Postmenopausal osteoporosis (PMOP) is by far the most common cause of age-related bone loss and is recognised as one of the leading health care problems in the world( Reference Devareddy, Hooshmand and Collins 1 ). PMOP is characterised by increased bone resorption, which in turn leads to increased bone fragility and fractures( Reference Alexander, Bab and Fish 2 ). In addition, hip and spine fractures are associated with particularly high morbidity and mortality rates in POMP patients( Reference Doherty, Sanders and Kotowicz 3 ).
It is well known that ovarian hormone deficiency is responsible for the development of PMOP. There is a strong association between POMP and sex, and the female:male ratio of hip fractures is greater than 2:1 in people aged above 50 years( Reference Curran, Maravic and Kiefer 4 ).
The worldwide health and economic burden of osteoporosis is likely to increase in the future, as improvements in life expectancy will lead to an increase in the population of elderly people with a high risk of fractures. The population of people aged above 65 years has been predicted to increase from 58 million in 1995 to 108 million in 2040 in fifteen member states of the European Union. In particular, it has been estimated that the population aged above 80 years, in whom the incidence of osteoporotic fractures is highest, will increase from 8·9 million women and 4·5 million men in 1995 to 26·4 million women and 17·4 million men in 2050( Reference Curran, Maravic and Kiefer 4 ). It has also been estimated that 10 million Americans aged above 50 years have osteoporosis and that a further 34 million are at a risk of the disease. An estimated 1·5 million fragility fractures occur every year. While most American women aged below 50 years have normal bone mineral density (BMD), 27 % are osteopenic and 70 % are osteoporotic at the hip, lumbar spine or forearm by the age of 80 years( Reference Holroyd, Cooper and Dennison 5 ).
Modern therapy recommended for the treatment of PMOP includes supplementation with oestrogen and calcitonin, etc. However, the therapeutic management has several downsides, and available evidence suggests that the long-term use of oestradiol replacement therapy (ERT) may lead to serious side effects such as breast or uterus cancer( Reference Beral, Reeves and Bull 6 , Reference Chlebowski, Anderson and Manson 7 – Reference Zhao, Liu and Zhang 9 ). Guidelines state that oestrogen is to be used for menopausal symptom relief and be given for the shortest duration possible( Reference Grady and Sawaya 10 ). Scientists throughout the world are looking for better alternative therapeutic management, especially from natural resources that can provide greater symptom relief with minimal risks, and preventive strategies of optimising early gains of bone mass are being preferred( Reference Cheng, Wang and Fan 11 ).
Resveratrol (3,5,4′-trihydroxystilbene, Res), a non-flavonoid polyphenol that is abundant in grapes and a variety of medicinal plants, exhibits multiple biological activities( Reference de la Lastra and Villegas 12 , Reference Delmas, Jannin and Latruffe 13 ). It belongs to the group of phyto-oestrogens called selective oestrogen receptor modulators and exhibits a weak oestrogenic activity( Reference Tasci, Bilgili and Altunay 14 ). In addition, Res has been shown to have significant antioxidant properties( Reference Berrougui, Grenier and Loued 15 – Reference Fan, Liu and Qian 17 ). Furthermore, previous studies have reported that Res can enhance proliferation and osteoblast differentiation in human mesenchymal stem cells via ER-dependent extracellular-signal-regulated kinases 1/2 activation( Reference Dai, Li and Quarles 18 ). He's study( Reference He, Andersson and Lindgren 19 ) has indicated the preventive effect of Res on the receptor activator of nuclear factor κB ligand (RANKL)-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through the inhibition of reactive oxygen species production. Other studies have reported that Res suppresses microcirculation levels in the osteal tissue( Reference Faitel'son, Koklina and Gudyrev 20 ) and promotes osteogenesis in human mesenchymal stem cells( Reference Tseng, Hou and Chen 21 ). Furthermore, Res can attenuate ovariectomy (OVX)-induced hypertension and bone loss in stroke-prone spontaneously hypertensive rats and regulate bone morphogenetic protein-2( Reference Mizutani, Ikeda and Kawai 22 , Reference Su, Yang and Zhao 23 ).
Based on the available information( Reference Liu, Li and Yu 24 , Reference Sehmisch, Hammer and Christoffel 25 ), we presumed that Res would prevent bone loss in ovarian hormone deficiency. Therefore, the present study was designed to examine the extent to which Res prevents bone loss leading to osteopenia in OVX rats and the underlying mechanisms. Furthermore, the pathological change in the endometrium and lumen of the uterus of the Res-treated rats compared with that in the uterus of the ERT rats was investigated.
