov et al. ( Reference Ejlerskov, Jensen and Christensen 36 ). Whole body resistance, reactance and impedance were measured with the child in a supine position using a single-frequency (50 kHz) tetrapolar BIA (Quantum III; RJL Systems). On the right foot, the signal electrode (LMP3 Diagnostic Tab Electrodes; Kendall, Covidien) was placed over the distal portion of the second metatarsal (the base of the second toe), and the detecting electrode was placed at the anterior ankle on an imaginary line bisecting the medial malleolus. On the right hand, the signal electrode was placed above the metacarpophalangeal joint of the middle finger. The detecting electrode on the right hand was placed on an imaginary line bisecting the ulnar head, as specified by the manufacturer. These measurements were performed twice consecutively, and the mean values of resistance, reactance and impedance were registered. A prediction equation for the FFM of the study's cohort of 3-year-old children was developed in advance using BIA, height and weight, with dual-energy X-ray analysis (DXA) as a reference method( Reference Ejlerskov, Jensen and Christensen 36 ). In short, high quality DXA scans were achieved for a sub-group of the SKOT children (n 101), and a prediction equation was obtained via linear regression models using a 10-fold cross validation approach with BIA, weight, height and sex as independent variables (adjusted R 2 0·84)( Reference Ejlerskov, Jensen and Christensen 36 ). FFM and FM were calculated using the following equations:
where FFM and FM are in grams, RI is the resistance index (height (cm)2/resistance (Ω), weight is digital weight computed in kg, height is in cm, and sex is recorded as male = 1 and female = 0.
Feeding patterns and other information
At the 9-month examination, the participants' parents filled out questionnaires on annual household income, age, height and weight of parents, infant gestational age at birth, gestational weight gain and smoking during pregnancy. In all three examinations, parents filled out follow-up questionnaires with updated information regarding their educational status, from which a variable of the mothers' educational levels at the time of the 3-year examination was generated. At the 36-month examination, we measured the height and weight of the accompanying parent using the same measuring equipment as used for the children. Data on feeding patterns, including duration of full and partial breastfeeding and age of introduction to complementary foods were obtained in interviews at each examination. Full breastfeeding was defined as receiving only breast milk, water and vitamins, though allowed for exceptional bottle feeding (e.g. if a child had been babysat for a single night), which increased the duration of full breastfeeding for fifteen infants. Partial breastfeeding was defined as a baby receiving some breast milk along with other food, weaning foods or formula milk. Age of introduction to solids was defined as the earliest age in months at which an infant first received one of nineteen food categories. Information on formula intake (g/d) at 9 months was derived from pre-coded dietary records developed for infants as described in Gondolf et al. ( Reference Gondolf, Tetens and Michaelsen 37 ).
Ethics
The parents of all participants received verbal and written information about the study and written consent was obtained from all. The study was approved by The Committees on Biomedical Research Ethics for the Capital Region of Denmark (H-KF-2007-0003).
Statistical analysis
The study sample only included data with complete records on FM and FFM. Missing values were considered missing at random, and available-cases analyses were carried out.
To account for natural variation in FM and FFM due to body size, we calculated FMI as FM/height (kg/m2), and FFMI as FFM/height (kg/m2)( Reference Wells 38 ). Linear regression confirmed that FMI and FFMI were not associated with height (P>0·5). In the absence of well-established reference data for FM or FMI to identify cut-off values for overweight-ness in young children, we grouped FMI and FFMI according to sex-specific quartiles.
Differences of weight-for-age z-scores (WAZ), height/length-for-age z-scores (HAZ), BMI-for-age z-scores (BAZ) and weight-for-height/length z-scores (WFH) in the study sample were evaluated to the WHO standard using one-sample t tests. Differences in the feeding patterns between sexes were assessed either using two-sample t tests or, if not normally distributed, Wilcoxon rank tests. Anthropometry at 3 years of age, parental characteristics and infant feeding practices were evaluated according to FMI and FFMI quartiles using mean values and standard deviations, and medians and interquartile ranges (if not normally distributed). Testing of trends across quartiles of FMI and FFMI was based on linear regression.
