Serum levels of liver enzymes such as alanine transaminase (ALT), aspartate transaminase (AST) and γ-glutamyltransferase (GGT) are the main markers of liver dysfunction and are considered as the important indicators of non-alcoholic fatty liver disease (NAFLD) in the general population(Reference Gowda, Desai and Hull1–Reference Chalasani, Younossi and Lavine3). NAFLD as the most common chronic liver disease refers to excess fat accumulation in the liver (more than 5 % of liver weight) and is closely linked to the metabolic syndrome and type 2 diabetes mellitus(Reference Kim and Younossi4,Reference Leite, Villela-Nogueira and Cardoso5) . The global prevalence of NAFLD is 25·24 %(Reference Araújo, Rosso and Bedogni6). In the pathogenesis of NAFLD, gut microbiota dysbiosis, insulin resistance, inflammation, oxidative stress, dyslipidaemia and obesity are the main contributing factors(Reference Sangouni, Ghavamzadeh and Jamalzehi7–Reference Sangouni and Ghavamzadeh9). Adherence to the Western dietary patterns which are high in red and processed meats, full-fat dairy products, SFA and refined sugars is directly correlated with the risk of NAFLD(Reference Asrih and Jornayvaz10). Lifestyle modification is the main strategy to manage NAFLD(Reference Zelber-Sagi, Godos and Salomone11,Reference Tomasiewicz, Flisiak and Halota12) .
The Mediterranean diet (MedDiet) contains high amounts of vegetables, fruits, whole grains, fibre, dairy products, fish, seafood and olive oil, as well as low amounts of red and processed meats and refined sugars(Reference Bach-Faig, Berry and Lairon13). Moreover, this plant-based diet is high in PUFA and the ratio of MUFA:SFA(Reference Torres, Aghemo and Lleo14). MUFA and PUFA can improve inflammation, insulin resistance and obesity(Reference Wardhana and Datau15–Reference Chrysohoou, Panagiotakos and Pitsavos18). In addition, high intake of fibres and limited intake of refined sugar modulate gut microbiota and improve dyslipidaemia, insulin resistance and obesity(Reference Parnell, Raman and Rioux19,Reference Macdonald20) . Med-style diet improves glycaemic control, obesity and cardiovascular risk factors(Reference Kastorini, Milionis and Esposito17,Reference Ndanuko, Tapsell and Charlton21,Reference Huo, Du and Xu22) . MedDiet reduces steatosis and hepatic fat accumulation(Reference Abenavoli, Greco and Milic23,Reference Ryan, Itsiopoulos and Thodis24) . Several randomised controlled trials (RCT) have been conducted to evaluate the effect of MedDiet on liver enzymes; however, results are contradictory. Some RCT showed a protective effect of MedDiet on liver enzymes(Reference Biolato, Manca and Marrone25–Reference Properzi, O’Sullivan and Sherriff28), but some others found no significant effects(Reference Abenavoli, Greco and Milic23,Reference Ryan, Itsiopoulos and Thodis24,Reference Abenavoli, Greco and Nazionale29–Reference Pintó, Fanlo-Maresma and Corbella32) . Therefore, for the first time, we conducted a systematic review and meta-analysis on RCT investigating the effect of the MedDiet on liver enzymes including AST, ALT and GGT.
Methods
Registration of the present study was performed in the PROSPERO (http://www.crd.york.ac.uk/PROSPERO), an international prospective register of systematic reviews, under registration number CRD42021233214.