Materials and methods
Resveratrol
Res was obtained from Xi'an Helin Biological Engineering Company, Limited. Its purity was greater than 98 % when checked by HPLC, and the analysis was carried out by Xi'an Helin Biological Engineering Company, Limited.
Animals and treatments
A total of sixty virgin adult (3–4 months old) female Wistar rats weighing 200–220 g were used, and the present study was approved by the animal service of Shanxi Medical University. The rats were bred under conditions of controlled temperature (22 ± 0·5°C), humidity (50 ± 10 %) and lighting (12 h light–12 h dark cycle, light period began at 07.00 hours). All the rats were given free access to distilled water and fed standard rat chow. The animal care and experimental procedures were carried out in accordance with the Guidelines for Animal Experimentation of Shanxi Medical University, with the approval of the Institutional Animal Care and Use Committee.
The acclimatised rats were subjected to either bilateral laparotomy (Sham, n 10) or bilateral OVX (OVX, n 50). After recovery from surgery, the OVX rats were randomly divided into five groups: OVX rats as a control group (OVX, n 10); OVX rats receiving oestradiol valerate (ERT, n 10, 0·8 mg/kg); OVX rats receiving Res at graded doses (Res 20 mg/kg, n 10; Res 40 mg/kg, n 10; and Res 80 mg/kg, n 10). Based on the Human Rat Equivalent Dose Conversion Principle( Reference Huang, Huang and Chen 26 , 27 ), the experimental dose of oestradiol valerate used in the present study was equivalent to the corresponding clinical prescription dose for a 60 kg human subject. Res and oestradiol valerate were orally administered through a custom-made stomach tube for 12 weeks. During the experiment, the weight of the rats was measured once a week.
Tissue preparation
After Res treatment, rats in all the groups were subjected to an overnight fast, and blood samples were collected from the external jugular vein( Reference Ege, Parra and Johnson 28 – Reference Terry, Kim and Li 30 ). The blood samples were collected in microcentrifuge tubes and allowed to clot at room temperature. The blood samples were centrifuged (3000 rpm for 5 min), and then serum was separated and stored at − 80°C until the determination of alkaline phosphatase (ALP), tartrate-resistant acid phosphatase (TRAP), transforming growth factor-β1 (TGF-β1), fibroblast growth factor (FGF), IL-6 and TNF-α concentrations. Both femurs of each rat were dissected for the measurements of BMD and morphological and physical parameters.
Analysis of serum and urine chemistry
An increase in urinary Ca excretion as well as a decrease in Ca absorption efficiency might lead to the reduction of BMD. P plays an important role in the process of bone metabolism, and it can promote the synthesis of bone matrix and the deposition of inorganic salts. In addition, ALP and TRAP are considered to be bone turnover markers( Reference Mizutani, Ikeda and Kawai 22 ). Bone destruction and inflammation are closely associated( Reference Moon, Ahn and Jung 31 ).
Serum Ca and serum P concentrations were measured by the ortho-cresol phthalein complexone method and the ammonium molybdate method using an autoanalyser, respectively( Reference Tsuruoka, Wakaumi and Yamamoto 32 ). Urinary Ca and urinary P concentrations were measured by the method used for the serum samples.
ALP and TRAP concentrations were measured using a commercial kit and a semi-autoanalyser (Jiancheng Institute of Biotechnology). In addition, TGF-β1, FGF, IL-6 and TNF-α concentrations were measured using an ELISA kit (Senxiong Biotech) according to the manufacturer's instructions. All assays were carried out in duplicate.
Femur physical parameters
Fresh isolated left femur was weighed using an electronic scale. Femoral length and external diameter were measured using digital slide calipers. Femoral length was measured from the proximal tip of the head of the femur to the distal tip of the medial condyle. The external diameter of the femur was measured at the midshaft. The dry weight of bone can indirectly reflect the mineral content in the bone, so the femur was dried at 110°C for 12 h, and the weight of the dried femur was determined after measuring the above-mentioned parameters( Reference Zhang, Yu and Ao 33 ).
Bone densitometry
After removing the soft tissue, the right femur of each rat was subjected to densitometric (dual-energy X-ray absorptiometry technique) measurements of BMD. For the measurements, the bone was placed on its posterior surface in a thin-walled plastic container filled with 0·9 % NaCl and scanned using a Lunar Prodigy densitometer with a built-in small animal mode (GEMedical Systems). BMD was measured at the distal end of the femur (region 1 cm in length from the most distal point of the bone), femoral neck and femoral diaphysis (mid-diaphyseal region). The CV for the measurements (determined by thirty separate scans) was < 3 %( Reference Xu, Han and Li 34 ). All the measurements were taken by the same investigator.