Associations between body composition at 3 years of age and weight gain in four age intervals (0–5 months, 5–9 months, 9–18 months and 18–36 months) were analysed using multiple linear regression. Body composition outcomes were regressed on change in WAZ in each of the four age intervals in both a simple model, only adjusting for sex and birth weight z-score (BWZ), and in a fully adjusted model controlling for sex, BWZ, household income, educational level of mother, smoking during pregnancy, gestational weight gain and parental BMI or height or weight according to the outcome of interest. The educational level of the mother was grouped into five categories: ‘no education above school level’, ‘trainee or vocational education’, ‘short academic education < 3 years of age’, ‘academic education 3–4 years’ and ‘long academic education >4 years’. Data on household incomes were collected in categories ranging from ‘less than 200 000 Danish Kroner’ to ‘more than 800 000 Danish Kroner’, with fourteen intervals of 50 000 Danish Kroner (approximately 8700 US$) in between, and these groupings were used as quantitative variables in the analyses. As only few mothers reported smoking in pregnancy, this variable was included as a dichotomous one (yes/no). Model assumptions were evaluated using residual and normal probability plots. Robust standard errors were employed, in case substantial departures were found.
For each quartile of FMI, the development of mean WAZ across the four time points was visualised in scatter plots. The same was done for each quartile of FFMI. Differences between quartiles of mean WAZ over time were evaluated using linear mixed models while controlling for sex, household income, educational level of mother, smoking during pregnancy, gestational weight gain and parental BMI, including child-specific random effects.
To identify the impact of birth weight and early growth on FMI and FFMI, we grouped birth weight into four categories: ‘ < 3000 g’ (n 23), ‘3000–3499 g’ (n 92), ‘3500–3999 g’ (n 83) and ‘ ≥ 4000 g’ (n 35). Only two children were born with a weight below 2500 g. Change in WAZ from 0 to 5 months was also divided into four categories: ‘ < − 0·67’, ‘ − 0·67–0’, ‘0–0·67’ and ‘>0·67’. A change in WAZ exceeding 0·67 represents upward centile crossing at standard growth curves, which is a threshold that has been found clinically relevant( Reference Ong and Loos 39 ). Differences in the proportion of children in the highest quartile of FMI and FFMI across the categories were tested using χ2 test or Fisher's exact test.
Associations between FMI or FFMI, duration of full and partial breastfeeding, and age of introduction to solids were assessed, using multiple regression in simple models adjusted only for sex, and in fully adjusted models while controlling for BWZ, WAZ change from 0 to 5 months, educational level of mother, gestational weight gain and maternal BMI. We included the combined effects corresponding to the BWZ-modified effect of full breastfeeding, the BWZ-modified effect of age of introduction to solids, the effect modification of full breastfeeding by WAZ change from 0 to 5 months, and the effect modification of age of introduction to solids by WAZ change from 0 to 5 months in a fully adjusted regression model with FMI or FFMI as outcome variables. For these analyses, full breastfeeding was categorised as ‘ < 1 month’ ( < 31 d, n 36), ‘1–3 months’ (31–120 d, n 39), ‘4–5 months’ (121–180 d, n 137) and ‘6 months’ (>180 d (range 183–197 d), n 21). Age of introduction to solids was grouped as ‘3–4 months’ (n 138), ‘5 months’ (n 63) and ‘6 months’ (n 32). Backwards stepwise elimination was used for removing non-significant combined effects one at a time by means of likelihood ratio tests. The same procedure was applied to a similar model with the variables of partial breastfeeding used in interaction terms (‘ < 4 months’ ( < 121 d, n 30), ‘4–5 months’ (121–182 d, n 30), ‘6–8 months’ (183–273 d, n 60), ‘9–11 months’ (274–364 d, n 56) and ‘ ≥ 12 months’ (>365 d, n 54)).
Analyses were carried out using STATA version 11.0 (StataCorp LP). The significance level was set at α = 0·05.
Results
Out of the 330 children initially recruited for SKOT, 263 (79·7 %) completed the 36-month examination. FFM and FM were calculated for the 233 children with complete BIA, height and weight data. Children without BIA data were taller at 3 years of age than children with complete BIA data (P= 0·008, data not shown), and yet no differences were seen in terms of weight and BMI at 3 years of age. Data for weight and length at 5 months was missing for sixty-six children. A comparison of the children with or without 5-month values showed no differences in weight and length at birth and 9 months of age (data not shown). Selected characteristics of parents are presented in Table 1.