Search strategy
PubMed, Web of Science, Scopus and Google scholar databases were searched until December 2020 with no language or date restriction using Medical Subject Heading and non-Medical Subject Heading terms. We used the following keywords: (Diet, Mediterranean OR Mediterranean Diets) AND (liver OR liver enzyme OR Transaminases OR Aminotransferase OR transpeptidase OR Alanine Transaminase OR Alanine Aminotransferase OR ALT OR SGPT OR Aspartate Aminotransferases OR AST OR SGOT OR Alkaline phosphatase OR ALP OR Gamma-Glutamyltransferase OR GGT OR lactate dehydrogenase OR L-Lactate Dehydrogenase OR “Dehydrogenase L-Lactate OR Dehydrogenase Lactate OR LDH OR AST-to-ALT ratio OR AST to ALT ratio OR liver enzyme abnormality OR liver enzyme activity OR liver function tests OR LEA OR AST/ALT).
Study selection
Based on titles and abstracts, the relevant studies were screened by two investigators separately (A. S. and Sh. H.). All completed RCT evaluating the effect of a Med-style diet compared with any control diet on liver enzymes in adults (age ≥ 18 years) were eligible for inclusion. The meta-analysis included RCT evaluating MedDiet adherence and using the standard instruments such as a 24-h dietary recall, food record and FFQ. Studies involving pregnant and lactating women, as well as examining nutrients, food groups and supplements rather than dietary pattern were excluded.
Data extraction
Data extraction was conducted independently by two authors (Sh. H. and A. S.). The following data were extracted: first author’s family name, publication year, sex and mean age of participants, study design, country, intervention duration, number of participants in the MedDiet and the control groups, type of MedDiet and control diet, reported data and levels or changes in liver enzymes (ALT, AST and GGT) for the intervention and control groups.
Quality assessment
The quality assessments were performed by two independent authors (A. S. and Sh. H.). The quality assessment of articles was performed using the Cochrane Risk of Bias Tool for RCT(Reference Higgins, Altman and Sterne33). This tool consisted of seven domains as follows: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other sources of bias. As blinding is almost impossible for dietary intervention trials, we did not consider the blinding of participants and investigators as a key domain to assess the risk of bias. We classified these domains as low risk of bias, high risk of bias or unclear. Finally, if at least three domains were low risk of bias, the study was considered to have good quality.
Data synthesis and statistical analysis
To calculate the effect sizes of variables, the mean difference and standard deviation of each study were extracted for the intervention and control groups. To calculate the weighted mean differences (WMD) and their CI (95 % for continuous outcomes) in the present meta-analysis, random effects model was conducted(Reference Borenstein, Hedges and Higgins34). Cochran’s Q test and quantified using the inconsistency index (I-squared) were used to evaluate the heterogeneity among the studies(Reference Higgins, Altman and Gøtzsche35). High heterogeneity among the studies was defined as I 2 ≥ 75 % and P-value of Q statistic < 0·1(Reference Higgins, Thompson and Deeks36). To find out the possible sources of heterogeneity, subgroup analyses were performed based on the control group diet and participants’ health status. Sensitivity analysis was performed by removing a study from the meta-analysis and calculating the effect size with the remaining studies(Reference Egger, Smith and Altman37). This process was performed for all studies. To evaluate the publication bias, Begg’s test and Egger’s test were used(Reference Egger, Smith and Altman37). STATA, version 11.2 (Stata Corp) was used to perform statistical analyses. P < 0·05 was considered significant.
Results
Literature search
Our electronic search of PubMed, Web of Science, Scopus and Google Scholar led to 365 articles, and ten studies were eligible for inclusion in our systematic review (Fig. 1).

Fig. 1. Flow chart of the studies selection process.
