Introduction
The balsam twig aphid Mindarus abietinus Koch (Hemiptera: Aphididae) is one of the most important pests of the Christmas tree industry in the province of Québec and other parts of eastern Canada, and the United States of America (Deland et al. Reference Deland, Berthiaume, Hébert and Cloutier1998; Berthiaume et al. Reference Berthiaume, Hébert and Cloutier2001; Fondren and McCullough Reference Fondren and McCullough2003). Studies on the phenology of M. abietinus are sparse and focus on distantly related populations throughout North America, including natural and commercial fir (Abies Miller; Pinaceae) plantations (Varty Reference Varty1968; Bradbury and Osgood Reference Bradbury and Osgood1986; Deland et al. Reference Deland, Berthiaume, Hébert and Cloutier1998; Fondren and McCullough Reference Fondren and McCullough2003). With the potential ecological stresses inflicted upon plant and insect populations by global warming in the 21st century (Romero-Lankao et al. Reference Romero-Lankao, Smith, Davidson, Diffenbaugh, Kinney and Kirshen2014), studying the interactions between this phytophagous insect and its host plant in a hotter environment could illustrate their potential effects on the phenology, population dynamics, and future infestation levels of insect pests (reviews of Joschinski et al. Reference Joschinski, Hovestadt and Krauss2015; Tang et al. Reference Tang, Körner, Muraoka, Piao, Miaogen, Thackeray and Yang2016). In addition, the question if whether the growth rates of M. abietinus colonies could vary between host species has not yet been addressed, due in part to a lack of knowledge on the resistance of conifers to their potential insect pests, and the relatively complex life cycle of aphids (DeHayes Reference DeHayes1981; Edwards et al. Reference Edwards, Jesson, Quiring, Weng, Johns and Park2016).
The life cycle of the holocyclic, non-host-alternating M. abietinus is complex, including five morphs and three to four partly overlapping generations. In southern Québec, the stem mother (fundatrix) hatches from the overwintering egg in early May, typically before fir bud break has occurred (Deland et al. Reference Deland, Berthiaume, Hébert and Cloutier1998). Shortly after bud break, immature stem mothers migrate onto the new shoot to start feeding on the growing needles and complete nymphal development. The adult stem mother produces viviparously the second generation, which develop into mature aphids of two distinct morphs: the wingless viviparous daughters (G2 apterae or fundatrigeniae) and the alate viviparous daughters (G2 alatae or sexuparae). Both morphs feed and develop colonially on current-year needles of the shoot selected by their mother (Bradbury and Osgood Reference Bradbury and Osgood1986; Deland et al. Reference Deland, Berthiaume, Hébert and Cloutier1998), stunting the growth of new shoots and distorting needles into a curled shape. Once matured, the apterous daughters (G2) of the stem mother produce an additional generation of alate aphids (G3 sexuparae), thus greatly increasing potential colony size, as the fecundity of these daughters is similar to that of the stem mother (Varty Reference Varty1968). According to previous studies, no more than 10% of the second generation mature into G2 apterae, a proportion that may nevertheless strongly influence final colony size (Varty Reference Varty1968; Deland et al. Reference Deland, Berthiaume, Hébert and Cloutier1998). However, ambient temperature, predation, crowding conditions, and other factors may affect the proportion of apterous and alate daughters in an aphid colony (review of Müller et al. Reference Müller, Williams and Hardie2001). Towards the end of June, the winged G2 and G3 sexuparae migrate onto new shoots, where they produce the last, sexually reproducing generation of the cycle, as oviparous females (oviparae) and non-feeding males (both G3 or G4 sexuales). Males and oviparae mate and, during the first week of July, the oviparae each lay one or two overwintering eggs. Hence, the continuous feeding of a colony on the sap of the host tree can damage the host tree at high aphid densities. The deformation of the shoot and its needles usually forms a protective pseudogall around the growing aphid colony, which reduces the aesthetic value of Christmas trees, and causes economic losses for growers (Nettleton and Hain Reference Nettleton and Hain1982; Bradbury and Osgood Reference Bradbury and Osgood1986; Kleintjes et al. Reference Kleintjes, Lemoine, Schroeder and Solensky1999).
