Introduction
The global decline in cheetah Acinonyx jubatus populations has led to their current IUCN Red List categorization as Vulnerable in most of Africa and Critically Endangered in north-west Africa and Iran (Durant et al., Reference Durant, Mitchell, Ipavec and Groom2015). Namibia is a stronghold for the cheetah and possibly holds > 25% of the estimated global cheetah population (Marker et al., Reference Marker, Cristescu, Dickman, Nghikembua, Boast, Morrison, Nyhus, Marker, Boast and Schmidt-Küntzel2018a,Reference Marker, Cristescu, Morrison, Flyman, Horgan, Sogbohossou, Nyhus, Marker, Boast and Schmidt-Küntzelb) of 7,100 adult and adolescent individuals (Durant et al., Reference Durant, Mitchell, Ipavec and Groom2015). In Namibia, 90% of the cheetah population lives outside protected areas and occupies livestock and game farmlands, resulting in a high risk of loss as a result of human–wildlife conflict (Morsbach, Reference Morsbach1987; Marker-Kraus et al., Reference Marker-Kraus, Kraus, Hurlbutt and Barnett1996; Marker et al., Reference Marker, Dickman, Wilkinson, Schumann and Fabiano2007, Reference Marker, Cristescu, Dickman, Nghikembua, Boast, Morrison, Nyhus, Marker, Boast and Schmidt-Küntzel2018a,Reference Marker, Cristescu, Morrison, Flyman, Horgan, Sogbohossou, Nyhus, Marker, Boast and Schmidt-Küntzelb). The Cheetah Conservation Fund rescues cheetahs throughout Namibia orphaned as a result of human–wildlife conflict and, where possible, helps rescued cheetahs transition back into the wild.
The release of cheetahs and other large carnivores for conservation purposes (rehabilitation, reintroduction and reinforcement translocation, hereafter referred to as ‘release’) is not a new practice and numerous releases have occurred across Africa (Hayward et al., Reference Hayward, Adendorff, O'Brien, Sholto-Douglas, Bissett and Moolman2007; Marnewick et al., Reference Marnewick, Hayward, Cilliers, Somers, Hayward and Somers2009; Houser et al., Reference Houser, Gusset, Bragg, Boast and Somers2011; Weise et al., Reference Weise, Lemeris, Bowden, Venter, van Vuuren and van Vuuren2015; Boast et al., Reference Boast, Chelysheva, van der Merwe, Schmidt-Küntzel, Walker, Cilliers, Nyhus, Marker, Boast and Schmidt-Küntzel2018). Previously, release efforts of cheetahs were hindered by issues with design and decision-making, lack of monitoring, and inadequate reporting (Boast et al., Reference Boast, Chelysheva, van der Merwe, Schmidt-Küntzel, Walker, Cilliers, Nyhus, Marker, Boast and Schmidt-Küntzel2018). However, improved knowledge and expertise garnered from continual effort and improved pre- and post-release management has led to high levels of success (Marnewick et al., Reference Marnewick, Hayward, Cilliers, Somers, Hayward and Somers2009; Buk et al., Reference Buk, van der Merwe, Marnewick and Funston2018; Vebber et al., Reference Vebber, Noack, Heyns, Rodenwoldt and Edwards2020).
The release of captive-bred or captive-raised individuals presents an added layer of difficulty as such individuals have shown poor survival post-release (Jule et al., Reference Jule, Leaver and Lea2008; Hunter & Rabinowitz, Reference Hunter and Rabinowitz2009). This has mainly been attributed to these animals lacking natural behaviours associated with survival, foraging success and reproductive fitness (Snyder et al., Reference Snyder, Derrickson, Beissinger, Wiley, Smith, Toone and Miller1996; Vickery & Mason, Reference Vickery and Mason2003). Yet, the few studies that documented successful cheetah rehabilitations found that post-release ranging and prey selection behaviour were similar to those of their wild counterparts (Houser et al., Reference Houser, Gusset, Bragg, Boast and Somers2011; Vebber et al., Reference Vebber, Noack, Heyns, Rodenwoldt and Edwards2020).
As wildlife populations continue to decline, the value of releasing captive-bred and captive-raised individuals may grow. By increasing post-release survival, use of these individuals for release efforts vs wild-caught individuals could mitigate the pressure on vulnerable and/or dwindling wild populations (Wilson & Price, Reference Wilson, Price, Olney, Mace and Feistner1994; Schwartz et al., Reference Schwartz, Gusset, Crosier, Versteege, Eyre, Tiffin, Kotzé, Nyhus, Marker, Boast and Schmidt-Küntzel2018). Cheetahs naturally live at low population densities and are at risk of further decline as a result of human–wildlife conflict, habitat loss and illegal trade (Marker et al., Reference Marker, Cristescu, Dickman, Nghikembua, Boast, Morrison, Nyhus, Marker, Boast and Schmidt-Küntzel2018a,Reference Marker, Cristescu, Morrison, Flyman, Horgan, Sogbohossou, Nyhus, Marker, Boast and Schmidt-Küntzelb). Rehabilitation of orphaned/trapped individuals could therefore be a complementary approach for cheetah conservation.