Study characteristics
Characteristics of all included studies are represented in Table 1. All studies were published from 2008 to 2020. Most of the studies were conducted in Europe(Reference Abenavoli, Greco and Milic23–Reference Biolato, Manca and Marrone25,Reference Georgoulis, Yiannakouris and Kechribari27,Reference Abenavoli, Greco and Nazionale29–Reference Pintó, Fanlo-Maresma and Corbella32) , and two were carried out in Asia(Reference Fraser, Abel and Lawlor26) and Australia(Reference Properzi, O’Sullivan and Sherriff28). All RCT had a parallel design, except two studies that had a cross-over design(Reference Ryan, Itsiopoulos and Thodis24,Reference Biolato, Manca and Marrone25) . The duration of the interventions ranged from 1·5 to 72 months. Six studies investigated the effect of MedDiet in subjects with NAFLD(Reference Abenavoli, Greco and Milic23–Reference Biolato, Manca and Marrone25,Reference Properzi, O’Sullivan and Sherriff28,Reference Abenavoli, Greco and Nazionale29,Reference Katsagoni, Papatheodoridis and Ioannidou31) , one in patients with obstructive sleep apnoea(Reference Georgoulis, Yiannakouris and Kechribari27), one in obese patients with diabetes(Reference Fraser, Abel and Lawlor26), one in subjects with high cardiovascular risk(Reference Pintó, Fanlo-Maresma and Corbella32) and one in subjects without CVD(Reference Cueto-Galán, Barón and Valdivielso30). In addition, there were several different control groups: low-fat diet(Reference Ryan, Itsiopoulos and Thodis24,Reference Biolato, Manca and Marrone25,Reference Properzi, O’Sullivan and Sherriff28,Reference Cueto-Galán, Barón and Valdivielso30,Reference Pintó, Fanlo-Maresma and Corbella32) , energy restriction diet(Reference Georgoulis, Yiannakouris and Kechribari27,Reference Katsagoni, Papatheodoridis and Ioannidou31) , American Diabetes Association diet(Reference Fraser, Abel and Lawlor26) and without any dietary treatment(Reference Abenavoli, Greco and Milic23,Reference Abenavoli, Greco and Nazionale29) .
Table 1. Characteristics of included randomised controlled clinical trials in the systematic review

M, male; F, female; RCT, randomised clinical trial; CHO, carbohydrate; Pro, protein; ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma-glutamyl transpeptidase; NAFLD, non-alcoholic fatty liver disease.
Quality of studies
All studies in our systematic review had a good quality based on the Cochran criteria. Score quality of the included studies was from 3 to 6. Four studies did not report using any method to conceal the allocation of participants(Reference Fraser, Abel and Lawlor26–Reference Properzi, O’Sullivan and Sherriff28,Reference Cueto-Galán, Barón and Valdivielso30) . For the blinding of assessors or analysts, five of the studies tried to address this potential source of bias; however, as all included studies were dietary trials, they could not blind the intervention protocols. All studies have evaluated selective reporting and incomplete outcome data (online Supplemental Table 1).
Meta-analysis
Effect of Mediterranean diet on aspartate aminotransferase level
Our findings on four studies (n 168 participants) demonstrated that MedDiet significantly decreases AST level (WMD = −0·38 IU/l, 95 % CI − 0·73, −0·03 IU/l; P = 0·03) (Fig. 2). Subgroup analysis based on health status reported stronger results on participants with NAFLD (WMD = −0·62 IU/l, 95% CI − 0·99, −0·25 IU/l; P = 0·001; Cochran’s Q = 0·04, P = 0·97, I 2 = 0·00). However, analysis on parallel studies showed no significant association between this diet and AST level (WMD = −0·22 IU/l, 95% CI − 0·57, 0·11 IU/l; P = 0·19; Cochran’s Q = 2·24, P = 0·32, I 2 = 10·81). Due to the limited number of available studies, we could not conduct the subgroup analysis based on other potential factors like control group diet. Sensitivity analysis revealed that the overall effects of a MedDiet on AST levels were significantly influenced by removing some studies(Reference Abenavoli, Greco and Milic23,Reference Biolato, Manca and Marrone25,Reference Abenavoli, Greco and Nazionale29) (online Supplementary Fig. 1). No significant heterogeneity was observed among the studies (Cochran’s Q = 4·68, P = 0·19, I 2 = 36·01). The analyses with different pre-post correlation (r = 0·2, and r = 0·8) showed contradictory results in some subgroups (Table 2).