Table 1 Baseline characteristics (Median values and 25th–75th percentiles, number of participants and percentages)

Sample characteristics
The mean WAZ of the SKOT children was above average compared with WHO growth standards in all examinations (all P< 0·001; Table 2). HAZ was measured at birth and 5 months by midwives and practitioners and was substantially higher than the WHO standards, most likely due to inaccurate length measures in the primary healthcare sector. At 3 years of age, nineteen children were overweight (8·2 %), and none was obese according to the International Obesity Task Force criteria. Forty-six children (19·7 %) were at risk of becoming overweight (with a BAZ of above 1 sd), four children (1·7 %) were overweight with a BAZ above 2 sd and none obese according to the WHO growth standards. A total of 158 children (67·8 %) were fully breastfed for 4 months or more, while ninety-nine children (42·5 %) were still partially breastfed at 9 months. Five children (2 %) were introduced to solids earlier than 4 months, 133 (57 %) at 4 months, 63 children (27 %) at 5 months and 32 children (14 %) at 6 months.
Table 2 Anthropometric characteristics in the Småbørns Kost og Trivsel (SKOT) cohort according to WHO z-scores( 35 ) * (Mean values and ranges (minimum–maximum))

WAZ, weight-for-age z-score; HAZ, height/length-for-age z-score; WFH, weight-for-height/length z-score; BAZ, BMI-for-age z-score.
* Differences between WAZ, HAZ, WFH and BAZ in the study sample, as well as the WHO standards, were evaluated by means of one-sample t tests.
† Measured by midwives at the hospital.
‡ Measured by general medical practitioners.
§ Missing values for three children.
Feeding patterns were found not to differ between boys and girls (all P>0·3, data not shown); however, infants who were no longer fully breastfed at 4 months were introduced to solids earlier than infants fully breastfed at 4 months (P< 0·001) (data not shown). No trends across FMI or FFMI quartiles were found for the majority of the infant feeding variables (Tables 3 and 4), and yet a negative trend across FMI quartiles was seen for the number of children fully breastfed at 4 months (P= 0·028) (Table 3). There was also a trend of higher maternal BMI in the higher FMI quartiles (P= 0·07). Maternal age, height, gestational weight gain, smoking during pregnancy, parental educational level, paternal height and BMI, and household incomes were not related to the FMI quartiles (all, P>0·13, data not shown). Positive trends across FFMI quartiles were seen for parental BMI (both P< 0·02), while negative trends were seen for parental education (both P< 0·05, data not shown). Maternal age, height, gestational weight gain, smoking during pregnancy, paternal height and household incomes were not related to the FFMI quartile (all, P>0·18, data not shown).
Table 3 Characteristics of the Småbørns Kost og Trivsel (SKOT) children according to fat mass index (FMI, kg/m2) quartile at 3 years of age* (Mean values and standard deviations, medians and interquartile ranges (IQR) (if not normally distributed), number of participants and percentages)

FM, fat mass; FFM, fat-free mass; BF, breastfed.
* Trends across FMI quartiles were assessed using linear regression models adjusted for sex.
† Not adjusted for sex.
Table 4 Characteristics of the Småbørns Kost og Trivsel (SKOT) children according to fat-free mass index (FFMI, kg/m2) quartile at 3 years of age* (Mean values and standard deviations, medians and interquartile ranges (IQR) (if not normally distributed), number of participants and percentages)

FM, fat mass; FFM, fat-free mass; BF, breastfed.
* Trends across FFMI quartiles were assessed using linear regression models adjusted for sex.
† Not adjusted for sex.
Association between early weight gain and body composition at 3 years of age
BWZ and change in WAZ from 0 to 5 months were found to be positively related to height, weight, BMI, FMI and FFMI at 3 years of age in both the simple and adjusted models (Table 5). In addition to this, change in WAZ from 0 to 5 months was positively associated with the sum of skin folds (P< 0·001). The different growth patterns over time for the mean WAZ according to the FMI quartiles are shown in Fig. 1(a). There was significant interaction between the FMI quartiles and time (P< 0·001), with the most evident difference existing between growth patterns from 0 to 5 months. Controlling for the educational level of the mother, smoking during pregnancy, gestational weight gain and parental BMI, differences between the quartiles showed that the mean WAZ of the highest FMI quartile was significantly higher than that of the other FMI quartiles at 5 months (P< 0·002) and 9 months (P< 0·05). At 18 months, only the WAZ of the first and second FMI quartiles were significantly lower than the highest (P< 0·002). At 36 months, the third FMI quartile was only just lower than the fourth FMI quartile (P= 0·08), and yet the others remained even lower (P< 0·001). Growth patterns over time did not differ between the FFMI quartiles (P= 0·17), but the mean WAZ of the fourth quartile was significantly higher than all the other quartiles (P< 0·001) (Fig. 1(b)).
