INTRODUCTION
Renal and perinephric abscesses (RA) are potentially lethal complications of urinary tract infection. Clinical studies conducted in earlier years reported a case-fatality rate in the range of 39–50%, even when treated with aggressive drainage. The variable constellation of symptoms and insidious clinical course at the time of evaluation leads to difficulty in the diagnosis of RA. It has been reported that only 15–25% of patients were correctly diagnosed at the time of admission, and a lower case-fatality rate has been found to be associated with accurate diagnosis at an earlier stage [Reference Salvatierra, Bucklew and Morrow1, Reference Thorley, Jones and Sanford2]. The progress in imaging studies and the development of percutaneous drainage of retroperitoneal abscess substantially decreased the case-fatality rate. However, case-fatality rates are still high, varying from 5% to 15% [Reference Yen3–Reference Coelho5].
Diabetes mellitus (DM) is the most common endocrine disease. Diabetic patients suffer more frequently from complicated infections than non-diabetic patients. A large study of bacteraemic patients demonstrated that two thirds of these patients had a history of DM, and the urinary tract was the most prevalent infection site [Reference Carton6]. Some previous studies reported that diabetes may increase the risk of RA [Reference Yen3, Reference Shu, Green and Orihuela7, Reference Lee8]. However, nearly all of the previous studies were hospital-based, and population-based studies that investigated the relationship between diabetes and RA are rare. Using a nationally representative diabetic cohort retrieved from the National Health Insurance (NHI) database of Taiwan, this study aimed to investigate the effect of diabetes on the incidence of RA between 1997 and 2007.
METHODS
Study design and data source
This is a registry-based cohort study, between 1997 and 2007, of RA in association with diabetes. Data were obtained from the NHI database, which has been routinely collected by the National Health Research Institutes (NHRI), and supervised by the state-run Bureau of NHI (BNHI). The NHI programme is a universal health insurance programme in Taiwan implemented in March 1995. The NHI programme enrolled about 96% of the Taiwanese population, and the BNHI had contracted with 97% of hospitals and clinics throughout the country by the end of 1996 [Reference Chang9]. The BNHI performs expert reviews on a random sample of every 50–100 ambulatory and in-patient claims in each hospital and clinics on a quarterly basis to ensure the accuracy of the claims data. False reports of diagnosis receive severe penalty from the BNHI [10]. After ethical approval of the NHRI, diabetic ambulatory care claims (1997–2007), all in-patient claims (1997–2007), and the updated registry for beneficiaries (1997–2007) were used in this study. Using each individual's personal identification number (PIN), we were able to inter-link all datasets.
Selection of the diabetic and control groups
Details of the claims data and selection methods of diabetic and control groups were described in our previous report [Reference Chen, Ho and Li11]. In summary, an individual was classified as a diabetic patient if he/she had an initial diabetes diagnosis (ICD-9-CM: 250 or A-code 181), at any time in 1997, and experienced another one or more diagnoses within the subsequent 12 months. In addition, the first and last outpatient visits during the 12-month period had to be separated by at least 30 days to avoid accidental inclusion of miscoded patients. The patient's index date was set to be the date of the first outpatient visit with a diabetic code in 1997.
The control group was identified from the registry of beneficiaries after excluding data of the diabetic group. It was selected by matching to the diabetic group on frequency distributions of both sex and age (in every 5 years from 0–105 years). After stratification according to the pre-determined sex and age classification, a simple random sampling technique was applied to select control subjects from each stratum. The index date for the control subject was the first date of enrolment in the NHI. If the first date of enrolment was before 1 January 1997, we set the index date as 1 January 1997.