Fig. 2. Forest plot displaying mean difference (represented by the black square) and 95 % CI (represented by horizontal line) for aspartate aminotransferase (AST) level and Mediterranean diet. Weights are from random effects analysis. The area of the black square is proportional to the specific study weight to the overall meta-analysis. The centre of the diamond displays the pool mean difference, and its width shows the pooled 95 % CI.
Table 2. Analysis with different pre-post correlation
(Odds ratio and 95 % confidence intervals)
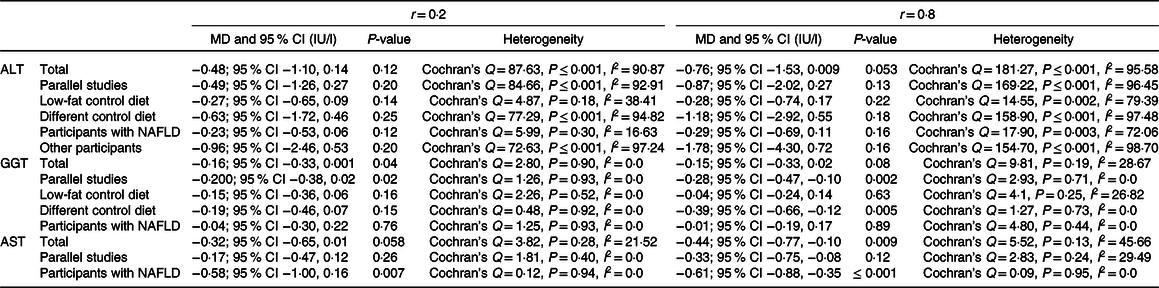
Effect of Mediterranean diet on γ-glutamyltransferase level
Our analysis on eight studies (n 484 participants) indicated that MedDiet significantly decreases GGT level (WMD = −0·16 IU/l, 95 % CI − 0·32, −0·006 IU/l; P = 0·04) (Fig. 3). Analysis on parallel studies reported similar findings (WMD = −0·23 IU/l, 95 % CI − 0·41, −0·05 IU/l; P = 0·01; Cochran’s Q = 1·53, P = 0·90, I 2 = 0·00). However, our findings in participants with NAFLD were contradictory (WMD = −0·03 IU/l, 95 % CI − 0·27, 0·20 IU/l; P = 0·78; Cochran’s Q = 2·03, P = 0·84, I 2 = 0·00). In addition, no significant decrease in GGT was reported based on the control group diet (WMDlow fat diet = −0·12 IU/l, 95 % CI − 0·32, 0·07 IU/l; P = 0·23; Cochran’s Q = 2·88, P = 0·41, I 2 = 0·00) and (WMD other diets = −0·24 IU/l, 95 % CI − 0·51, 0·02 IU/l; P = 0·07; Cochran’s Q = 0·75, P = 0·86, I 2 = 0·00) (online Supplementary Fig. 2). Sensitivity analysis represented that the overall effects of MedDiet on GGT levels were significantly influenced by excluding of some studies(Reference Georgoulis, Yiannakouris and Kechribari27,Reference Abenavoli, Greco and Nazionale29–Reference Katsagoni, Papatheodoridis and Ioannidou31) (online Supplementary Fig. 3). No heterogeneity was observed among the studies (Cochran’s Q = 4·15, P = 0·76, I 2 = 0·00). The analyses with different pre-post correlation (r = 0·2, and r = 0·8) revealed contradictory results in some subgroups (Table 2).