Despite recent successful releases of cheetahs, the need for improved understanding and methodology, and for evidence-based decision-making and management in reintroduction practices for carnivores, is well established (Hayward, et al., Reference Hayward, Adendorff, O'Brien, Sholto-Douglas, Bissett and Moolman2007; Gusset et al., Reference Gusset, Ryan, Hofmeyr, van Dyk, Davies-Mostert and Graf2008; Jule et al., Reference Jule, Leaver and Lea2008; Taylor et al., Reference Taylor, Canessa, Clarke, Ingwersen, Armstrong, Seddon and Ewen2017). Here we present a methodological framework for cheetah rehabilitation and post-release management based on the outcome of release trials conducted by the Cheetah Conservation Fund during 2004–2018. We present the characteristics of release sites and a detailed description of the selection process of release candidates, followed by an overview of our pre- and post-release management protocol. Then, we analyse post-release survival in light of the main biological (age when orphaned, sex), ecological (group size, release site) and management-related (captivity time, training–release) factors that we expected to affect post-release survival. These findings are incorporated into our management recommendations for the rehabilitation and release of wild-born, captive-raised cheetahs, and we encourage scientists and managers to implement, expand and refine this protocol throughout the cheetah's current and historical range.
Methods
Release site characteristics
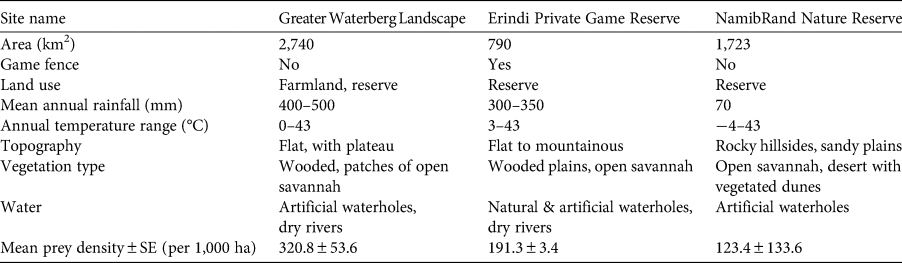
The study area included three release sites in Namibia within the cheetah's current and historical range (Table 1, Fig. 1, Supplementary Table 1). Assessed by surveys and expert knowledge, all sites had sufficient prey available, suitable cheetah habitat, and natural and artificial water points that provided water year-round. Release sites were sufficiently large to meet the ecological requirements of cheetahs (Lindsey et al., Reference Lindsey, Tambling, Brummer, Davies-Mostert, Hayward, Marnewick and Parker2011). Competing carnivores were present in all release sites. Cheetahs may persist well in systems with dominant competitors through fine-scale avoidance strategies (Durant, Reference Durant1998, Reference Durant2000; Broekhuis et al., Reference Broekhuis, Cozzi, Valeix, Mcnutt and Macdonald2013; Swanson et al., Reference Swanson, Caro, Davies-mostert, Mills, Macdonald and Borner2014, Reference Swanson, Arnold, Kosmala, Forester and Packer2016), thus the presence of other carnivores was not considered to be a limiting factor. Resident conspecifics may negatively influence release efforts, but established, long-term monitoring was present in all release sites and none had large resident cheetah populations. In addition, both the NamibRand Nature Reserve and the Greater Waterberg Landscape facilitate natural regulation of existing cheetah populations through natural dispersal, as they are open systems. NamibRand Nature Reserve was established as an open system in 1984. The Greater Waterberg Landscape is an open system with mixed land use of livestock and game farming and includes the Bellebenno release training camp. Erindi Private Game Reserve is a private fenced reserve that was converted from a cattle farm in 2008.

Fig. 1 Locations of the three sites where cheetahs Acinonyx jubatus were released: (a) Greater Waterberg Landscape in north-central Namibia, including the Bellebenno release training camp; (b) Erindi Private Game Reserve in north-central Namibia; and (c) NamibRand Nature Reserve in south-central Namibia.
Table 1 Summary information for the three sites in Namibia were cheetahs Acinonyx jubatus were released (Fig. 1).
Release candidate selection
The selection of cheetahs to be released was based on the following parameters:
Age at orphaning
We considered individuals orphaned at an age of 6 months or older as release candidates. At this age, they have experienced 4 months or more out of the natal den with their mother, learning survival skills (Caro, Reference Caro1994). This enabled us to minimize their habituation to humans during captivity, as they did not need the close care that younger orphans required. Individuals orphaned at 3–5 months were considered for release if bonded to older orphans that qualified. Once bonded, the older orphans help minimize habituation levels in the younger orphans.