Bone morphology and histology
Samples were obtained from the right distal femur of rats in each group and used as decalcified specimens for bone histomorphometric analysis. The femur samples were fixed in 10 % phosphate-buffered formalin for 24 h, dehydrated in a vacuum desiccator with graded ethanol, and then defatted in xylene and embedded in methyl methacrylate. Sections (4 μm thick) were cut with a rotary microtome (Weswox Optik) and stained with haematoxylin–eosin for trabecular bone histomorphometric analysis( Reference Jing, Shen and Huang 35 ).
In the present study, the static measurements included the following parameters: the percentage of trabecular area (Tb.Ar); trabecular thickness (Tb.Th); trabecular number (Tb.N); trabecular separation (Tb.Sp).
Determination of the expressions of IL-6, TNF-α, osteoprotegerin and receptor activator of nuclear factor κB in the femur by immunohistochemistry
To determine the expressions of IL-6, TNF-α, osteoprotegerin (OPG) and RANKL in the femur, sections were deparaffinised using xylene and rehydrated using a graded series of alcohol concentrations. Endogenous peroxidase was quenched using 3 % hydrogen peroxide for 10 min. Non-specific binding of epitopes was blocked using 1:10 normal blocking serum. Slides were incubated at 4°C overnight with a 1:100 dilution of rat IL-6, TNF-α, OPG and RANKL primary antibodies (Boster Bio-Engineering Company, Limited). Sections were washed and incubated with biotinylated secondary antibodies (Boster Bio-Engineering Company, Limited) for 30 min followed by incubation with peroxidase substrate for 10 min. The sections were washed and incubated in deionised water for 5 min, counterstained with haematoxylin–eosin and observed under a light microscope. The expressions of these proteins were estimated by counting immunostained cells (percentage of cells), and the average score was used for the statistical analysis. Femurs of three rats were studied. For each rat, five slides and five fields in every slide were read, and all the slides were scored by two observers in a blinded fashion( Reference Cheng, Wang and Fan 11 ).
Histological evaluation of the uterus
The body of the uterus was cut right/just above the junction of the cervix. The uterus was stripped of fat and connective tissues. In addition to absolute weight, the relative weight (uterus weight/body weight (BW)) of the uterus of each rat was also calculated (mg/g).
Samples collected from the middle portions of both uterine horns were fixed in 10 % neutral buffered formalin for 24 h. Paraffin-embedded sections (6 mm; Leica SM2000R; Wyzner) were mounted on silanised slides, deparaffinised in xylene (3 × 5 min), hydrated in a series of graded ethanol solutions, and washed in Tris–HCl buffer (pH 7·6). The general appearance and the pathological changes in the endometrium and lumen of the uterus were observed using a light microscope.
Statistical analysis
All the data are expressed as means and standard deviations. Statistical analysis was carried out using one-way ANOVA, followed by the least significant difference (LSD) test (equal variances or homogeneity of variance assumed after variable transformation) or Dunnett's T3 (equal variances not assumed after variable justification) for post hoc testing between groups. Data were considered to be statistically significant when a P value < 0·05 was achieved. All the tests were carried out using the SPSS 13.0 software.
Results
Effect of resveratrol on body weight and food intake
No deaths or obvious clinical signs (such as activity decrease, hair loss and diarrhoea) were observed in any of the groups throughout the experimental period. Daily food intake per rat did not differ among any of the groups: OVX: 21·47 (sd 1·19) g/d; Sham: 19·55 (sd 1·69) g/d; Res 20 mg/kg: 20·49 (sd 3·23) g/d; Res 40 mg/kg: 20·77 (sd 1·72) g/d; Res 80 mg/kg: 23·15 (sd 1·17) g/d; ERT 20·03 (sd 1·98) g/d.
BW was analysed using two-way ANOVA with repeated measures and the factors of group and time. The six groups of rats had a similar initial mean BW (P>0·05 among all the groups). However, during week 4 after surgery, the BW of the OVX group was significantly higher than that of the Sham group. The BW of the OVX group continued to increase significantly throughout the study period when compared with that of the Sham group. Oestradiol administration completely prevented the increase in BW associated with oestradiol deficiency, and the BW levels returned to levels observed in the Sham group 4 weeks after treatment. In the Res 20, 40 and 80 mg/kg groups, no significant effects of Res on BW were observed (OVX and Res 20, 40, 80 mg/kg v. ERT and Sham: P <0·05; Res 20, 40, 80 mg/kg v. OVX: P>0·05; ERT v. Sham: P>0·05) (Fig. 1).