Table 5 Relation between weight gain during four time periods (Δ weight-for-age z-score (Δ WAZ)) and body composition outcomes at 3 years of age expressed as regression coefficients† (β Coefficients and standard errors)

FMI, fat mass index; FFMI, fat-free mass index; Σ SF, sum of triceps and subscapular skin folds; BWZ, birth weight z-score.
* P< 0·05, **P< 0·01, ***P< 0·001.
† Each cell represents a multiple regression model with body composition at 3 years of age as the dependent variable and the left row showing explanatory variables. The simple model was adjusted for sex and BWZ. The adjusted model was adjusted for sex, BWZ, income, mother's educational level, smoking during pregnancy and gestational weight gain. Height was also adjusted for parental height, weight was adjusted for parental weight, and BMI, FMI, FFMI and sum of skin folds were all adjusted for parental BMI.

Fig. 1 Change in weight-for-age z-scores (WAZ) from 0 to 36 months according to (a) fat mass index (FMI) and (b) fat-free mass index (FFMI) quartiles at 3 years of age. ![]() , First quartile;
, First quartile; ![]() , second quartile;
, second quartile; ![]() , third quartile;
, third quartile; ![]() , fourth quartile. Differences between quartiles were assessed via mixed model analyses controlling for the educational level of the mother, smoking during pregnancy, gestational weight gain, household income and parental BMI. * Mean value of the fourth quartile (reference) was significantly higher than that of all other quartiles (P< 0·05). † Mean value of the fourth quartile was significantly higher than that of the first and second quartiles (P< 0·002). ‡ Mean value of the fourth quartile was borderline higher than that in the third quartile (P< 0·08).
, fourth quartile. Differences between quartiles were assessed via mixed model analyses controlling for the educational level of the mother, smoking during pregnancy, gestational weight gain, household income and parental BMI. * Mean value of the fourth quartile (reference) was significantly higher than that of all other quartiles (P< 0·05). † Mean value of the fourth quartile was significantly higher than that of the first and second quartiles (P< 0·002). ‡ Mean value of the fourth quartile was borderline higher than that in the third quartile (P< 0·08).
Fat mass index and fat-free mass index according to birth weight and early weight gain
The probability of being in the highest quartile of FMI and FFMI because of birth weight and early weight gain is shown in Table 6. We categorised birth weight into four categories and analysed the share of children from each category that were placed in the highest quartile of FMI and FFMI at 3 years of age. The same approach was taken for change in WAZ from 0 to 5 months. More children with a birth weight above 4000 g were placed in the highest quartile of FMI compared to children with birth weights from 3000 to 3500 g (40 % compared to 17 %, P< 0·007). For the group of children undergoing rapid growth in their first five months of life (change in WAZ >0·67), 49 % were placed in the highest quartile of FMI at 3 years of age, which was more than that of children with weight gains not exceeding 0·67 WAZ from 0 to 5 months (all groups, P< 0·031). A significantly larger proportion of the children with birth weights above 4000 g, when compared to 3000–3500 g and less than 3000 g were found to be in the highest FFMI quartile at 3 years of age (40 % compared to 17 and 13 %, respectively, both P< 0·04). WAZ change from 0 to 5 months did not affect the probability of being in the highest FFMI quartile at 3 years of age.
Table 6 Prevalence of children in the fourth quartile of fat mass index (FMI, kg/m2) and fat-free mass index (FFMI, kg/m2) according to birth weight and weight-for-age z-score change (Δ WAZ) from 0 to 5 months (Number of participants and percentages)
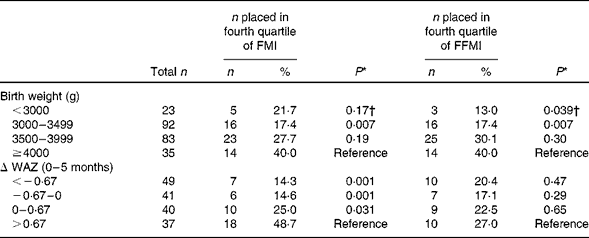
* Comparison of group difference against reference by Pearson's χ2 test.