In natural forests, M. abietinus is found mainly on the balsam fir, Abies balsamea (Linnaeus) Miller (Pinaceae), a conifer species of Holarctic distribution common to the boreal forest (Patch Reference Patch1910; Varty Reference Varty1966; Martineau Reference Martineau1984; Farjon Reference Farjon1990). Mindarus abietinus has also been reported on at least a dozen other conifer species (Bradbury and Osgood Reference Bradbury and Osgood1986), but in Christmas tree plantations they are known to infest both the balsam fir and the related Fraser fir Abies fraseri (Pursh) Poiret (Pinaceae) (Deland et al. Reference Deland, Berthiaume, Hébert and Cloutier1998; Fondren and McCullough Reference Fondren and McCullough2003), a species occurring naturally at altitudes >1200 m on the southeastern Appalachians (Farjon Reference Farjon1990). Genetic differences between these two host firs and the biogeographical history they share with M. abietinus may play a key role in their susceptibility to this aphid and/or their capacity to tolerate its presence (reviews of Hanover Reference Hanover1975; Strong Reference Strong1979). Differing fir bud break phenology and lack of synchronicity between the hatching, development, and migration of the stem mothers from previous-year foliage to a shoot from the current year may strongly inhibit colony growth, considering that aphid nymphs may not have early access to burgeoning shoots (DeHayes Reference DeHayes1981; Desrosiers Reference Desrosiers1998; Fondren and McCullough Reference Fondren and McCullough2003). For Fraser firs, bud break occurs usually later in the season than balsam firs (Eidt and MacGillivray Reference Eidt and MacGillivray1972), a phenological difference between host tree and aphid that may partly explain the common belief amongst Christmas tree growers that colonies of M. abietinus infest balsam firs in greater densities per shoot than Fraser firs in southern Québec (Dominique Choquette, Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec-Estrie, personal communication). From the many other fir varieties available for Christmas tree growers (e.g., Douglas fir, Concolor fir, Korean fir, etc.), the Canaan fir, Abies balsamea var. phanerolepis (Fernald) (Pinaceae), can be cultivated in Québec for its unique tolerance to cold temperature and soil moisture variation (McKinley Reference McKinley1995). Its close relation to the balsam fir and its later bud break may place this variety between the other two fir species in regard to M. abietinus colony growth and infestation levels.
In southern Québec, the average annual temperature has increased between 0.8 °C and 1.6 °C since the 1960s, accompanied by a longer growing season for plants (Yagouti et al. Reference Yagouti, Boulet, Vincent, Vescovi and Mekis2008). For the coming decades, an increase between 2.4 °C and 4.6 °C of the average annual temperature is predicted if greenhouse gas emissions continue at the current rate throughout the 21st century (Ouranos 2015). An increase in air temperature directly affects the natural history and physiology of poikilotherms such as M. abietinus (van Baaren et al. Reference van Baaren, Le Lann and van Alphen2010), possibly altering their performance relative to the host plant (Flores-Mejia et al. Reference Flores-Mejia, Fournier and Cloutier2014). Even though phenological plasticity can temper the effects of climate change on living organisms (Joschinski et al. Reference Joschinski, Hovestadt and Krauss2015), it could desynchronise the complex life cycles of insects such as M. abietinus and its host trees (Visser and Holleman Reference Visser and Holleman2001). Because of its close dependency on its host, warming temperatures could affect this aphid at any moment throughout the year, especially during the establishment of the stem mother on young shoots and early development of her offspring in pseudogalls. Increasing air temperatures can accelerate the development rates of these aphids and could modify the proportion of G2 apterae, which may lead to much higher colony densities per shoot, thus potentially resulting in greater damage to Christmas trees.
The main objective of this two-year field study was to test the effects of warmer temperatures on colony growth rates and proportions of G2 apterae produced by stem mothers, in field experiments involving semi-protected balsam fir shoots bearing M. abietinus colonies. We hypothesised that warmer ambient temperatures could positively affect the colony growth rate of M. abietinus and also the proportion of G2 apterae. Our second objective was to study the current phenology of M. abietinus in commercial fir plantations of southern Québec, providing an updated understanding on the life history dynamics of this species in relation to the currently grown host fir tree species in Québec plantations. This was achieved by field sampling in four experimental plots situated within three commercial Christmas tree plantations. We hypothesised that colony growth rates and eventual size could be higher on balsam fir than for those found on Fraser and Canaan firs.
Materials and methods
Field study sites and experimental plots
Sampling was performed in four experimental plots situated in three commercial Christmas tree plantations, located within 12 km of each other in the southern part of Québec, Canada. Two of these plantations were located in the Estrie administrative region (plot B1; 45.905026°N, 71.036177°W and plots B2 and C; 45.847515°N, 70.908675°W); the fourth plot was located in the Chaudière-Appalaches administrative region (plot F; 45.941219°N, 70.953575°W). Plot F was composed of Fraser fir, plots B1 and B2 were composed of balsam fir, and plot C was composed of Canaan fir. Each experimental plot consisted of 15 rows of 45 trees averaging 2 m in height, between eight and 10 years old, for a total of 675 trees per plot. Each plot was divided into 45 sections of 15 trees (i.e., three sections per plot row), from which one sample shoot was collected on a randomly selected tree from within each section every collection date, for a total of 45 samples per plot per collection date. Local temperatures were monitored with a HOBO® Pro v2 data logger (Onset, Cape Cod, Massachusetts, United States of America) installed in a tree in the southwestern corner of the plot. During the summer, no pesticides were used, except for herbicides between tree rows.