Fig. 1 Effect of resveratrol (Res) on body weight. The body weight (BW) of the rats was recorded weekly during the experimental period. Values are means, with standard deviations represented by vertical bars, and they were analysed by two-way ANOVA with repeated measures and the factors of group and time. All the six groups of rats had a similar initial mean BW (P>0·05 among all the groups). However, during week 4 after surgery, the BW of the ovariectomy (OVX, ![]() ) group was significantly higher than that of the Sham (
) group was significantly higher than that of the Sham (![]() ) group. The BW of the OVX group continued to increase significantly throughout the study period when compared with that of the Sham group. Oestradiol administration completely prevented the increase in BW associated with oestradiol deficiency and the BW levels returned to the levels observed in the Sham group 4 weeks after treatment. All the three doses of Res (Res 20 mg/kg (
) group. The BW of the OVX group continued to increase significantly throughout the study period when compared with that of the Sham group. Oestradiol administration completely prevented the increase in BW associated with oestradiol deficiency and the BW levels returned to the levels observed in the Sham group 4 weeks after treatment. All the three doses of Res (Res 20 mg/kg (![]() ), Res 40 mg/kg (
), Res 40 mg/kg (![]() ) and Res 80 mg/kg (
) and Res 80 mg/kg (![]() ) groups) had no significant effects on BW (OVX and Res 20, 40 and 80 mg/kg groups v. oestradiol replacement therapy (ERT,
) groups) had no significant effects on BW (OVX and Res 20, 40 and 80 mg/kg groups v. oestradiol replacement therapy (ERT, ![]() ) and Sham groups: P <0·05; Res 20, 40 and 80 mg/kg groups v. OVX group: P>0·05; ERT group v. Sham group: P>0·05). * Mean value was significantly different from that of the OVX group (P <0·05). † Mean value was significantly different from that of the Sham group (P <0·05). (A colour version of this figure can be found online at http://journals.cambridge.org/bjn).
) and Sham groups: P <0·05; Res 20, 40 and 80 mg/kg groups v. OVX group: P>0·05; ERT group v. Sham group: P>0·05). * Mean value was significantly different from that of the OVX group (P <0·05). † Mean value was significantly different from that of the Sham group (P <0·05). (A colour version of this figure can be found online at http://journals.cambridge.org/bjn).
Femoral length, diameter, wet weight and dry weight
ANOVA indicated that the wet weight and dry weight of the femurs were significantly different among all the groups (F(5,36) = 2·871, P <0·05, for wet weight; F(5,36) = 3·124, P <0·05, for dry weight). However, femoral length and diameter were not significantly different among all the groups (F(5,36) = 1·021, P>0·05, for femoral length; F(5,36) = 1·924, P>0·05, for femoral diameter). In addition, the dry weights of the femurs in the Res 80 mg/kg group and the ERT group were significantly higher than those of the femurs in the OVX group (Table 1).
Table 1 Effects of resveratrol (Res) on the femoral size and weight of rats‡ (Mean values and standard deviations)
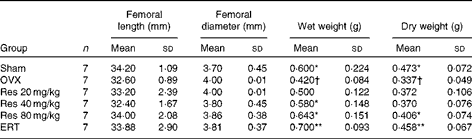
OVX, ovariectomy; ERT, oestradiol replacement therapy.
Mean values were significantly different from those of the OVX group: *P< 0·05, **P< 0·01.
† Mean values were significantly different from those of the Sham group (P< 0·05).
‡ Wet weight, dry weight and femoral length and diameter were determined at the time of sampling of the left femur.
Femoral bone mineral density
The BMD of the neck and distal end of the femur in the Res 40 and 80 mg/kg groups and the ERT group was significantly higher than that in the OVX group, with no significant changes being observed in the femoral diaphysis. No statistical significance was observed for BMD between the Res 20 mg/kg group and the OVX group (Fig. 2).

Fig. 2 Effect of resveratrol (Res) on bone mineral density. After Res treatment, the right femur of each rat was dissected and bone mineral density was measured by dual-energy X-ray absorptiometry. Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the ovariectomy (OVX, ■) group (P <0·05). †† Mean value was significantly different from that of the OVX group (P <0·01). ![]() , Sham control group;
, Sham control group; ![]() , Res 20 mg/kg group;
, Res 20 mg/kg group; ![]() , Res 40 mg/kg group;
, Res 40 mg/kg group; ![]() , Res 80 mg/kg group;
, Res 80 mg/kg group; ![]() , oestradiol replacement therapy group.