† Comparison by Fisher's exact test.
Associations between early feeding practices and body composition at 3 years of age
Both in the unadjusted and adjusted regression analyses, full breastfeeding, partial breastfeeding, age of introduction to solids and age of introduction to cow's milk were found not to be related to FMI or FFMI at 3 years of age (data not shown). However, when testing for possible interactions between early feeding practices and early growth, we found two effect modifications of duration of full breastfeeding (Table 7). The effect of BWZ on FMI was eliminated by full breastfeeding for 6 months compared to less than 1 month (from β = 0·64 to β = 0·02, P= 0·009) (Table 7 and Fig. 2(a)). A duration of full breastfeeding for 1–3 months and 4–5 months borderline attenuated the positive effect of change in WAZ from 0 to 5 months on FMI by 41 % compared to less than 1 month (from β = 0·73 to β = 0·44, P= 0·13 and β = 0·43, P= 0·10, respectively), while 6 months of full breastfeeding eliminated the effect of WAZ on FMI (from β = 0·73 to β = − 0·03, P= 0·010) (Table 7 and Fig. 2(b)). The effect of age of introduction to solids on FMI did not interact with duration of full breastfeeding (P>0·25), and no interactions were found between sex and age of introduction to solids or duration of full breastfeeding (P>0·23).
Table 7 Multiple regression model of the association between birth weight, weight gain from 0 to 5 months, infant feeding and fat mass index (FMI) at 3 years of age (n 156)* (β Coefficients and standard errors)

BWZ, birth weight z-score; WAZ, weight-for-age z-score.
* A multiple regression model with FMI (kg/m2) at 3 years of age as the dependent variable controlling for BWZ, WAZ change from 0 to 5 months, educational level of mother, gestational weight gain and maternal BMI. The intercept represents the mean value of FMI at 3 years of age in the reference groups.

Fig. 2 Predicted mean fat mass index (FMI, kg/m2) at 3 years of age according to (a) birth weight z-score (BWZ) and (b) change in weight-for-age z-scores (Δ WAZ) from 0 to 5 months categorised into subgroups of breastfeeding duration in 156 children in the Småbørns Kost og Trivsel (SKOT) cohort. Figure lines are drawn from the coefficients in question derived from the multiple regression model presented in Table 7. (a) Illustrates the interaction between BWZ and duration of full breastfeeding and (b) illustrates the interaction between Δ WAZ from 0 to 5 months and duration of full breastfeeding. Duration of full breastfeeding < 1 month (![]() , n 36), 1–3 months (
, n 36), 1–3 months (![]() , n 39), 4–5 months (
, n 39), 4–5 months (![]() , n 137) and 6 months (
, n 137) and 6 months (![]() , n 21).
, n 21).
Neither age of introduction to solids nor duration of partial breastfeeding modified the effect of BWZ, or change in WAZ from 0 to 5 months on FMI (P>0·3). No interactions were found between BWZ, WAZ change from 0 to 5 months and early feeding practice on FFMI (P>0·6).
Discussion
In this prospective cohort, we found that high birth weight and rapid growth in the first 5 months of life independently affected body composition and measures of adiposity at 3 years of age, while there were no effects of weight gain in the following age periods. Full breastfeeding for 6 months considerably attenuated the effect of birth weight and weight gain from 0 to 5 months on FMI at 3 years compared to less than 1 month. The inherent limitations of an observational study granted, the results of the present study indicate that full breastfeeding for 6 months, which is a modifiable factor, can attenuate the effect of birth weight and early growth on FM at 3 years of age.
After controlling for parental height, weight or BMI, gestational weight gain, mother's educational level and smoking during pregnancy, the results show that birth weight and growth in the first 5 months of life are strong predictors of body composition at 3 years of age. A birth weight above 4000 g increased the probability of being in the highest quartile of FMI and FFMI at 3 years of age by approximately 50 %, compared to children with birth weights ranging from 3000 to 3500 g. Rapid weight gain above 0·67 z-scores from 0 to 5 months was strongly related to FMI, but not FFMI. Other studies have also found rapid early weight gain being associated with FM rather than FFM( Reference Singhal, Kennedy and Lanigan 3 – Reference Ekelund, Ong and Linne 5 , Reference Larnkjaer, Schack-Nielsen and Molgaard 40 – Reference Ong, Emmett and Northstone 42 ), though studies on the effect of birth weight on later body composition support a stronger association with FFM than with FM( Reference Singhal, Wells and Cole 43 , Reference Wells, Chomtho and Fewtrell 44 ). It is possible that low birth weight is particularly responsible for a smaller proportion of FFM later in life( Reference Singhal, Wells and Cole 43 ). However, one reason why we found birth weight to be equally related to FFM and FM could be due to the very small number of children with birth weights below 2500 g in this cohort.