Climate-warming field experiment
In 2015, 50 shoots bearing an overwintering egg or a newly hatched stem mother nymph were identified in plot B1 (balsam fir) on 8 May, during the egg eclosion period. For logistic reasons, this site was chosen as the experimental plot for its greater density of eggs per shoot and because balsam firs are the most widely cultivated variety in Québec. When an egg or a nymph was found, a 50.8×63.5 cm sheet of Dura-Lar® with a thickness of 0.13 mm (Grafix, Maple Heights, Ohio, United States of America) was wound into a cone-shaped shelter around the shoot. The vertex of the cone was fixated to the branch with strong adhesive tape, leaving a small opening around the stem for potential predators to enter. The base of the cone was closed off with a white polypropylene bouffant cap to allow air circulation (Emerson Summers, Mississauga, Ontario, Canada). These semi-protective shelters provided a miniaturised greenhouse effect around each shoot.
Samples (n=10) randomly selected among previously identified shoots bearing an aphid, consisting of the entire shoot and its semi-protective shelter, were collected weekly during the following five weeks in 2015 (15 May, 20 May, 28 May, 4 June, and 11 June), along with an equal number of unprotected shoots bearing a stem mother and/or an aphid colony, depending on the collection date (i.e., the control group). Collected shoots were then stored and counted in the laboratory like those sampled for field phenology (see below). In the 2016 experiment, a slightly different sampling schedule was adapted to an earlier spring with an increased sample size. On 5 May, 60 shelters were installed in the same plot during the eclosion period and the first sample collection was carried out three weeks later on 26 May. Samples (n=20) were collected once every two weeks for a total of three collection dates (26 May, 9 June, and 23 June) along with an equal number of control shoots. The goal here was to avoid collecting/destroying samples during stem mother development, when shelters still contained only the stem mother and no progeny. Two temperature loggers (HOBO® Pro v2) were installed during both experiments to measure the difference in ambient temperature between a semi-protected shoot and an unprotected one.
Phenology of Mindarus abietinus on different fir species
In 2015, shoot samples (n=45 per plot) were collected biweekly during the development of the stem mother and her colony, from May to July in all four experimental plots (described above). A specific objective here was to more precisely determine the sequence of aphid generations in M. abietinus colonies, so sampling was increased to a daily basis during the first five days of June in all four plots. This period was deemed critical in the determination of the generation to which aphids belong in the colony, based on previous observations (unpublished data). One sample consisted of the two subterminal buds/nodal shoots and the terminal bud/shoot of the current year (Powell Reference Powell1982), and the terminal shoot of the previous year, on which overwintering eggs and newly hatched stem mothers are usually located. Each sample shoot was cut from the middle crown level on the southern sector of the tree and placed separately in 160 mL plastic vials (VWR International, Radnor, Pennsylvania, United States of America), modified with a fine polyester mesh at its base for air circulation. In order to arrest development, all samples were kept at ~2 °C overnight until counted in the laboratory the following day, when aphid morphs were identified and development stage (instar) was determined. For the first two instars, we could not differentiate the apterous from the alate daughters (G2) of the stem mother. Only when G2 daughters reached the third instar could the alate morph be identified to their wingbuds.
Statistical analyses
Data were analysed with SAS 9.4 (SAS Institute, Cary, North Carolina, United States of America), with the following procedures: PROC MIXED was used to compare colony growth rates and proportions of G2 apterae in the natural versus the semi-protected environment, where fixed effects were treatment (i.e., the semi-protected versus control shoots), date, and the interaction treatment×date, with fir shoot nested within date as random effects. PROC GLIMMIX was used to compare colony growth rates in 2015 depending on host species, where fixed effects were host species, Julian day, and the interaction host species×Julian day, with experimental plot nested within host species as random effects. When required, square root transformations were applied to the data in order to meet the assumptions underlying linear regression analysis.