Level of habituation
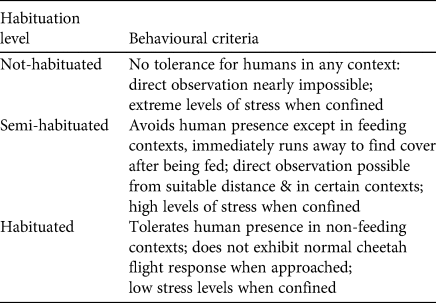
We assessed the degree of habituation of each release candidate with the classification criteria described by Weise et al. (Reference Weise, Lemeris, Bowden, Venter, van Vuuren and van Vuuren2015) (Table 2), adapted for this study. Individuals classified as habituated were not considered releasable. We aimed for candidates to be semi-habituated at the time of release, to allow the monitoring team to track and observe the individual post-release reliably. This level of habituation could be maintained for most individuals orphaned at ≥ 6 months of age (and their younger coalition members), as those candidates only had exposure to human activity during husbandry routines (e.g. feeding, health check, management training). By maintaining the optimal habituation level for all candidates, we aimed to reduce the impact of captivity time on survival probability (Weise et al., Reference Weise, Lemeris, Bowden, Venter, van Vuuren and van Vuuren2015).
Table 2 Classification criteria for determining habituation level of release candidates. Adapted from Weise et al. (Reference Weise, Lemeris, Bowden, Venter, van Vuuren and van Vuuren2015).
Social grouping
Adult female cheetahs live solitarily except when breeding or raising cubs, whereas males form lifelong groups with their brothers, known as coalitions (Caro, Reference Caro1994; Wachter et al., Reference Wachter, Broekhuis, Melzheimer, Horgan, Chelysheva, Marker, Nyhus, Marker, Boast and Schmidt-Küntzel2018). Living in a coalition may improve male survival probability (Durant et al., Reference Durant, Kelly and Caro2004). If bonded at an early age, both male and female cheetah cubs can form coalitions with unrelated individuals in captivity. When possible, we bonded release candidates to form artificial coalitions, with the goal of improving each member's survival probability (Hunter, Reference Hunter and Penzhorn1998; Gusset et al., Reference Gusset, Ryan, Hofmeyr, van Dyk, Davies-Mostert and Graf2008; Boast et al., Reference Boast, Chelysheva, van der Merwe, Schmidt-Küntzel, Walker, Cilliers, Nyhus, Marker, Boast and Schmidt-Küntzel2018; Marneweck et al., Reference Marneweck, Becker, Beverley, Davies-Mostert, du Plessis and Forssman2019).
Age at release
To increase their ability to integrate into their new environment, we only released fully-grown candidates that were at least 2.5 years old.
Pre-release protocol
Husbandry
Release candidates were housed in large off-exhibit enclosures and were kept in good physical and mental condition through daily exercise by allowing them to chase the feeding vehicle (see Supplementary Material 1 for additional information).
Release candidate preparation
One month prior to a planned release, feeding was changed to a diet consisting of whole carcasses of natural prey. This provided the release candidates with the experience to open and feed from intact carcasses and prepared their system to handle less frequent, larger quantities of food. A veterinarian registered with the Veterinary Council of Namibia performed a full health examination under anaesthesia on all candidates prior to release to verify good health status and fit cheetahs with a GPS/VHF collar to enable post-release monitoring. All releases were done under permission from Namibia's Ministry of Environment, Forestry and Tourism. For additional details on release preparation and collaring, see Supplementary Material 2.
Release type
We used two release types (hard and soft). In a hard release individuals are freed into the new environment immediately upon arrival, whereas in a soft release individuals are allowed a period of acclimation to their new environment in a temporary holding facility (a boma) before release (Hunter et al., Reference Hunter, Pretorius, Carlisle, Rickelton, Walker, Slotow and Skinner2007). A soft release aims to suppress the tendency to return to the location of capture, known as homing, thereby encouraging the establishment of a home range within the release site (Hayward et al., Reference Hayward, Adendorff, O'Brien, Sholto-Douglas, Bissett and Moolman2007; Briers-Louw et al., Reference Briers-Louw, Verschueren and Leslie2019). Cheetahs soft-released in this study were kept in holding bomas for 1–5 months. Any animal released into the Greater Waterberg Landscape from an enclosure located within the landscape was considered soft-released. For some individuals, we used both release types as some cheetahs were released more than once during the study (Table 3). Pre-release hunting training may increase post-release survival (Houser et al., Reference Houser, Gusset, Bragg, Boast and Somers2011). Therefore, we trialled a release preparation technique, training–release, on some individuals, providing them with hunting experience in a monitored and relatively safe environment (Bellebenno release training camp; Fig. 1) prior to release. Training–release trials took place early in our study and were used for individuals or groups that had characteristics prompting concern for their survival chances (e.g. long periods of time in captivity, physical issues). A given candidate group or individual was released into the training camp and monitored intensely during daylight hours, facilitated by the manageable size (4,000 ha) of the camp. During these trials cheetahs were fed smaller portions more frequently (every 2–3 days) than during an actual release (Supplementary Material 3). Once an individual or coalition achieved independence in terms of hunting, preparation began for the final release. Individuals that failed to transition towards the expected routine of a wild cheetah were returned to permanent captivity.