Length measures of the children at 0 and 5 months resulted in very high HAZ scores, most likely due to a tendency on the part of midwives and general medical practitioners to overestimate length measurements in the primary healthcare sector, as has been demonstrated by other studies( Reference Rifas-Shiman, Rich-Edwards and Scanlon 45 ). The WAZ values were found to be above the WHO standards at all ages. Disregarding WFH and BAZ at birth and 5 months, those values were also found to be above the WHO standards at 9, 18 and 36 months of age. Part of the explanation for this is that the cohort of the present study included infants who had only been breastfed for a short period. However, we have previously shown that the WAZ and BAZ of children from the SKOT cohort who were breastfed at 9 months were closer to the median of the WHO growth standards at 9 and 18 months or less compared to children who were no longer breastfed at 9 months( Reference Madsen, Larnkjaer and Molgaard 32 ). However, duration of full or partial breastfeeding was not found to be directly associated with BMI, FMI or FFMI at 3 years of age in the present study.
Early feeding patterns as effect modifiers
The effect of birth weight on FMI at 3 years of age was eliminated if the child had been fully breastfed for 6 months, as no effect modification was found for less than 6 months' duration of full breastfeeding. Duration of full breastfeeding was found to have a modifying effect on the impact of early growth on FMI at 3 years of age, although this effect modification was only of borderline significance for 1–3 and 4–5 months. These findings indicate that, apart from the direct effect of breastfeeding on early growth( Reference Dewey 11 , Reference Victora, Morris and Barros 12 , Reference Madsen, Larnkjaer and Molgaard 32 ), a longer duration of full breastfeeding directly influences the extent to which early rapid weight gain adversely affects FM development. The Dortmund Nutritional and Anthropometric Longitudinally Designed Study (DONALD)( Reference Karaolis-Danckert, Gunther and Kroke 46 ) showed a similar protective effect of full breastfeeding for at least 4 months with respect to body fat percentage among 249 infants, ranging from 2 to 5 years of age with early rapid weight gain. Preliminary data from the Promotion of Breastfeeding Intervention Trial (PROBIT) also showed a strong relationship between early rapid weight gain and obesity at 6–7 years of age, to be higher among children who had been exclusively formula-fed from 1 month of age, compared to other infants( Reference Manasseh, Kramer and Dewey 47 ).
As the present study is observational, we cannot say for certain whether the effect modifications observed can be explained by physiology, decisions made by parents or advice given by healthcare workers. Also unrecognised confounders might still be operating. The duration of full breastfeeding and age of introduction to solids are by nature interdependent. Infants in the SKOT cohort partially breastfed still at 9 months had been introduced to complementary foods later than non-breastfed infants at 9 months( Reference Gondolf, Tetens and Michaelsen 37 ). Moreover, at this point of time, non-breastfed infants in this cohort had a higher intake of protein in their complementary diet, compared to partially breastfed infants( Reference Gondolf, Tetens and Michaelsen 37 ) who were also found to modify the effect of early growth on FM development( Reference Karaolis-Danckert, Gunther and Kroke 46 ). The fact that we did not see a direct association between early feeding and FMI could be due to the insufficient power and the low number of overweight children in the present study.
The relationships between FFMI, birth weight and early growth were not affected by infant nutrition in the present study. Thus, we hypothesise that factors in pregnancy, the child's level of physical activity and dietary quality beyond infancy have a greater impact on FFMI.