Results
Climate-warming field experiment (plot B1)
In 2015, a total of 30 semi-protected shoots were collected, along with 44 unprotected shoots (i.e., the control group) bearing a stem mother and/or its aphid colony. Similarly in 2016, a total of 42 semi-protected shoots were collected, along with 39 unprotected shoots. For the semi-protected shoots, the presence of hoverfly larvae (Diptera: Syrphidae: Syrphinae), easily identifiable by their vibrant yellow or green pigmentation, accounted for 6.9% sample loss and strong winds, for 25.4% sample loss. The global average temperature difference inside the semi-protected shelter versus nearby unprotected shoots in the plantation was measured at 1.04±0.09 °C in 2015 and 2.55±0.15 °C in 2016. During the night, temperature differences were smaller than 1 °C during both experiments, as could be expected, but average daily temperatures in the semi-protective shelters often exceeded that of the unaltered ambient temperatures around the unprotected shoots by 5 °C, especially during hot and sunny days (Fig. 1). In 2016, mortality rates observed in the semi-protective shelters averaged 20.0±3.5% per colony (no mortality was observed in 2015).
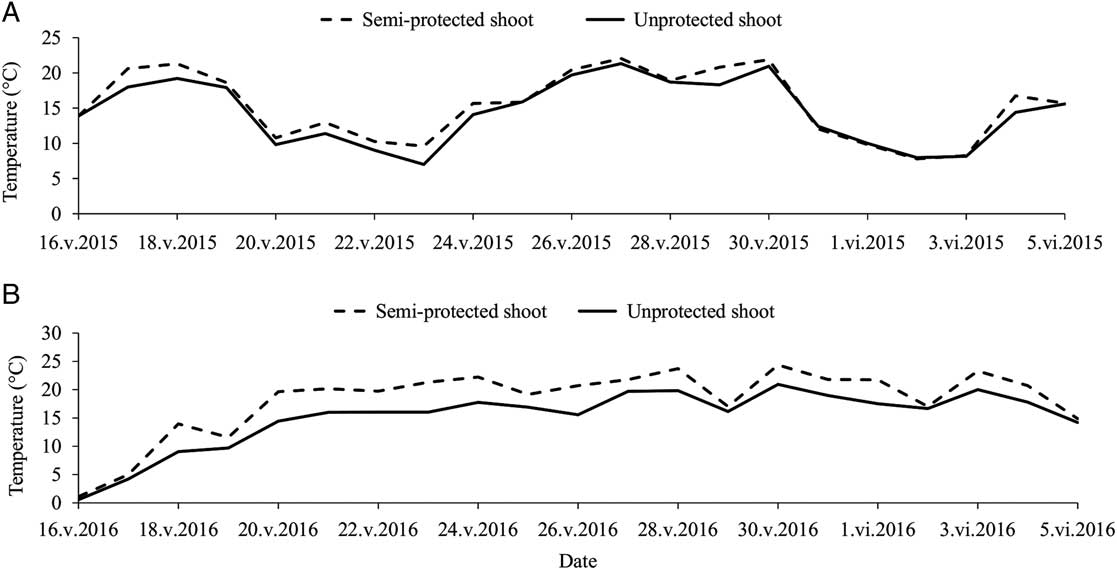
Fig. 1 Average daily temperatures around semi-protected and unprotected balsam fir shoots in spring 2015 (A) and 2016 (B), during development of Mindarus abietinus stem mothers and their offspring.
Colony growth rate in a warmer environment
For both years, warmer air temperatures inside the semi-protective shelter very significantly increased the average number of aphids per infested shoot when compared with the control group (F(1,12)=16.65, P=0.0015 in 2015; F(1,16)=45.07, P<0.0001 in 2016). As expected, initial colonies grew rapidly as the season advanced (F(2,25)=14.97, P<0.0001 in 2015; F(1,20)=24.14, P<0.0001 in 2016), and the colony growth rate was clearly positively affected by the semi-protected environment (F(2,12)=7.46, P=0.0078 in 2015; F(1,16)=8.76, P=0.0092 in 2016) (Fig. 2A–B). In 2016, a notably smaller increase in colony size in early season was observed when compared with a similar date in 2015 (Fig. 2A–B).