Table 3 Breakdown of releases by release site, release type and released cheetahs.
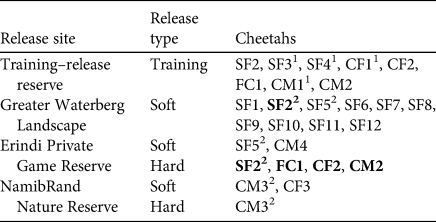
1 Indicates cheetahs that failed their training–release and were returned to captivity; bold font indicates individuals that underwent training–release prior to release.
2 Indicates cheetahs released more than once.
Post-release monitoring protocol
Monitoring routine/schedule
We attempted to locate each individual or group twice daily (during 05.00–09.00 and 15.00–19.00) and recorded all actions during the monitoring period. The monitoring team used collar GPS data each morning to find the cheetahs or began tracking from the individual's last known location if it only had a VHF collar. The team determined and prioritized monitoring activity based upon the overnight developments captured by the GPS data or on the events of the previous day. Additionally, as cheetahs rarely scavenge, we visited GPS cluster points to identify carcass remains as evidence for any kill made by the individual or coalition.
Supplemental feeding/water and medical aid
Supplemental feeding and watering occurred as necessary with all released cheetahs. If a cheetah failed to make a kill when expected or necessary (based on its body condition), we fed a large meal (5–8 kg per cheetah of either horse/donkey Equus sp. or game meat) to that individual or group. If an individual or group had still not made a successful kill after the second or third week of release, we started decreasing the frequency of feedings to promote hunting. Minimal feeding frequency was maintained to ensure that cheetahs were not at risk of loss of condition or starvation. Supplemental feeding stopped as soon as cheetahs began making successful kills at regular intervals (Supplementary Material 3). A registered veterinarian was on standby for any emergency situation.
End of intensive post-release monitoring period
Once a cheetah settled into a behavioural routine expected of a wild cheetah (i.e. no longer requiring regular supplemental feeding, visiting water points, and stabilization of initial exploratory behaviour; Marker et al., Reference Marker, Cristescu, Dickman, Nghikembua, Boast, Morrison, Nyhus, Marker, Boast and Schmidt-Küntzel2018a,Reference Marker, Cristescu, Morrison, Flyman, Horgan, Sogbohossou, Nyhus, Marker, Boast and Schmidt-Küntzelb; Wachter et al., Reference Wachter, Broekhuis, Melzheimer, Horgan, Chelysheva, Marker, Nyhus, Marker, Boast and Schmidt-Küntzel2018), the monitoring team would end the intensive post-release monitoring period. From then on, cheetahs were only monitored via their GPS/VHF collars and observed when they were easy to find or when they were seen opportunistically.
Assessment of release success
We evaluated the success of released cheetahs based on achieved independence and survival.
Independence achieved
We considered a released cheetah to have achieved independence once they began making kills at regular intervals and no longer required supplemental feeding for survival. If an individual or group failed to reach independence or displayed behaviour suggesting they would fail to achieve independence, we considered their release a failure and returned them to permanent captivity.
Survival
To assess cheetah survival, we calculated Kaplan–Meier survival estimates with the product limit estimator using a staggered entry design (Kaplan & Meier, Reference Kaplan and Meier1958; Pollock et al., Reference Pollock, Winterstein, Bunck and Curtis1989). This method allowed the inclusion of animals entering the study at irregular intervals and accommodates data from censored individuals (individuals with unknown fates when collars fail or deplete). Survival estimates were based on the time individuals survived during both the training–release (if applicable) and the post-release monitoring period. We did not include released individuals that returned to captivity after a failed training–release or release in our survival analyses. We used log rank tests to compare survival curves between social grouping (male coalition, reserve; female coalition, reserve; single females, reserve; single females, farmland), and trained/non-trained individuals (Pollock et al., Reference Pollock, Winterstein, Bunck and Curtis1989). Two continuous variables were also investigated, after being transformed into categorical factors. Age when orphaned was split into individuals orphaned at < 6 months of age and individuals orphaned at ≥ 6 months of age, to assess whether individuals < 6 months old could be considered for release when bonded to older individuals. Captivity time was divided into individuals in captivity < 1,500 days and ≥ 1,500 days. Survival was estimated for consecutive 3-month periods to investigate differences throughout the post-release period, as Weise et al. (Reference Weise, Lemeris, Bowden, Venter, van Vuuren and van Vuuren2015) detected increased mortality in the first 3 months after the release of translocated cheetahs. Analysis was done in R 4.0.4 (R Core Team, 2021).