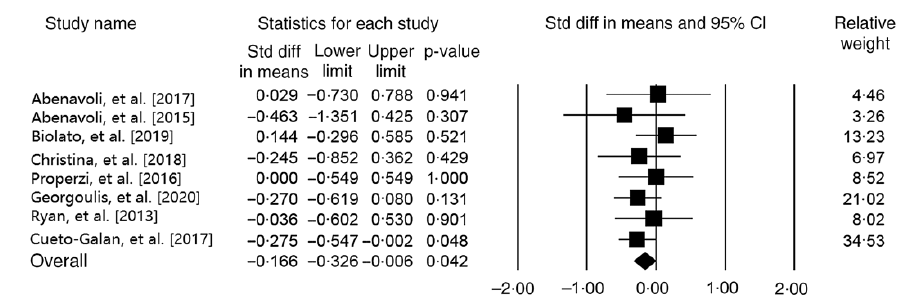
Fig. 3. Forest plot displaying mean difference (represented by the black square) and 95 % CI (represented by horizontal line) for γ-glutamyltransferase (GGT) level and Mediterranean diet. Weights are from random effects analysis. The area of the black square is proportional to the specific study weight to the overall meta-analysis. The centre of the diamond displays the pool mean difference, and its width shows the pooled 95 % CI.
Effect of Mediterranean diet on alanine transaminase level
Our meta-analysis on nine studies (n 465 participants) indicated no significant relationship between MedDiet and ALT level (WMD = –0·55 IU/l, 95 % CI –1·25, 0·13 IU/l; P = 0·11) (Fig. 4). Another analysis on studies with parallel design showed the same results (WMD = –0·59 IU/l, 95 % CI –1·49, 0·30 IU/l; P = 0·19; Cochran’s Q = 112·84, P ≤ 0·001, I 2 = 94·68). The results demonstrated that the subgroup analysis based on the control group diet (WMDlow-fat diet = –0·28 IU/l, 95 % CI –0·69, 0·11 IU/l; P = 0·07; Cochran’s Q = 6·94, P ≤ 0·001, I 2 = 56·78) and (WMD other diets = –0·78 IU/l, 95 % CI –2·08, 0·52 IU/l; P = 0·24; Cochran’s Q = 103·85, P ≤ 0·001, I 2 = 96·14), and participants’ health status (WMDParticipants with NAFLD = –0·26 IU/l, 95 % CI –0·60, 0·07 IU/l; P = 0·12; Cochran’s Q = 8·74, P = 0·12, I 2 = 42·79) and (WMDOther participants = –1·18 IU/l, 95 % CI –3·01, 0·64 IU/l; P = 0·20; Cochran’s Q = 99·18, P ≤ 0·001, I 2 = 97·98) have no significant effect on ALT (online Supplementary Fig. 4 and 5). Moreover, the sensitivity analysis showed that our finding in this field was significantly influenced (WMD = –0·23 IU/l, 95 % CI –0·47, –0·004 IU/l; P = 0·04) by excluding the study of Fraser et al. (Reference Fraser, Abel and Lawlor26) (online Supplementary Fig. 6). We observed evidence of heterogeneity among the effect sizes of the included studies (Cochran’s Q = 117·76, P ≤ 0·001, I 2 = 93·20). The analyses with different pre-post correlation (r = 0·2, and r = 0·8) showed similar results (Table 2).
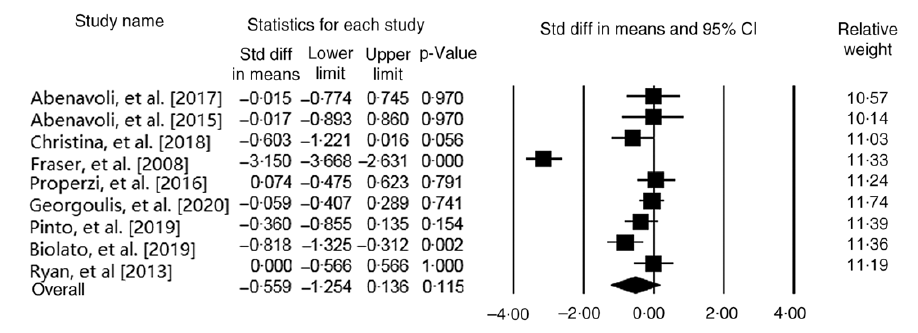
Fig. 4. Forest plot displaying mean difference (represented by the black square) and 95 % CI (represented by horizontal line) for alanine transaminase (ALT) level and Mediterranean diet. Weights are from random effects analysis. The area of the black square is proportional to the specific study weight to the overall meta-analysis. The centre of the diamond displays the pool mean difference, and its width shows the pooled 95 % CI.