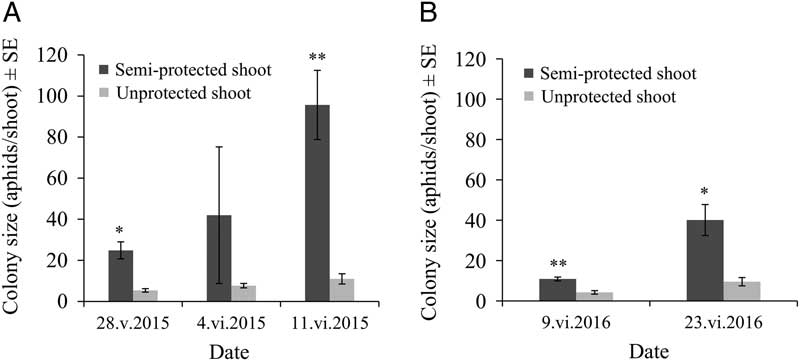
Fig. 2 Mindarus abietinus colony size (mean±standard error) in semi-protected and unprotected balsam fir shoots for 2015 (A) and 2016 (B), where * indicates a significant effect (P=0.0001) and ** indicates a highly significant effect (P<0.0001) of the treatment (increased air temperature around the shoot in the semi-protective shelter). SE, standard error.
Proportion of G2 apterae in a warmer environment
For both years, the semi-protective shelter, causing increased colony size, had no significant effect on the proportion of G2 apterae (F(1,12)=0.04, P=0.8392 in 2015; F(1,21)=2.99, P=0.0983 in 2016), neither did the date (F(2,25)=3.31, P=0.0529 in 2015; F(1,24)=0.16, P=0.6889 in 2016) nor their interaction (F(2,12)=0.31, P=0.7408 in 2015; F(1,21)=0.34, P=0.5642 in 2016) (Fig. 3A–B). Average proportions of G2 apterae for untreated (control) aphid colonies from unprotected shoots varied from 56.2±17.5% to 88.5±6.15% in 2015 (Fig. 3A) and from 50.0±16.7% to 53.9±14.4% in 2016 (Fig. 3B), according to date.
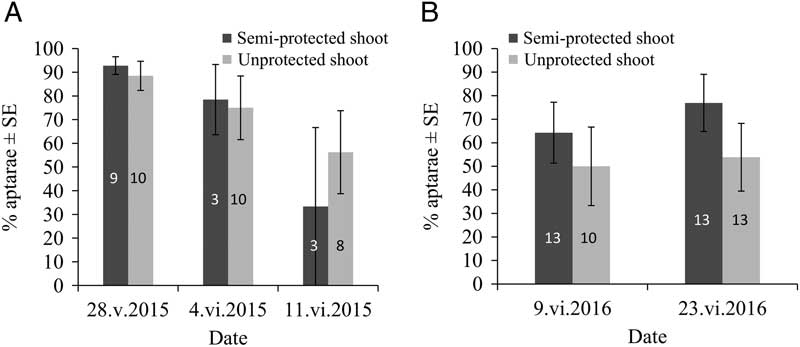
Fig. 3 Mindarus abietinus proportion (mean±standard error) of G2 apterae in semi-protected and unprotected balsam fir shoots for 2015 (A) and 2016 (B). Sample sizes are indicated in the rectangles. SE, standard error.
Phenology of Mindarus abietinus (all four plots)
Phenology on balsam fir
During the 2015 growing season, as aphid densities were most abundant in plot B1 (balsam fir), data from this site best illustrate M. abietinus phenology and density fluctuations in plantations (Fig. 4). During the season, all morphs were observed, except for the adult oviparae, but immature oviparae were observed in mid to late June. Immature stem mothers were observed on the first collection date, and mature individuals were observed in mid-May (see Fig. 4 for details). Overwintering eggs were first observed on current-year shoots at the end of June. Even though aphid densities varied considerably between experimental plots, similar tendencies in phenology were observed (Supplementary Material Figs. S1–S3).

Fig. 4 Variations of average aphid density per shoot (n=45) in 2015 balsam fir plot B1, with percentage of shoots bearing ⩾1 aphids (A); seasonal composition (morph, age group) for Mindarus abietinus colonies and colony size (mean±standard error) (B). Note: the x-axis is not continuous and only represents the series of collection dates. SE, standard error.
Generational separation of G2 and G3 alate daughters
In preliminary studies in 2014 for the same experimental plots (unpublished data), the young nymphs produced by the ageing stem mother (young G2 nymphs) and those from her mature apterous offspring (young G3 nymphs) appeared concurrently and thus could not be separated, despite belonging to successive generations of the cycle. In order to attempt separating the resulting two overlapping generations of alate nymphs in this complex cycle (G2 and G3 alatae), samples from five consecutive dates were collected between 1 and 5 June 2015, when it was possible to directly follow late development of the G2 apterae and the subsequent production of young G3 nymphs (Fig. 4). By correctly separating the G2 and G3 generations of alate daughters, the first young progeny of G2 apterae (i.e., first and second instars), subsequently maturing as G3 alatae, were first observed between 3 and 5 June 2015, depending on experimental plot. Proportions of G2 apterae were thus estimated to vary between 60.0% and 100%, depending on collection date.