The age of each study subject was calculated by the difference in time between the index date and the date of birth. The geographic area of each study subject's NHI unit, either the beneficiaries' residential area or the location of their employment, was classified into four geographic areas (North, Central, South, East) or three levels of urbanization (metropolis, satellite city, rural area) according to the National Statistics of Regional Standard Classification [12]. We also calculated the prevalence of admission in 1997 due to complicated urinary tract infection [UTI, including acute pyelonephritis (ICD-9-CM: 590·1), acute cystitis (ICD-9-CM: 595·0), cystits (ICD-9-CM: 595·9), non-specific urinary tract infection (ICD-9-CM: 599·0), and urethritis (ICD-9-CM: 597·80)], coronary artery disease [CAD, including acute myocardial infarction (ICD-9-CM: 410) and ischaemic heart disease (ICD-9-CM: 411–414)], or stroke [including non-traumatic haemorrhagic stoke (ICD-9-CM: 430–432) and ischaemic stroke (ICD-9-CM: 433–438)] for both diabetic and control groups to evaluate the history of UTI- and diabetes-related macrovascular complications prior to the occurrence of RA.
Study endpoints
With the unique PIN, we linked ambulatory claims data of study subjects to in-patient claims records (1997–2007) in order to identify the episodes of admission due to RA (ICD-9-CM: 590·2). The date of encountering the clinical endpoint of interest was the first day of hospitalization. The study period was between 1 January 1997 and 31 December 2007. Only the first-time episode of RA admission during follow-up was analysed.
Statistical analysis
The sex-, age-, geographic area- and urbanization level-specific incidence density (ID) of RA according to diabetes was calculated with person-years as the denominator under Poisson assumption. In the analysis of RA admission, the study subjects who did not encounter RA admission were considered censored and the date of censoring was the date of NHI policy termination [date of death, date of their last withdrawal from NHI, or the date of study termination (i.e. 31 December 2007)]. To assess the independent effect of diabetic status on the risk of RA, we conducted Cox proportional hazard regression models with sex, age, geographic area, and urbanization status adjusted simultaneously in the model. We adjusted the latter two regional variables because there is urban–rural difference in accessibility to medical care in Taiwan [Reference Tan13]. All statistical analyses were performed with SAS (version 9.1; SAS Institute, USA). A P value <0·05 was considered statistically significant. Access to the claims data was approved by the Review Committee of the NHRI. Because all PINs were scrambled, no informed consent from individual participants was required.
RESULTS
The mean age (±s.d.) of the diabetic group was 61·0±12·5 years, while that of the control group was 60·9±12·6 years. The percentage of study subjects aged <45, 45–54, 55–64, and >64 years was 10·0%, 18·1%, 28·2%, and 43·7%, respectively, in both groups. Female patients slightly predominated in both groups. The prevalence of complicated UTI admission in 1997 in the diabetic and control groups was 4·7% and 1·4%, respectively. The corresponding figures for CAD and stroke were 4·9% vs. 1·8% and 5·4% vs. 1·9%. The distributions of geographic areas and urbanization level for residences were similar between diabetic and control groups (Table 1).
Table 1. Characteristics of the study subjects
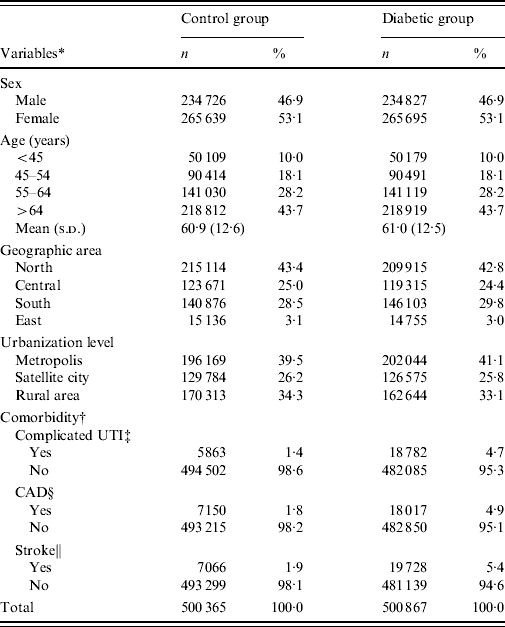
* Inconsistency between total population and population summed for individual variable was due to missing information.