Meta-regression
There is no relationship between increasing the duration of the trial and AST (Slope: −0·002, 95 % CI −0·006, 0·0007; P = 0·11) and GGT (Slope: −0·00009, 95 % CI −0·00002, 0·00001; P = 0·36) (online Supplementary Fig. 7 and 8).
Publication bias
Publication bias was not observed on the basis of asymmetry tests for the effect of MedDiet on AST, ALT and GGT level (AST: Begg’s test P = 1·00 and Egger’ test P = 0·30), (ALT: Begg’s test P = 0·75 and Egger’ test P = 0·95) and (GGT: Begg’s test P = 1·00 and Egger’ test P = 0·44).
Discussion
To the best of our knowledge, the present study was the first systematic review and meta-analysis on the effect of MedDiet on liver enzymes including AST, ALT and GGT. This systematic review and meta-analysis showed that adherence to the MedDiet improves levels of AST and GGT but has no significant effect on ALT. Moreover, the efficacy of MedDiet on ALT was sensitive to exclusion of the study that conducted by Fraser et al. (Reference Higgins, Thompson and Deeks36). In addition, sensitivity analyses showed that the effects of MedDiet on AST and GGT were significantly influenced by removing some RCT. Evidence confirmed that the lifestyle modification (aerobic exercise, weight loss and adherence to the healthy dietary patterns) can improve the features of NAFLD(Reference Zelber-Sagi, Godos and Salomone11,Reference Tomasiewicz, Flisiak and Halota12) . We evaluated the net effect of MedDiet on liver enzymes, independent from physical activity as well as weight loss. In the RCT that were included to our meta-analysis, the participants were asked not to change their physical activity during interventions and if there was a significant difference in the mean change of exercise between the groups during the follow-up, the mean change of body exercise was considered as a cofactor.
Evidence showed that adherence to the healthy dietary patterns containing high amounts of fruits, vegetables, white meat and olive oil is inversely associated with the risk of NAFLD(Reference Adriano, De Carvalho Sampaio and Arruda38). In the same vein, a meta-analysis on observational studies confirmed that higher adherence to the MedDiet decreases the risk of NAFLD(Reference Hassani Zadeh, Mansoori and Hosseinzadeh39). Previously, some systematic reviews demonstrated the beneficial effects of MedDiet and its components on main causes of NAFLD such as obesity, dyslipidaemia, insulin resistance and oxidative stress(Reference Buckland, Bach and Serra-Majem40–Reference Amiot, Riva and Vinet42). The therapeutic effects of adherence to the MedDiet on NAFLD features like liver enzymes and steatosis can be attributed to the high intake of MUFA and PUFA, fibres, phytochemicals and antioxidants, as well as the low intake of red and processed meats, and refined sugars(Reference Bach-Faig, Berry and Lairon13,Reference Sofi and Casini43) . MUFA and PUFA improve pathways involved in lipid metabolism and oxidative stress, increase hepatic sensitivity to insulin and decrease liver fat content(Reference Jump, Lytle and Depner44,Reference Scorletti and Byrne45) . A meta-analysis showed that n-3 PUFA can decrease liver fat, ALT, AST and GGT in patients with NAFLD(Reference Yan, Guan and Gao46). On the other hand, Zhao et al. (Reference Zhao, Yang and Mao47) found an inverse correlation between dietary fibre intake and NAFLD risk in the general population. Dietary fibre intake can delay gastric emptying and reduce postprandial blood glucose, increase excretion of lipids, reduce fat accumulation and produce SCFA like propionic acid and butyric acid, which improve insulin resistance, dyslipidaemia, gut microbiota dysbiosis, obesity and consequent features of NAFLD(Reference Surampudi, Enkhmaa and Anuurad48–Reference De Carvalho, De Paula and Viana53). It has been shown that fruit fibre consumption improves liver enzymes and hepatic steatosis(Reference