Results
Selected release candidates
Of 86 wild-born, captive-raised orphaned cheetahs rescued during 2001–2012, 42% were selected for rehabilitation based on age on arrival and health status. The 36 individuals (15 males, 21 females) were divided into 20 release groups (Table 3); four male coalitions (CM1 to CM4), three female coalitions (CF1 to CF3), 12 single females (SF1 to SF12), and one female with cubs (FC1). Candidates selected for release were orphaned at 3–13 months of age (mean 7.28 ± 2.61 SD months), spent 1.5–9 years in captivity (mean 4.76 ± SD 1.87 years) before release, and were released at 2.5–10 years of age (mean 5.56 ± 1.81 years).
Releases
Releases took place during 2004–2018. Eight training–releases involving 17 individuals were attempted. Three (CF2, FC1, CM2) of the eight groups were hard-released in Erindi, and a fourth (SF2) was soft-released into the Greater Waterberg Landscape prior to being hard-released into Erindi (Table 3). Of the 12 groups that did not undergo training, nine were soft-released into the Greater Waterberg Landscape, one (CF3) was soft-released into NamibRand, one (SF5) was soft-released twice, first into the Greater Waterberg Landscape and later into Erindi following veterinary treatment for an injury sustained in the first release, and one (CM3) was first hard-released into NamibRand, but recaptured as they began moving out of the Reserve, and then soft-released back into NamibRand (Table 3).
Evaluation of release success
Training–release outcomes
Of the 17 (7 M, 10 F) cheetahs that underwent training–release, 52% (n = 9; 4 M, 5 F) successfully reached independence and were subsequently released, and 48% (n = 8; 4 M, 4 F) failed to reach independence and were returned to captivity. Of these eight, two females (CF1) escaped the training camp and killed a goat, two females (SF3, SF4) failed to make successful kills and throughout their training–release period showed abnormally low interest in trying to hunt, and four males (CM1) remained at the release enclosure and did not explore the training camp or attempt to hunt.
Time to independence
Of the 36 cheetahs selected for release, 75% (10 M, 17 F) achieved independence. Eight individuals failed to achieve independence during the training–release, and one male (#NAAJU1616 of CM4) was killed by a leopard Panthera pardus before the coalition had achieved independence. Considering only cheetahs that underwent final release, independence was achieved by 96% of released individuals. Each individual or group achieving independence was supplemental fed on average 17 times (median 7 feedings, range 1–112 feedings) and made its first known kill after 18 days (median 7 days, range 3–113 days). FC1 struggled post-release, probably because of her dependent cubs, and as a result was fed more frequently (112 feedings) to ensure that the cubs were properly nourished. Most individuals or groups (25 cheetahs) achieved independence within 2 weeks after release and only required 1–3 supplemental feedings before making their first kills.
Survival
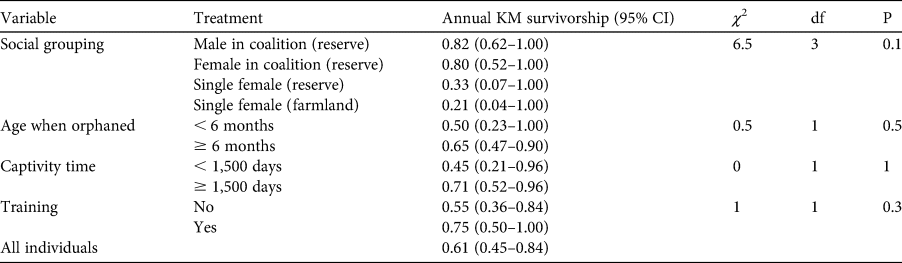
The eight individuals that failed their training–release trials were not included in the survival analyses, reducing the total number of individuals used for analysis to 28 (11 M, 17 F). Of these, 68% (6 M, 13 F) were recorded dead at the time of analysis, and the fate of 32% (5 M, 4 F) was unknown because of collar failure or depleted batteries. The annual Kaplan–Meier survival estimate for all released individuals was 0.61 (95% CI 0.45–0.84; Fig. 2, Table 4). Social grouping did not significantly affect survival, but males in coalitions tended to live longer compared to females in coalitions and single females. The six deaths recorded on farmland were attributed to both anthropogenic (34%) and natural causes (64%). Of the four cheetahs that died of natural causes, two were from hunting injuries and two were killed by leopards. For the 13 recorded deaths in a reserve, 69% were attributed to natural causes and 31% to unknown causes. Of the nine cheetahs that died of natural causes, two died of old age, two of hunting injuries, one was killed by baboons Papio ursinus and four by competing predators (two leopard, one lion Panthera leo, one spotted hyaena Crocuta crocuta). No differences in survival were found for the variables age when orphaned, time spent in captivity, and training–release (Fig. 2, Table 4). No deaths occurred as a result of starvation, suggesting that cheetahs, with strategic support provided by post-release monitoring, can acquire the skills required to support themselves despite their history. Overall survival estimates at 3, 6, 9 and 12 months remained stable, without any increased mortality in the first 3 months post-release (Fig. 3). Survival in the first and second years did not differ significantly (χ 2 = 0, df = 1, P = 0.8). The increase in survivorship after 15 months is a result of five individuals (18%) surviving beyond 2 years-post release.