, oestradiol replacement therapy group.
Effect of resveratrol on bone morphology and histology
The histology of the femurs of the Sham rats (demineralised with EDTA and stained with haematoxylin and eosin) showed normal bone microarchitecture, when compared with that of the femurs of the OVX rats, where pathological alterations were observed. However, restoration of bone microarchitecture was observed in the Res 80 mg/kg-treated rats, when compared with the OVX rats. The ERT rats also exhibited restoration (Fig. 3).

Fig. 3 Effect of resveratrol (Res) on bone architecture. After Res treatment, the right distal femur of each rat was dissected, and bone architecture was observed under a bright-field microscope after staining the sample with haematoxylin and eosin at 200 × magnification. (a) Normal bone architecture in the Sham group; (b) altered bone architecture in the ovariectomy group; (c) partially recovered bone architecture in the Res 80 mg/kg group; (d) the bone architecture in oestradiol replacement therapy group. (A colour version of this figure can be found online at http://journals.cambridge.org/bjn).
The histomorphometric quantitative changes of the trabecular bone mass and bone architecture in the sections of the right distal femur are summarised in Table 2. The OVX group exhibited a remarkable decrease in Tb.Ar, Tb.Th and Tb.N (P <0·05 for Tb.N; P <0·01 for Tb.Th and Tb.Ar) and an increase in Tb.Sp (P <0·01) compared with the Sham group. Res administration partially reversed these changes (Res 40 mg/kg v. OVX: P <0·05; Res 80 mg/kg v. OVX: P <0·05 for Tb.Sp). Furthermore, in the ERT group, a better efficacy for the prevention of the decrease in Tb.Ar, Tb.Th and Tb.N and the increase in Tb.Sp was observed when compared with the Res groups (ERT v. OVX: P <0·01 for all the indices).
Table 2 Changes in the trabecular bone histomorphometric parameters of rats† (Mean values and standard deviations)
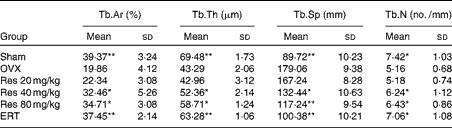
Tb.Ar, trabecular area; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; Tb.N, trabecular number; OVX, ovariectomy; Res, resveratrol; ERT, oestradiol replacement therapy.
Mean values were significantly different from those of the OVX group; *P< 0·05, **P< 0·01.
† After Res treatment, samples were obtained from the right distal femur and Tb.Ar, Tb.Th, Tb.Sp and Tb.N were determined. Femurs of three rats were studied. For each rat, five slides and five fields in every slide were read, and all the slides were scored by two observers in a blinded fashion.
Serum and urine chemistry
There were no significant differences in serum Ca, serum P and TRAP concentrations among all the groups (F(5,36) = 0·434, P>0·05, for serum Ca; F(5,36) = 0·762, P>0·05, for serum P; F(5,36) = 1·561, P>0·05, for TRAP), while urinary Ca, urinary P and ALP concentrations in the OVX group were significantly increased compared with those in the Sham group (P <0·01 for both). In addition, Res 40 and 80 mg/kg administration significantly attenuated the increase in urinary Ca, urinary P and ALP concentrations in the OVX group (P <0·05 or 0·01) (Table 3).
Table 3 Effects of resveratrol (Res) on the biochemical parameters of rats† (Mean values and standard deviations)
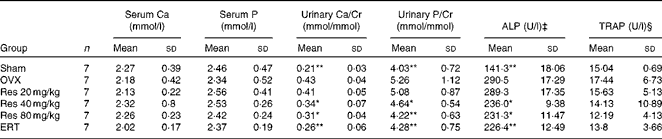
Cr, creatinine; ALP, alkaline phosphatase; TRAP, tartrate-resistant acid phosphatase; OVX, ovariectomy; ERT, oestradiol replacement therapy.
Mean values were significantly different from those of the OVX group: *P< 0·05, **P< 0·01.
† After Res treatment, blood and urine samples were collected and the biochemical parameters were measured.
‡ 1 μ ALP = 1 mg phenol produced when 100 ml serum interacts with substrate for 15 μm at 37°c.
§ 1 μ TRAP = 1 mg phenol produced when 1 litre serum interacts with substrate for 1 min at 37°c.