Strengths and limitations
In the present study, FM and FFM were predicted using predictive equations generated from a large number of DXA scans in the same cohort. This was done to facilitate inclusion of more children in the analyses, as we had acceptable DXA scans from only 101 children and bioelectrical impedances of 233 children( Reference Ejlerskov, Jensen and Christensen 36 ). Compared with FFM and FM measured by DXA, the predicted FFM and FM showed a prediction error of 2·8 % (333 g) for FFM, and a prediction error of 11·3 % (284 g) for FM( Reference Ejlerskov, Jensen and Christensen 36 ). The observed associations are based on body composition of 3-year-old children, and it is possible that the effect modifications of infant feeding on early growth observed in the present study do not persist beyond childhood. However, we surmise that FMI of 3-year-old children is a good predictor of later risk of obesity. Other studies have shown a high level of tracking of FM from 2 to 7 years( Reference Karaolis-Danckert, Buyken and Bolzenius 41 ) and 4 to 9 years( Reference Goulding, Taylor and Jones 48 ), with a higher increase of FMI among those who acquired a high fat percentage earlier in life( Reference Karaolis-Danckert, Buyken and Bolzenius 41 , Reference Goulding, Taylor and Jones 48 ). A positive feature of the present study is the prospective and detailed information it obtained with regard to growth. The data of the study on the duration of breastfeeding and age of introduction to solids was collected retrospectively at 9 months. Unfortunately, the data on age of introduction to solids was crude (in months) and did not allow for a more detailed grouping of the ages of introduction. The main limitation of the present study is its small number of children fully breastfed for 6 months (n 21), as this group appears to drive the significant effect modifications. Breastfeeding is commonly associated with other beneficial life-style factors in western societies( Reference Toschke, Martin and von Kries 19 ), and with an observational study design, it will always be a risk to conjecture that the children breastfed for 6 months differ from children being breastfed for a shorter period of time with other characteristics.
In contrast to many other countries, full breastfeeding for 4–6 months is common in Denmark. Further, the families in the SKOT study are characterised by high education levels and high annual incomes, both the factors associated with longer durations of breastfeeding and later ages of introduction to solids( Reference Toschke, Martin and von Kries 19 ). In contrast to other studies( Reference Ong, Emmett and Noble 28 , Reference Tarrant, Younger and Sheridan-Pereira 29 ), most children in the SKOT study were introduced to solids at 4 months of age or later. Despite this relative homogeneity, infant feeding practices were found to modify the effect of early growth. The effect modifications of early feeding are likely to depend on alternatives to breastfeeding that differ from country to country. The infant feeding practices observed in the SKOT cohort are likely to differ from other populations worldwide. We desire that further studies be undertaken to investigate similar effect modifications of infant feeding on early growth that might lead to a global generalisation of the findings of the present study, which might be applicable to populations of different characteristics.
Conclusion
In conclusion, the present study found that birth weight and weight gain in the first 5 months of life had a strong influence on later body composition both in terms of FM and FFM. None of the other periods, from 5 months to 3 years of age, showed significant relations with FM and FFM at 3 years of age. Moreover, we have demonstrated that full breastfeeding for 6 months eliminated the impact on FMI at 3 years of age of both high birth weight and early growth in the first 5 months. Since the results are based on an observational study, causal effects can only be conjectures. However, the complex relation suggests that breastfeeding could have an additional protective effect apart from a direct effect on early growth. Thus, if infants with high birth weight or with rapid weight gain during the first few months are still fully breastfed, it seems reasonable to encourage the mother to continue full breastfeeding for about 6 months. Infant feeding was found not to modify the effect of early growth on FFMI. The results of our investigation suggest that early feeding can be important to modify the effect of high birth weight and excessive weight gain in early infancy, and support current recommendations concerning the duration of full breastfeeding.
Acknowledgements
We are very grateful to the children and families of the SKOT cohort for their involvement in the study. We are thankful as well to Anja Lykke Madsen and Laurine Bente Schram Harsløf, technical staff of the Department of Nutrition, Exercise and Sports, for their involvement in the data collection.
The SKOT study was supported by grants from The Danish Directorate for Food, Fisheries and Agri Business as part of the ‘Complementary and young child feeding (CYCF) – impact on short- and long-term development and health’ project (to K. F. M.). The Danish Directorate for Food, Fisheries and Agri Business had no role in the design, analysis, findings or writing of this article.
The authors' contributions are as follows: the study was designed by K. F. M., C. M., and K. T. E., and L. B. C. conducted the 3-year data collection examination. K. T. E., K. F. M., and C. M. formulated the research question. K. T. E. analysed the data and wrote the first draft of the manuscript. S. M. J. and C. R. supervised the quality standards of the statistical analyses. All authors contributed to the interpretation of the results and commented on the drafts. K. T. E. has primary responsibility for the final content. All authors have read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.