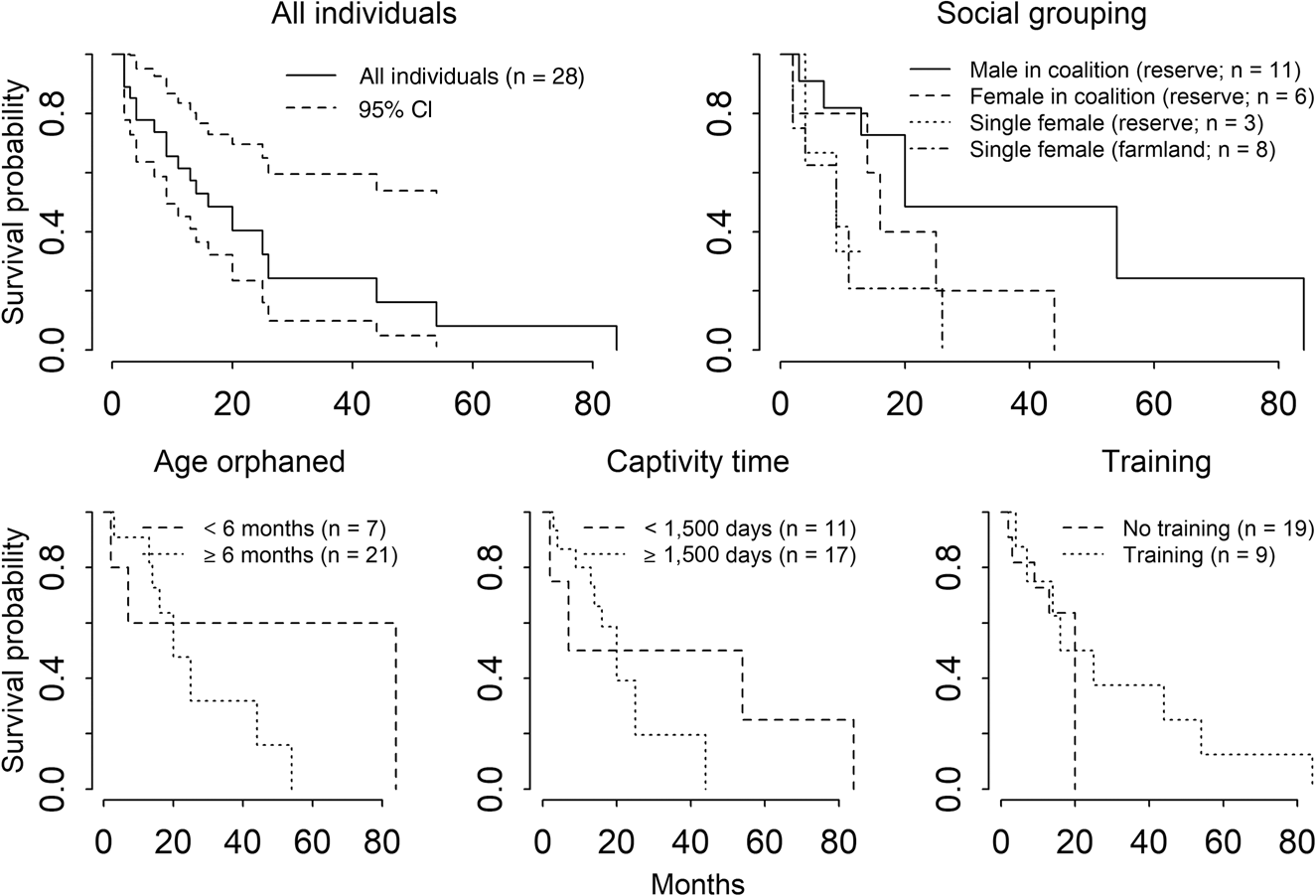
Fig. 2 Kaplan–Meier survivorship curves for all the released captive-reared cheetahs and comparisons for the variables social grouping, age when orphaned, captivity time and training–release.

Fig. 3 Three-month interval Kaplan–Meier survival analysis for released cheetahs. Error bars show 95% CI of the Kaplan–Meier estimate.
Table 4 Annual Kaplan–Meier (KM) survivorship estimates with 95% confidence intervals of released captive-reared cheetahs. χ 2 values, degrees of freedom and probability are reported for the log rank test comparing survivorship for different variables.
Reproductive success
Successful reproduction is the ultimate measure of the success of release efforts. Although our data is insufficient for statistical analyses, the following observations were made: SF5, SF6, SF10 and SF11 mated with and were impregnated by wild males. SF5 raised two female cubs to independence, SF10 gave birth to a litter of cubs before her collar failed. During the necropsy on SF6, five unborn cubs were found. SF11 gave birth to two litters, lost the first but raised the second to 11 months of age before being killed by a leopard. CM4 were seen on several occasions with female cheetahs, and were observed mating with a wild female.