IL-6, TNF-α, transforming growth factor-β1 and fibroblast growth factor concentrations in the serum determined by ELISA
The concentrations of IL-6 and TNF-α were significantly different among the groups (F(5,36) = 19·24, P <0·01, for IL-6; F(5,36) = 11·40, P <0·01, for TNF-α). The concentrations of IL-6 in the ERT group and the Res 20 and 40 mg/kg groups were significantly decreased compared with those in the OVX rats (ERT and Res 20 mg/kg v. OVX: P <0·01; Res 40 and 80 mg/kg v. OVX: P <0·05). However, the concentrations of IL-6 and TNF-α in the Res 40 and 80 mg/kg groups were higher than those in the ERT group. The concentrations of TNF-α exhibited the same trend. The three Res dosages increased TGF-β1 concentrations, but exhibited no effect on FGF concentrations in the serum (Table 4).
Table 4 Concentrations of IL-6, TNF-α, transforming growth factor-β1 (TGF-β1) and fibroblast growth factors (FGF) in the serum‡ (Mean values and standard deviations)
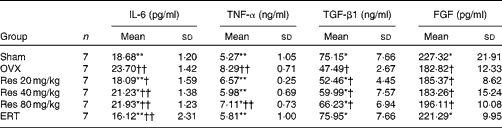
OVX, ovariectomy; Res, resveratrol; ERT, oestradiol replacement therapy.
Mean values were significantly different from those of the OVX group: *P< 0·05, **P< 0·01.
Mean values were significantly different from those of the Sham group: †P< 0·05, ††P< 0·01.
‡ After Res treatment, serum was separated from the blood samples collected from the rats. The concentrations of IL-6, TNF-α, TGF-β1 and FGF in the serum were determined.
IL-6, TNF-α, osteoprotegerin and receptor activator of nuclear factor κB ligand expressions in the femur determined by immunohistochemical analysis
Immunohistochemical analysis indicated that the expressions of IL-6, TNF-α and RANKL were significantly decreased in the Sham, the ERT and the three Res-treated rats compared with those in the OVX rats. However, the expression of OPG was increased in the Sham, the ERT and the three Res-treated rats compared with that in the OVX rats (Figs. 4 and 5). The quantitative analysis results are given in Table 5.

Fig. 4 Effect of resveratrol on the expressions of IL-6 and TNF-α. After resveratrol treatment, femur samples were collected and the expressions of IL-6 and TNF-α were analysed by immunohistochemical methods. (a, b) Representative photomicrographs of femur sections showing IL-6 expression in the different groups ((a) the Sham group and (b) the OVX group). Images were acquired at 200 × magnification. Arrows indicate IL-6-positive cells. (c, d) Representative photomicrographs of femur sections showing TNF-α expression in the different groups ((c) the Sham group and (d) the OVX group). Images were acquired at 200 × magnification. Arrows indicate TNF-α-positive cells. (A colour version of this figure can be found online at http://journals.cambridge.org/bjn).

Fig. 5 Effect of resveratrol (Res) on the expressions of osteoprotegerin (OPG) and receptor activator of nuclear factor κB ligand (RANKL). After treatment, femur samples were collected and the expressions of OPG and RANKL were analysed by immunohistochemical methods. ((a)–(f)) Representative photomicrographs of femur sections showing OPG expression in the different groups : (a) the ovariectomy (OVX) group; (b–d) the Res 20, 40 and 80 mg/kg groups; (e) the oestradiol replacement therapy (ERT) group; (f) the Sham group. ((g)–(l)) Representative photomicrographs of femur sections showing RANKL expression: (g) the OVX group; ((h)–(j)) the Res 20, 40 and 80 mg/kg groups; (k) the ERT group; (l) the Sham group. Images were acquired at 200 × magnification. Arrows indicate the OPG- or RANKL-positive cells. (A colour version of this figure can be found online at http://journals.cambridge.org/bjn).
Table 5 Effects of resveratrol (Res) on the expressions of IL-6, TNF-α, osteoprotegerin (OPG) and receptor activator of nuclear factor κB ligand (RANKL) in the femur‡ (Mean values and standard deviations)
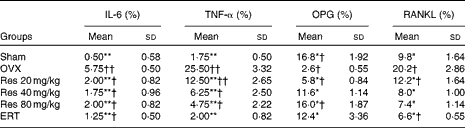
OVX, ovariectomy; ERT, oestradiol replacement therapy.
Mean values were significantly different from those of the OVX group: *P< 0·05, **P< 0·01.
Mean values were significantly different from those of the Sham group: †P< 0·05, ††P< 0·01.