Impact of host species (all four plots)
Bud break on balsam fir occurred approximately two weeks before Fraser fir in 2015 (Fig. 5), while the buds of the Canaan fir started to break at the same time as the closely related balsam fir, but in smaller proportions. The relatively low aphid densities and great variability in colony composition between experimental plots made it difficult to precisely quantify colony growth rates according to host tree species (see Fig. 1 and Supplementary Material Figs. S1–S3). We used simple linear regressions of colony size (i.e., the number of aphids per infested shoot) on time (date), which were compared between tree species. Sampling dates occurring later than the first appearance of adult alate daughters (G2 and G3) were excluded from the regression, as these aphids leave the colony by flying and no longer contribute to colony size and growth. No effects included in the regression for colony growth in 2015 were significant (F(2,106)=0.60, P=0.5512, interaction host species×Julian day), nor was the direct effect of host species (F(2,1)=0.59, P=0.6774), and Julian day (F(1,106)=0.04, P=0.8340), indicating that colony size varied little in the early season and was similar between all three host fir species.
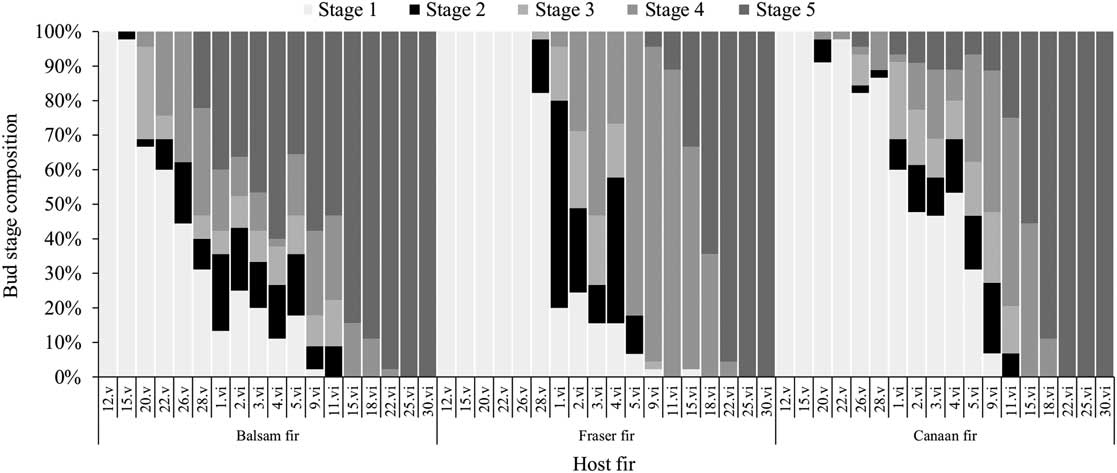
Fig. 5 Terminal bud break phenology according to host species (balsam, Fraser, or Canaan fir) in 2015 (plots B1, F, and C); fir stages 1–5 are based on Osawa et al. (Reference Osawa, Shoemaker and Stedinger1983), wherein stage 2 (i.e., the black rectangles) is when immature Mindarus abietinus stem mothers usually migrate onto the newly exposed shoot of the current year.
Discussion
The positive effect of ambient temperature in the semi-protective shelters on M. abietinus colony size during the early season is demonstrated in the climate-warming field experiment, as the effect of the semi-protective shelters increased colony size substantially. Initial colony growth rates were significantly higher in the experimentally warmer environment, as one would expect for these small insects. However, in 2016, mid-day temperatures exceeding 45 °C were measured in the semi-protected shelter on four separate dates, varying slightly between semi-protected shoots, which could account for the recorded numbers of aphid deaths (i.e., ranging from 3.1–23.9% per colony) in the semi-protected colonies. Such high temperatures are abnormal and could be very stressful for aphids and their nutritional symbionts (Wernegreen Reference Wernegreen2012). They also indicate that our shelters had some limitations in recreating natural climate warming, as it is unlikely that control aphids on unprotected shoots experienced such high temperatures. It is worth noting that factors other than warming may have positively influenced colony size in the semi-protective shelter. For example, a colony of M. abietinus inside a pseudogall may be naturally protected against adverse weather conditions, like heavy rainfall and strong winds (review of Stone and Schönrogge Reference Stone and Schönrogge2003). The semi-protective shelters most likely offered additional protection throughout these unfavourable conditions, resulting in fewer individuals falling from the shoot during rough weather, although, this additional contribution to colony size may be negligible.