Discussion
Following our release protocol, the success rate of animals achieving independence was high. Furthermore, annual post-release survival rate was similar to other cheetahs translocated into a free-ranging reserve (NamibRand) in Namibia (annual Kaplan–Meier survival estimate 0.57, 95% CI 0.35–0.76; Weise et al., Reference Weise, Lemeris, Bowden, Venter, van Vuuren and van Vuuren2015) and mortality was comparable to cheetahs in a fenced reserve in South Africa (Bissett & Bernard, Reference Bissett and Bernard2011). Independence was achieved quickly for most individuals (< 2 weeks) and we had evidence of successful reproduction events for some released individuals.
Neither age when orphaned nor time in captivity affected the survival probability of released cheetahs. Therefore, our protocol for pre-release management to achieve release candidate status for individuals orphaned younger than 6 months through bonding them to candidates orphaned at an older age seems effective. These findings are contrary to those of previous studies in which time in captivity had significant negative influence over survival, as they show success in releasing captive-raised individuals (Jule et al., Reference Jule, Leaver and Lea2008; Weise et al., Reference Weise, Lemeris, Bowden, Venter, van Vuuren and van Vuuren2015; Boast et al., Reference Boast, Chelysheva, van der Merwe, Schmidt-Küntzel, Walker, Cilliers, Nyhus, Marker, Boast and Schmidt-Küntzel2018).
Although the training–releases did not significantly improve survival, one advantage they may offer is the opportunity to screen candidates that meet requirements but that would benefit from additional assessment to confirm their ability to reach independence (Gusset et al., Reference Gusset, Slotow and Somers2006; Houser et al., Reference Houser, Gusset, Bragg, Boast and Somers2011). However, we suggest caution when employing such a technique to provide pre-release hunting training to large carnivores. As natural predator–prey interactions are difficult to recreate, the behaviour and strategy developed within a training environment may not be adequate for a final release. With intensive post-release monitoring according to our protocol, an animal can be reliably supported with supplemental feeding/water and other interventions (see Methods) as necessary until it has reached independence. Thus, we suggest that intensive post-release monitoring within the release site provides the animal with the safest and most stable conditions for acquiring the necessary hunting and survival skills relevant to that release site.
Past studies of carnivore releases have demonstrated the challenges associated with homing behaviour (Linnell et al., Reference Linnell, Aanes, Swenson, Odden and Smith1997; Yiu et al., Reference Yiu, Keith, Karczmarski and Parrini2015), which has also been observed in captive-raised and released animals of other species (Henshaw & Stephenson, Reference Henshaw and Stephenson1974; Ridgway & Robison, Reference Ridgway and Robison1985). Although we could not test for differences between soft and hard release, we recommend implementing soft releases based upon past findings and our experience with CM4. CM4 only established themselves after a soft release following a hard release that failed because of extensive exploratory roaming.
Although our results did not show significantly higher survival for releases into reserves, reserves generally exclude the risk of conflict with humans. Additionally, farmland restricted the capacity of our team to monitor and assist released cheetahs. If a cheetah crossed a farm border onto a property where we failed to obtain permission for monitoring, that individual ceased to be monitored until it moved onto a property where it could be monitored. Our inability to closely monitor some released cheetahs moving across property borders perhaps influenced survival probability, as we were unable to provide these individuals with as much support (e.g. supplemental feeding, veterinary assistance) as individuals released into a reserve, where monitoring ability is more secure.
The annual survival estimate obtained for cheetahs released into large or fenced reserves was similar to the annual survival estimate of 0.85 for cheetahs translocated into fenced reserves in South Africa (Marnewick et al., Reference Marnewick, Hayward, Cilliers, Somers, Hayward and Somers2009). Buk et al. (Reference Buk, van der Merwe, Marnewick and Funston2018) found predators to be responsible for 53.2% of known deaths for cheetahs translocated into fenced reserves in South Africa. Competing predators were responsible for 44.4% (4 cheetahs) of the deaths with known causes for cheetahs released into reserves in our study, suggesting that predator naivety is not more of a concern for rehabilitated cheetahs than for their wild counterparts. Unfortunately, reserves are often isolated and individuals are not able to contribute to the free-ranging gene pool unless intensive metapopulation management is employed to create gene flow between these isolated populations (Marnewick et al., Reference Marnewick, Hayward, Cilliers, Somers, Hayward and Somers2009; Boast et al., Reference Boast, Chelysheva, van der Merwe, Schmidt-Küntzel, Walker, Cilliers, Nyhus, Marker, Boast and Schmidt-Küntzel2018). Our findings will be applicable to future reintroduction efforts into reserves within the cheetah's historical range after problems that caused extirpation have been addressed (IUCN/SSC, 2013).