‡ After Res treatment, femur samples were collected and the expressions of IL-6 and TNF-α were analysed by immunohistochemical methods. Femurs of three rats were studied. The expression of these proteins was estimated by counting immunostained cells (percentage of cells), and the average score was used for the statistical analysis. For each rat, five slides and five fields in every slide were read, and all the slides were scored by two observers in a blinded fashion.
Examination of uterus weight and histopathology
Uterus weight and uterine index
Significant atrophy of uterine tissue was observed in the OVX group compared with the Sham group, indicating the success of OVX, and oestradiol administration significantly increased uterus weight compared with OVX (F(5,36) = 65·86, P <0·001, for uterus weight; F(5,36) = 88·65, P <0·001, for uterine index), whereas Res did not exert any uterotrophic effect in the Res 20, 40 and 80 mg/kg groups (Table 6).
Table 6 Uterus weight and uterine index at the end of the treatment period‡ (Mean values and standard deviations)
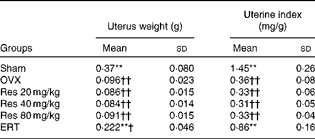
OVX, ovariectomy; Res, resveratrol; ERT, oestradiol replacement therapy.
** Mean values were significantly different from those of the OVX group (P< 0·01).
Mean values were significantly different from those of the Sham group: †P< 0·05, ††P< 0·01.
‡ Uterus samples were collected after killing the rats. The absolute weight and the relative weight (uterus weight/body weight) were determined.
Histological examination of the uterus
Qualitative analyses were carried out on the general appearance of the uterus and on the endometrium and lumen. In the OVX group, the blue-stained connective tissue and its fibrous structure were not detected, and the lumen periphery was fragmented (Fig. 6(a) and (e)). In the ERT group, oedema was observed in the endometrium, and an abnormal increase in the uterine diameter was detected (Fig. 6(b) and (f)). In the Res 80 mg/kg group, a healthy epithelial layer was observed; however, the increase in diameter was substantially less than that in the ERT group, and the infiltration of lymphocytes in the Res 80 mg/kg group was less than that in the ERT group, implying a reduced immune reaction against ERT (Fig. 6(c) and (g)). In the Sham group, blue-stained healthy connective tissue was easily detected, and the lumen periphery was intact (Fig. 6(d) and (h)).

Fig. 6 Effect of resveratrol on the uterus. After killing the rats, samples from the middle portions of both uterine horns were collected and a histological examination of the samples was carried out. The samples were studied at 40 × magnification (a–d) and 200 × magnification (e–h). The endometrium was destructed and the lumen periphery was not intact in the ovariectomy (OVX) group (a, e); the destruction of the endometrium in the Res 20, 40 and 80 mg/kg groups was much less than that in the OVX group and a healthy epithelial layer was observed. In addition, the increase in the uterine diameter was much less than that in the oestradiol replacement therapy (ERT) group (b, f); a healthy endometrium and an intact lumen periphery were observed, and oedema in the endometrium and an abnormal increase in the uterine diameter were also observed in the ERT group (c, g); healthy connective tissue and intact endometrium and lumen periphery were observed in the Sham control group (d, h). (A colour version of this figure can be found online at http://journals.cambridge.org/bjn).
Discussion
PMOP is an oestrogen-deficient state characterised by bone fragility, as the balance between bone resorption and bone formation shifts towards increased levels of bone resorption. The OVX rat has been widely used as a model for the evaluation of potential treatments for PMOP due to its similarities with humans with regard to bone loss. In the present study, we assessed the ability of Res to prevent bone loss in OVX rats and the underlying mechanisms. Oestradiol was included in the study as a reference compound to study the effect of oestrogen on bone modelling and remodelling.
The study results indicate that Res treatment may significantly prevent oestrogen deficiency-induced BW gain without affecting the weight of the uterus in the OVX rat model. The results also indicate that treatment with Res or oestradiol valerate for 12 weeks can prevent the increased rates of urinary Ca and urinary P excretion induced by OVX, suggesting that the rate of bone turnover is down-regulated by Res.
Although ALP is a non-specific isoenzyme, it is accepted as a phenotype marker for bone formation and resorption( Reference Cheng, Wang and Fan 11 ). In the present study, the increase in ALP concentrations was attenuated in the Res-treated groups (P <0·05 or 0·01) (Table 3). The results demonstrate the potential protective action of Res due to increased bone formation with reduction of bone resorption.