† Retrieved from the NHI admission data in 1997.
‡ UTI, Urinary tract infection includes acute pyelonephritis (ICD-9-CM: 590·1), acute cystitis (ICD-9-CM: 595·0), cystits (ICD-9-CM: 595·9), non-specific urinary tract infection (ICD-9-CM: 599·0), and urethritis (ICD-9-CM: 597·80).
§ CAD, Coronary artery disease includes: acute myocardial infarction (ICD-9-CM: 410) and ischaemic heart disease (ICD-9-CM: 411–414).
∥ Non-traumatic haemorrhagic stoke (ICD-9-CM: 430–432) and ischaemic stroke (ICD-9-CM: 433–438).
Table 2 presents overall, sex-, age-, geographic area-, and urbanization level-specific ID according to diabetes status. Diabetes increased the risk of RA irrespective of sex, age, geographic area, and urbanization level. The overall ID for the control group and the diabetic patients was 1·1 and 4·6/10 000 person-years (p-yr), respectively. The ID for male and female patients in the control group was 0·7 and 1·5/10 000 p-yr, respectively. The corresponding figures for the diabetic group were 2·2 and 6·7/10 000 p-yr, respectively. Female subjects were observed to be more susceptible to RA irrespective of diabetic status and the highest ID was found in diabetic females (6·7/10 000 p-yr). In control subjects, the age-specific ID of RA increased with age, and the highest ID was found in the >64 years age group (1·6/10 000 p-yr). The age-specific IDs were higher in diabetic patients compared to their non-diabetic counterparts. However, unlike the control group, the diabetic group showed a tendency for age-specific ID to decrease with age. With regard to geographic area and urbanization level, the IDs of RA in study subjects living in eastern Taiwan were much higher than those living in the other areas for both control subjects and diabetic patients. Compared to people living in the metropolis or satellite cities, those from rural areas tended to have a higher ID of RA (Table 2).
Table 2. Incidence densities of renal and perinephric abscess for the control group and diabetic groups
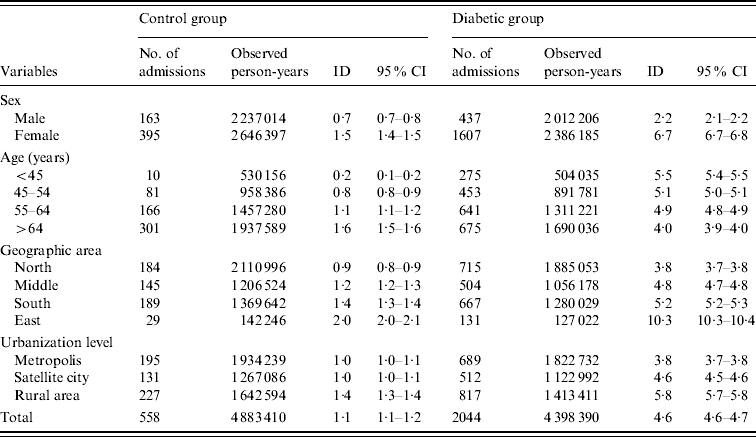
ID, Incidence density (per 10 000 person-years); CI, confidence interval.
Table 3 shows the cumulative event rates and hazard ratios (HRs) of RA over an 11-year study period. The adjusted HR (aHR) in relation to diabetes was significantly increased at 3·81 [95% confidence interval (CI) 3·44–4·23, P<0·0001]. Female patients were 2·78 (95% CI 2·51–3·08, P<0·0001) times more likely than males to be hospitalized due to RA. On the other hand, there was no difference in HR between different age groups. Compared to those living in the north, people from south (aHR 1·36, 95% CI 1·22–1·51) and east (aHR 2·14, 95% CI 1·72–2·66) were both associated with significantly increased aHRs of RA. Moreover, rural residents had a significantly increased aHR of RA (1·16), compared to those living in metropolitan areas.