Cantero, Abete and Monreal54). In addition, fruits and vegetables as the rich sources of antioxidants like polyphenols, and carotenoids, inhibit NF-κB activation, de novo lipogenesis, stimulate hepatic β-oxidation, reduce activation of hepatic stellate cells and decrease reactive oxygen species(Reference Del Rio, Rodriguez-Mateos and Spencer55–Reference Salomone, Godos and Zelber-Sagi57). A meta-analysis confirmed that consumption of green tea which is a rich source of polyphenols can decrease levels of liver enzymes in patients with NAFLD(Reference Mahmoodi, Hosseini and Kazemi58). However, another meta-analysis showed that supplementation with resveratrol as a polyphenol has no effect on liver enzymes in patients with NAFLD(Reference Elgebaly, Radwan and Aboelnas59). It seems, a set of polyphenols (which is an important feature of healthy dietary pattern) compared with a single polyphenol has more therapeutic effects on NAFLD.
The sensitivity analysis revealed that the effect of MedDiet on ALT was sensitive to exclusion of the study that conducted by Fraser et al. (Reference Fraser, Abel and Lawlor26), which means by removing this study, the overall results change to significant. Compared with the other RCT investigating the effect of MedDiet on ALT, type of the MedDiet and health status of participants are the key features of the trial of Fraser et al. (Reference Fraser, Abel and Lawlor26), such that the intervention group followed a modified MedDiet with 35 % carbohydrate (as low glycaemic index), 45 % fat and 20 % protein, and this study is the only RCT evaluating the effect of MedDiet on ALT among patients with diabetes. It should be noted that ALT is mainly found in the hepatocytes, but AST and GGT are not specific to the liver and are found in various organs(Reference Whitfield60–Reference Poelzl, Ess and Mussner-Seeber62). This can justify discrepancies came from results of the present study. Therefore, findings must be interpreted with caution.
Our study has two important strengths. First, the publication bias was not observed in the present study. Second, no heterogeneity and moderate (but not significant) heterogeneity was found among the effect sizes of trials investigating the effect of MedDiet on GGT and AST, respectively.
One of the limitations of this study was high heterogeneity among the effect sizes of studies evaluating the effect of MedDiet on ALT. The source of heterogeneity was not found by subgroup analyses or meta-regression. The high heterogeneity of the criteria used to select patients, and the components of MedDiets in RCT are the other limitations of the present study. Moreover, the effects of MedDiet can influence by genetic background of participants and polymorphisms, but there was no available data in this field. Finally, the efficacy of MedDiet on AST, GGT and ALT was sensitive to exclusion of some studies; Therefore, interpretation and conclusion came from results should be considered with caution.
Conclusion
MedDiet can significantly reduce levels of AST and GGT which are the important markers of liver function. However, MedDiet has no significant effect on ALT. Further RCT investigating the effect of MedDiet on features of NAFLD, especially in patients with NAFLD are needed to design a specific meta-analysis on main outcomes of patients with NAFLD.
Acknowledgements
The authors wish to thank the Nutrition Department in Shahid Sadoughi University of Medical Sciences in Yazd, Iran.
None.
M. H. and A. S. designed research; Sh. H. and A. S. collected the data; M. H., A. S. and Sh. H. analysed and interpreted the data; A. S. wrote the manuscript; M. H. and H. M.-K. critically revised the manuscript; and M. H. supervised the study. The final version of the manuscript was approved by all authors.
The authors have declared no conflict of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521002270