The regulation of osteoblast differentiation and bone mineral formation is complex and involves a variety of factors. The most investigated and stable isoform is TGF-β1, which acts at many stages ranging from the osteoblast precursor maturation to the mature osteoblast and matrix formation stage( Reference Hadjidakis and Androulakis 36 – Reference Li, Ji and Shen 38 ). In bone, TGF-β1 is secreted and stored in the synthesised mineral matrix and is subsequently released upon resorption by osteoclasts. In addition, some evidence indicates that FGF play important roles in the control of bone formation( Reference Mukherjee, Dong and Clemens 39 , Reference Rehn, Chalk and Wendel 40 ). In the present study, Res administration attenuated the decrease in TGF-β1 concentrations observed in OVX rats. However, the effect of Res on FGF concentrations was less obvious.
OVX led to a significant decrease in BMD due to an increase in bone turnover in the OVX rats compared with the Sham rats. In the present study, Res administration prevented the OVX-induced decrease of BMD in the proximal tibia and femoral neck in the Res 40 and 80 mg/kg groups. The results indicate that Res administration can attenuate the decrease in BMD usually observed after OVX. However, the BMD value for the midshaft did not differ among all the groups. The midshaft region is dominated by cortical bone, while the distal metaphysis is mainly cancellous bone. At skeletal sites containing cancellous bone, change in BMD is more likely to be caused by OVX. Skeletal sites containing mainly cortical bone have been shown to be not significantly altered by oestrogen deficiency in rodents( Reference Han, Xu and Wang 41 ).
The trabecular bone structure, the indices of which are good predictors of bone mass and bone strength, is more susceptible to bone loss due to OVX than the BMD. The histopathological analysis results indicate the preventive effect of Res on OVX-induced trabecular bone loss and microarchitecture deterioration. As expected, OVX significantly reduced the values of trabecular Tb.Ar, Tb.Th and Tb.N while increasing the value of Tb.Sp. Treatment with Res 40 and 80 mg/kg significantly inhibited these changes.
TNF-α and IL-6 stimulate differentiation independently or in a synergistic fashion in osteoclasts and their precursors. Res treatment may positively affect bone by suppressing the release of pro-inflammatory cytokines from bone cells, the concentrations of which are often elevated after OVX. These cytokines also regulate the production of both receptor activator of NF-κB ligand (RANKL) in osteoblasts and OPG. The activated RANKL interacts with RANK present on the osteoclasts for bone resorption( Reference Heymann, Riet and Le Goff 42 ). OPG is a novel anti-resorptive agent and a soluble secreted protein of the TNF receptor family that functions as a decoy receptor for RANKL. Therefore, the inhibition of the RANK/RANKL pathway inhibits osteoclast formation, differentiation and activation and bone resorption( Reference Deuell, Callegari and Giachelli 43 – Reference Takahashi, Tonami and Tachibana 45 ). To examine this hypothesis, we measured the concentrations of TNF-α, IL-6, RANKL and OPG in the serum and in the femur. In the present study, Res administration prevented the increase in IL-6 and TNF-α concentrations as well as the decrease in TGF-β1 concentrations in OVX rats. In addition, the higher doses of Res (40 and 80 mg/kg) exhibiting anti-osteoporosis activity involving osteoclasts may inhibit bone resorption (similar to OPG) by promoting the binding of RANKL to RANK to decrease osteoclastic activity.
Furthermore, the pathological changes in the endometrium and lumen of the uterus of the Res-treated rats compared with those in the uterus of the ERT rats were also investigated. A histological pattern of significant thickening of the endometrium was observed in the ERT group. However, none of the three Res-treated groups demonstrated any significant increase in the thickness of the endometrium compared with the ERT group. In addition, there were also less lymphocyte activities in the uterus of the three Res-treated groups compared with the ERT group. The reduced cell proliferation in the three Res-treated groups indicates that oestrogen antagonism of ERT's adverse effects in the uterus might be possible.
In conclusion, the present study demonstrates that daily oral administration of Res over a 12-week period in adult female OVX rats can prevent the oestrogen deficiency-induced bone loss without effects on the uterus. Therefore, Res might be a potential candidate for the treatment of PMOP.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (no. 81001245), Scientific and Technologial Innovation Programs of Higher Education Institutions in Shanxi of China (no. 2010009), and China Postdoctoral Science Foundation Grant (no. 2012M510778). All the funders contributed to the study design and conduct of the study.
The authors' contribution are as follows: H. Z. was responsible for the study concept, was in charge of the whole trial and wrote the manuscript; X. L., N. L., T. L., J. L., Z. L., H. X. and J. L. assisted in the animal trials and chemical analyses.
None of the authors has any conflicts of interest to declare.