Table 3. Relative hazards of admission due to renal and perinephric abscess according to diabetes, age, sex, geographic area, and urbanization level
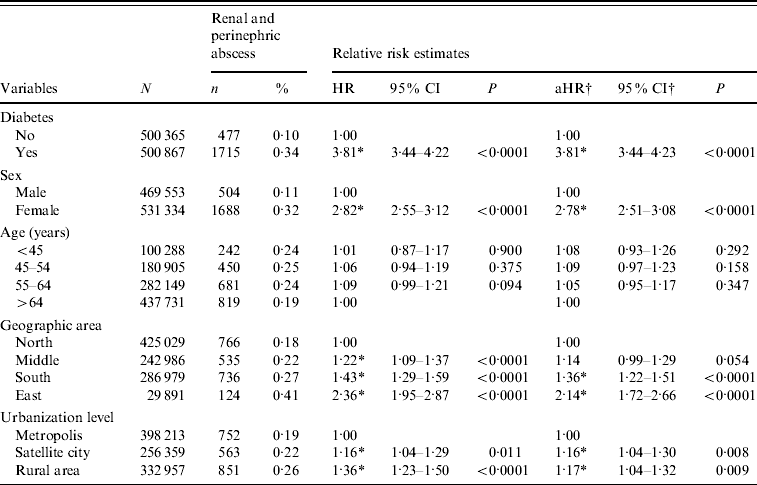
HR, Hazard ratio; CI, confidence interval; aHR, adjusted hazard ratio.
† Estimated from multivariate Cox proportional hazard model with diabetes, age, sex, geographic area, and urbanization level simultaneously included in the model.
* P<0·05.
To assess the severity of disease for the study subjects we further calculated the in-hospital mortality rate for study subjects. We observed that control subjects had a higher case-fatality rate during hospital stay than diabetic patients (3·4% vs. 2·3%), but such difference was not significant statistically (data not shown).
DISCUSSION
Some previous studies reported diabetes as a risk factor for RA and diabetic patients accounted for 28–78% of all cases suffering from RA [Reference Yen3, Reference Coelho5, Reference Shu, Green and Orihuela7, Reference Lee8]. There are some differences in host response to bacteria in the urinary tract in diabetic patients, including defects in leukocyte function [Reference Valerius14], and an increased bacterial adherence to uroepithelial cells of diabetic patients [Reference Hoepelman15]. Further, increased growth rates of Escherichia coli were observed in the presence of moderate or high amounts of glucose, as is found in the urine of diabetics [Reference Geerlings16]. In addition, local complications related to neuropathy, such as impaired bladder emptying, were observed in diabetic patients [Reference Hosking, Bennett and Hampton17]. As a result, diabetic patients tend to have higher incidence and prevalence rates of bacteriuria. Evidence from epidemiological studies suggested that asymptomatic bacteriuria and symptomatic UTIs occur more frequently in diabetic females than in non-diabetic females. Moreover, diabetic patients are at a greater risk for developing more serious consequences, such as emphysematous cystitis, pyelonephritis, renal papillary necrosis, and abscess [Reference Stapleton18]. Our results are in accord with these previous findings.
Although most of the previous studies reported that RA tended to occur more frequently in female patients than in males; female patients accounted for 48–80% of RA cases [Reference Yen3–Reference Coelho5, Reference Shu, Green and Orihuela7]. Nearly all of the previous studies were hospital-based and the differences in sex distribution may come from the differences in the manner of case ascertainment and the characteristics of the population served by the study hospitals. In our population-based study, female patients had higher IDs of RA than their male counterparts, irrespective of diabetes status, which provided support for previous findings. The UTI afflicts females more frequently than males at all ages, except in the first year of life [Reference Wettergren, Jodal and Jonasson19]. The pathological mechanisms for most RA are involved with ascending infections associated with infected urine, haematogenous spread, and proximity of the kidney to an infected area, or surgical contamination. The higher prevalence of cystitis in females associated with the ascending infection may also contribute to the higher IDs of RA in females.