Another contributing factor to aphid loss in this experiment was the presence of hoverfly larvae in the semi-protective shelters, which accounted for 6.9% sample loss during both experiments. These shelters were designed to be “semi-protective”, with the small opening at the apex of the cone-shaped shelter, where potential crawling predators such as hoverfly larvae could enter, allowing for natural biological control to occur in pseudogalls. We noted that when one or two hoverfly larvae, usually late instar individuals, were found within the pseudogall, absolutely every aphid in the colony had been consumed, suggesting a voracious appetite for these aphidophagous predators. Interestingly, adult hoverflies captured in Malaise traps deployed nearby in the plantation were identified as species predominantly from the Sphaerophoria LePeletier and Serville and Toxomerus Macquart genera (unpublished data). These hoverflies, along with several species of lady beetles (Coleoptera: Coccinellidae) and green lacewings (Neuroptera: Chrysopidae) can form a diverse community of aphid predators, each group having the potential to become effective biocontrol agents of M. abietinus in commercial Christmas tree plantations, notably because of their general abundance around fir trees (Fondren et al. Reference Fondren, McCullough and Walter2004; Berthiaume et al. Reference Berthiaume, Hébert and Cloutier2007, Reference Berthiaume, Hébert, Pelletier and Cloutier2016).
Average colony size, measured as the number of aphids per infested shoot, remained relatively low across all plantations, despite the absence of insecticidal aphid control in all four plots, when compared with those studied in the mid-1990s (Deland et al. Reference Deland, Berthiaume, Hébert and Cloutier1998). During that period, M. abietinus could sometimes be observed on all sampled shoots in some plantations, where colony size ranged from around 15 to 90 aphids on average per infested shoot at the height of the season. Since the late 1990s, when targeted insecticidal control of M. abietinus became more efficient, due to a better understanding of their phenology, frequent monitoring, and effective pesticide control (i.e., generally with Diazinon 500 EC), population densities for this aphid in commercial fir plantations have remained relatively low in southern Québec (Deland et al. Reference Deland, Berthiaume, Hébert and Cloutier1998; Berthiaume et al. Reference Berthiaume, Hébert and Cloutier2001; Dominique Choquette, Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec-Estrie, personal communication). This remains so for the current study, as the average colony size per infested shoot has dwindled to no more than 10 individuals. We cannot explain how colony size is now so reduced in comparison, but our observations support the idea that natural control by aphid predators could play a role under reduced pesticide control use. However, from a different perspective, studying now the phenology of the balsam twig aphid in commercial fir plantations with low population densities makes it more difficult than before to extract new and important information on the dynamics of this species in Christmas tree plantations.
Seasonal population trends in the complex cycle of M. abietinus did not vary remarkably since last studied almost 20 years ago in southern Québec (Deland et al. Reference Deland, Berthiaume, Hébert and Cloutier1998). However, spring emergence of stem mothers and the appearance of overwintering eggs were observed between three and nine days later than in the mid-1990s. This slight yet consistent difference in phenology could partly be explained by regional differences in temperature, as the experimental sites selected by Deland et al. (Reference Deland, Berthiaume, Hébert and Cloutier1998) were located ~80 km south of the ones used in this study, in a slightly warmer climate implying possibly faster development for the stem mother and her colony, which is also the case for M. abietinus populations studied in lower latitudes like North Carolina, United States of America (Nettleton and Hain Reference Nettleton and Hain1982) and southern Michigan, United States of America (Fondren and McCullough Reference Fondren and McCullough2003).
Preliminary field observations (2014, unpublished data) suggest that colony growth rates on balsam fir could be faster than on Fraser fir, but this was not observed in 2015, probably due to very low densities in all four sampled plots. As buds that break open earlier in the season show more damage than those that break later on (Desrosiers Reference Desrosiers1998; Fondren and McCullough Reference Fondren and McCullough2003), colony size can be notably affected by bud break phenology. Fraser fir buds break open at a later date than those of balsam firs (see Fig. 5), which can contribute to the overall susceptibility of this fir tree towards M. abietinus (DeHayes Reference DeHayes1981; Desrosiers Reference Desrosiers1998). For the upcoming decades, increasing air temperatures may hasten bud break phenology and the access of the developing stem mother to the nutritive young fir shoots, possibly resulting in more colonies of greater density. As overwintering eggs may possibly develop earlier in the future due to advanced diapause termination, well before bud break occurs, global warming may play a decisive role in the relative phenologies of M. abietinus and its host tree, and potential damage to fir shoots by possibly larger colonies.