Released coalitions tended to have a higher estimated survivorship than single individuals, which may be attributable to increased chances of obtaining core territories. For male cheetahs these are smaller sections of overall home ranges that reliably provide food, shelter and water for its holder (Caro, Reference Caro1994; Melzheimer et al., Reference Melzheimer, Streif, Wasiolka, Fischer, Thalwitzer and Heinrich2018). Moreover, living in coalitions may improve hunting success and facilitate defence against other cheetah coalitions (Caro, Reference Caro1994; Durant et al., Reference Durant, Kelly and Caro2004). Our small sample did not allow us to test for differences in survival between sexes, but males tended to live longer than females. Longevity of females is particularly important as they need a minimum of 2 years post-release to successfully raise offspring (Laurenson, Reference Laurenson1993). Males on the other hand may contribute their genes relatively quickly and may therefore be of higher conservation benefit in the short-term. Paying close attention to females during the post-release monitoring period is critical for maximizing the success of the release of females.
Although releases are time-intensive and costly, permanent captivity is also expensive (c. USD 5,000 annually per animal). Therefore releasing a captive-raised individual presents financial benefits in addition to the biological benefit of returning an individual to the wild gene pool. Thus, we argue that the benefits of releasing captive-raised individuals probably outweigh concerns regarding its cost (Weise et al., Reference Weise, Stratford and van Vuuren2014; Boast et al., Reference Boast, Chelysheva, van der Merwe, Schmidt-Küntzel, Walker, Cilliers, Nyhus, Marker, Boast and Schmidt-Küntzel2018) and, with appropriate planning, such releases could provide genetic benefits to wild populations. Our study challenges recent critique that using captive-raised large carnivores for release presents little value for conservation (Hunter & Rabinowitz, Reference Hunter and Rabinowitz2009; Weise et al., Reference Weise, Lemeris, Bowden, Venter, van Vuuren and van Vuuren2015).
By carefully selecting release candidates, artificially forming coalitions where possible, balancing habituation levels during captivity, and providing strategic support during post-release monitoring, we have demonstrated that captive-reared cheetahs orphaned at age of 6 months or older can transition fully back into the wild. Moreover, cheetahs orphaned at a younger age can successfully be released if bonded to qualifying individuals before release; this strategy may prove a valuable tool for the development of procedures to release captive-bred individuals successfully. The effectiveness and necessity of post-release monitoring and management is further highlighted by the absence of elevated mortality during the first 3 months post-release (Weise et al., Reference Weise, Lemeris, Bowden, Venter, van Vuuren and van Vuuren2015). Our monitoring team always consisted of at least one person with extensive experience working directly with cheetahs. This experience enabled the team to adapt dynamically and respond to the developments of each release, thus improving the survival probability of released cheetahs. Having a veterinarian on standby allowed the team to treat potentially life-threatening injuries, providing the individual with an additional chance to reach independence. Further investigation into the causes of deaths of rehabilitated cheetahs is warranted, to facilitate experimentation with potential mitigation measures. Improved understanding of the specific risks associated with a given release site and the development of post-release management actions to mitigate those risks would improve survival of released individuals.
Although previous dismissal of releasing captive-raised large carnivores is understandable considering past failed release attempts, we argue this conclusion is premature, as most studies have approached the release of captive-reared cheetahs with the same pre- and post-release treatment as for wild conspecifics (Jule et al., Reference Jule, Leaver and Lea2008; Hunter & Rabinowitz, Reference Hunter and Rabinowitz2009; Weise et al., Reference Weise, Lemeris, Bowden, Venter, van Vuuren and van Vuuren2015; Boast et al., Reference Boast, Chelysheva, van der Merwe, Schmidt-Küntzel, Walker, Cilliers, Nyhus, Marker, Boast and Schmidt-Küntzel2018). Such an approach ignores the fact that captive-reared cheetahs lack much of the learning and experience provided by their mother during dependency. Although a lot of survival skills are probably instinctual, they do require trial and error until being fully acquired; by appropriately accounting for this challenge during pre- and post-release management we have shown that orphaned cheetahs are capable of transitioning back into the wild. We recommend that any future release efforts of captive-raised large carnivores be based on design strategies that fully consider the experiential knowledge gaps such individuals possess. Despite the small sample, our study indicates that cheetahs rehabilitated, released and monitored according to our protocol have chances of survival similar to those of wild cheetahs and therefore this technique is a valuable tool for cheetah conservation.
Acknowledgements
We thank Namibia's Ministry of Environment, Forestry and Tourism for supporting this research, all Cheetah Conservation Fund supporters that made this study financially feasible, all Cheetah Conservation Fund staff and volunteers who contributed to this research, Lorraine Boast for her comments on the text, and all the partners and release sites (the Greater Waterberg Landscape, Erindi Private Game Reserve, and NamibRand Nature Reserve), who worked with us to make this research possible.
Author contributions
Study design: EHW, LM, AS-K; fieldwork: EHW, LM, SV; writing, data analysis, revision: all authors.
Conflicts of interest
None.
Ethical standards
This research adhered to IUCN definitions and guidelines for reintroduction and translocations and to the Guidelines for Ethical Treatment of Animals in Applied Animal Behaviour and Welfare Research standards of the International Society for Applied Ethology, and otherwise abided by the Oryx ethical standards. All research and work with animals was approved by Namibia's Ministry of Environment, Forestry and Tourism.