Although age posed little influence on the risk of RA when both diabetic and control groups were collectively analysed, our data did show an apparent dissimilarity of age-specific risk of RA in diabetic and control groups separately. Unlike the control group, for which the age-specific risk of RA increased with age, younger diabetic patients tended to have a higher risk of RA. In a study on variation in diabetes care by patient's age, O'Connor et al. [Reference O'Connor20] reported that compared to individuals aged ⩾65 years, those aged 45–64 years are more likely to have poor blood sugar control, poor health-related behaviour, less frequent clinic visits, and irregular assessment of diabetes-related complications, which could worsen the prognosis of young diabetic patients, which in turn could be responsible for the higher ID of RA in younger diabetic patients observed in our study. This could explain the higher ID in our results and suggests the need for more aggressive care to be delivered to younger diabetic patients to further reduce the incidence of RA.
Geographic- and urbanization-level differences in the relative hazards of RA were also observed in our study. Patients living or working in eastern Taiwan, a rather remote and isolated area longitudinally separated by the Central Mountains from the western part of Taiwan Island, mostly had elevated HR levels of RA. A considerable number of studies have documented that rural residents are at an especially high risk for inadequate healthcare [Reference Tan13]. Failure to obtain timely care may result in poorer health or increased the risk of medical complications, including RA.
Our study had several methodological strengths. First, we used the NHI dataset, which is representative nationally with selection or recall bias being most unlikely, there is also small likelihood of non-response and loss to follow-up of cohort members. Second, the advantage of using insurance claims data in clinical research is the easy access to longitudinal records for a large sample of geographically dispersed patients, which greatly increased the representativeness of the study sample. Third, such a large number of study subjects made it possible for us to make analyses concerning certain variables of interest including age, sex, geographic area, urbanization status, and treatment modality.
Several limitations should be noted in our study. First, exclusive reliance on the claims data might have resulted in potential misclassification bias in our study. The accuracy of single diabetes diagnosis in the NHI claims data in 2000 was reported to be 74·6% [Reference Lin21], but we used at least two diabetes-related diagnoses with the first and the last visits >30 days apart, which would greatly reduce the likelihood of disease misclassification. The control group might have been mixed up with new onset or undiagnosed diabetes cases. However, such misclassification bias, was likely to be non-differential, which would tend to underestimate rather than overestimate the true relative hazard. Second, we could not differentiate between type 1 and type 2 diabetes in the diabetic population included in our study. In Taiwan, type 1 diabetes constitutes only 1·8% of all types of diabetes [Reference Chuang22] and the ratio of newly diagnosed type 2 to type 1 diabetes in schoolchildren aged 6–18 years is about 6:1 [Reference Wei23]. Therefore, the majority of our young diabetic patients tend to be of type 2 diabetes. Last, using the linked administrative data, we were unable to make a comprehensive adjustment for risk factors of RA incidence.
In conclusion, diabetes may increase the risk of hospitalization for RA, especially in the younger age group. Female subjects were observed to be more susceptible to RA irrespective of diabetic status. Additionally, there was a tendency that patients from remote and least urbanized areas were at a greater risk of RA. More aggressive care should be administered to those high-risk segments of the patient population in order to further reduce the risk of RA in diabetes.
ACKNOWLEDGMENTS
This study was partially supported by a grant from the National Scientific Council (NSC 96-2314-B-227-005-MY2), who had no role in the study. The interpretation and conclusions contained herein are those of the authors and do not represent those of BNHI, Department of Health or NHRI.
DECLARATION OF INTEREST
None.