In 2015 and 2016, the proportion of G2 apterae produced by the stem mother was much higher than what was previously observed in New Brunswick, Canada during the 1960s, and in Québec in the 1990s, when it was <10% (Varty Reference Varty1968; Deland et al. Reference Deland, Berthiaume, Hébert and Cloutier1998). In this study, the correct separation of the two generations of sexuparous alatae (G2 and G3) highlights the potential of the G2 apterae to greatly affect final colony size, as each individual can potentially produce as many alate daughters as their stem mother (Varty Reference Varty1968). Notably, a proportion of G2 apterae that is several times higher than a few decades ago could have substantially increased maximum aphid densities per shoot and the number of alate daughters (G2 and G3) that ultimately migrate onto new shoots, which could increase local colony density and dispersion of the sexuales and the overwintering eggs. The mechanism for M. abietinus morph determination among stem mother progeny has not been studied, but a possible explanation for the higher proportions of G2 apterae observed here in the field are the initial crowding conditions during the development of the stem mother and that of her colonial offspring.
Tactile stimulation between stem mothers accessing newly opening buds (i.e., pre-natal control) and/or her nymphs (i.e., post-natal control) could favour wing development in G2 aphids, a plastic phenotypic trait that varies between Aphididae species (Johnson Reference Johnson1965; Lees Reference Lees1967; Mehrparvar et al. Reference Mehrparvar, Zytynska and Weisser2013). Though not studied to our knowledge for phylogenetically distant Mindarinae conifer aphids such as M. abietinus, this capacity to induce wing formation by contact between stem mothers and/or their progeny could explain why higher proportions of G2 apterae were observed here than in previous studies in nearby regions. When shoots were examined in the 1990s, before properly timed insecticidal control was practised in southern Québec, up to dozens of eggs could be observed on a single shoot (Deland et al. Reference Deland, Berthiaume, Hébert and Cloutier1998). If several M. abietinus stem mothers were simultaneously competing for access and feeding sites on the same opening bud or newly exposed shoot before starting a colony, their close contact and/or subsequent crowding among the early instar progeny could favour wing development and result in lower proportions of G2 apterae.
Other factors such as predation, nutrition, and temperature may also play a role in the induction of wing formation for developing aphids (Müller et al. Reference Müller, Williams and Hardie2001). It has been shown that relatively high temperatures favour apterous aphid morph determination (Schaefers and Judge Reference Schaefers and Judge1971; Liu Reference Liu1994), which contradicts the general assumption that activity, therefore tactile stimulation, increases in a hotter environment. But such an indirect effect of temperature can be ruled out in this study, as proportions of G2 apterae in semi-protective shelters (i.e., a hotter environment during the day) did not vary significantly from those on unprotected shoots. Thus, a combination of biotic and abiotic factors is probably necessary for morph determination in a colony of M. abietinus, but we suggest that initial density of stem mothers and their colonial offspring is a factor, which needs further study.
This study has shed new light on M. abietinus ecology and its complex interactions with host tree phenology and temperature variation in relation to seasonal and regional climate. We showed that semi-protective shelters, thus likely providing a warmer environment, has a positive effect on incipient colony growth rates of M. abietinus on balsam fir, which would increase damage to fir shoots. In the following decades, increasing air temperatures brought on by climate change may benefit this species and reinforce its status as an important pest in commercial Christmas tree plantations. For future studies, it would be interesting to test the effects of temperature on the development of the stem mother in relation to fir bud break. Colony morph composition as a function of stem mother density and fecundity should also be studied, as this may play an important role in determining the proportion of G2 apterae. This proportion could have a large effect on the eventual colony size and the density of M. abietinus overwintering eggs. Our preliminary field observations suggest that M. abietinus colonies could infest balsam fir shoots at a higher rate than Fraser fir shoots, which may be partly explained by the slight asynchrony between the development of the stem mother and the bud break phenology of Fraser firs, but this needs further study. For the first time, we report data that separate both overlapping generations of alate daughters in colonies initiated by stem mothers, which led to observing a much higher proportion of G2 apterae than previously reported for this aphid.
Acknowledgements
The authors thank Simon-Charles Blouin, William Champagne-Cauchon, and Jonathan Franchomme (Université Laval) for their assistance in the field and in the laboratory. For statistical help, the authors thank Aurélien Nicosia (Université Laval). They also thank Dominique Choquette from the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ-Estrie), and Émilie Turcotte-Côté (Club agroenvironnemental de l’Estrie) for their positive input throughout the project. The authors are thankful to Gérald Couture (Québec Balsams Export) for allowing them to use his installations during the project and to sample in his plantations. This study was supported by the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec – Programme Innov’Action agroalimentaire grant #IA113043 to Conrad Cloutier.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.4039/tce.2017.41







