Introduction
The Lower Siwalik Subgroup is well exposed in areas surrounding the town of Ramnagar, India, ~38 km northeast of Jammu city (Udhampur District, Jammu and Kashmir), and contains exposures of Middle Miocene deposits where a large number of fossil-bearing localities have been worked over the past century. In particular, the region is well known for fossil primates, such as Sivapithecus indicus Pilgrim, Reference Pilgrim1910; Sivaladapis palaeindicus (Pilgrim, Reference Pilgrim1932); Ramadapis sahnii Gilbert et al., Reference Gilbert, Patel, Singh, Campisano, Fleagle, Rust and Patnaik2017; and Kapi ramnagarensis Gilbert et al., Reference Gilbert, Ortiz, Pugh, Campisano, Patel, Singh, Fleagle and Patnaik2020; as well as associated large mammals (Brown et al., Reference Brown, Gregory and Hellman1924; Lewis, Reference Lewis1934; Colbert, Reference Colbert1935; Dutta et al., Reference Dutta, Basu and Sastry1976; Vasishat et al., Reference Vasishat, Gaur and Chopra1978; Thomas and Verma, Reference Thomas and Verma1979; Basu, Reference Basu2004; Gilbert et al., Reference Gilbert, Patel, Friedman, Pugh, Fleagle and Patnaik2014, Reference Gilbert, Patel, Singh, Campisano, Fleagle, Rust and Patnaik2017, Reference Gilbert, Sehgal, Pugh, Campisano, May, Patel, Singh and Patnaik2019, Reference Gilbert, Ortiz, Pugh, Campisano, Patel, Singh, Fleagle and Patnaik2020). In recent years, many fossil rodents, including Kanisamys cf. K. potwarensis Flynn, Reference Flynn1982a; Kanisamys indicus Wood, Reference Wood1937; Antemus chinjiensis Jacobs, Reference Jacobs1977; Sayimys sivalensis (Hinton, Reference Hinton1933); Megacricetodon daamsi Lindsay, Reference Lindsay1988; Megacricetodon sivalensis Lindsay, Reference Lindsay1988; Myocricetodon sivalensis Lindsay, Reference Lindsay1988; Myomimus sp.; Tamias urialis (Munthe, Reference Munthe1980); Punjabemys downsi Lindsay, Reference Lindsay1988; and Punjabemys mikros Lindsay, Reference Lindsay1988, have also been reported from Ramnagar (Parmar and Prasad, Reference Parmar and Prasad2006; Sehgal and Patnaik, Reference Sehgal and Patnaik2012; Sehgal, Reference Sehgal2013; Parmar et al., Reference Parmar, Prasad, Kumar, Malik and Norboo2015, Reference Parmar, Magotra, Norboo and Prasad2016, Reference Parmar, Magotra, Norboo and Prasad2017).
Despite growing evidence of a diverse micromammal fauna, to date there are no reports of any treeshrews from Ramnagar, and they are relatively rare in the Indian Siwaliks in general. Previously, Dutta (Reference Dutta1975) reported a rib cage, possibly attributable to Tupaia, from the Indian Upper Siwaliks, but this specimen has never been formally described or figured (Sargis, Reference Sargis2001). Chopra and Vasishat (Reference Chopra and Vasishat1979) recovered a partial cranium of a tupaiid from the Middle Siwaliks of Haritalyangar (Himachal Pradesh State, India), placing it in a new taxon, Palaeotupaia sivalicus Chopra and Vasishat, Reference Chopra and Vasishat1979, which firmly established tupaiids in the Indian Siwaliks by ca. 10–8.5 Ma (Pillans et al., Reference Pillans, Williams, Cameron, Patnaik, Hogarth, Sahni, Sharma, Williams and Bernor2005). However, most authors since then have followed Luckett and Jacobs (Reference Luckett and Jacobs1980) in considering Palaeotupaia as virtually identical to extant Tupaia, synonymizing the former into the latter genus. The hedgehog Galerix is also relatively rare in the Indian Siwaliks, although species such as Galerix rutlandae Munthe and West, Reference Munthe and West1980, and G. wesselsae Zijlstra and Flynn, Reference Zijlstra and Flynn2015, have been reported from the Lower Siwaliks of the Punjab as well as Sindh Province, Pakistan (Munthe and West, Reference Munthe and West1980; Zijlstra and Flynn, Reference Zijlstra and Flynn2015).
The present study reports newly recovered fossil teeth from the Dehari locality in the Ramnagar region that can be attributed to a new treeshrew genus and species (named below) and a relatively large, indeterminate species of Galerix. Additionally, we describe new dental specimens of previously documented rodents, including Kanisamys indicus and Sayimys sivalensis, as well as specimens of Murinae indet. preserving a mix of Antemus and Progonomys features. The murines, in particular, are biochronologically informative, and provide an updated age estimate for the Dehari deposits, specifically, and the Ramnagar region, more broadly.
Geological setting
Much of the Ramnagar region has long been considered to be roughly equivalent to the Chinji Formation on the Potwar Plateau, Pakistan (Brown et al., Reference Brown, Gregory and Hellman1924; Pilgrim, Reference Pilgrim1927; Colbert, Reference Colbert1935; Gregory et al., Reference Gregory, Hellman and Lewis1938; Vasishat et al., Reference Vasishat, Gaur and Chopra1978; Gaur and Chopra, Reference Gaur and Chopra1983; Nanda and Sehgal, Reference Nanda and Sehgal1993; Basu, Reference Basu2004). Recent rodent biochronological studies based on limited material suggest the area correlates to the middle or lower half of the Chinji Formation, with an age estimate of ca. 13.8–12.5 Ma (Parmar and Prasad, Reference Parmar and Prasad2006; Sehgal and Patnaik, Reference Sehgal and Patnaik2012; Patnaik, Reference Patnaik, Wang, Flynn and Fortelius2013; Gilbert et al., Reference Gilbert, Patel, Friedman, Pugh, Fleagle and Patnaik2014, Reference Gilbert, Ortiz, Pugh, Campisano, Patel, Singh, Fleagle and Patnaik2020; Parmar et al., Reference Parmar, Prasad, Kumar, Malik and Norboo2015, Reference Parmar, Magotra, Norboo and Prasad2017, Reference Parmar, Prasad and Norboo2018; Singh et al., Reference Singh, Gilbert, Patel and Patnaik2018; but see below). Fossil specimens presented here are from the Dehari locality (N32°46'59.4″N, 75°16′39.5″E), ~0.5 km northeast of Dehari village and ~5 km southwest of the town of Ramnagar (Fig. 1).This location is part of the same small area of exposures where previously described specimens from Dehari were mapped (Parmar and Prasad, Reference Parmar and Prasad2006; Parmar et al., Reference Parmar, Prasad, Kumar, Malik and Norboo2015, Reference Parmar, Magotra, Norboo and Prasad2017; Singh et al., Reference Singh, Gilbert, Patel and Patnaik2018) and are from the same general stratigraphic position. Lithologically, the sites are dominantly characterized by red to reddish-brown mudstones alternating with thick, fine-grained, gray sandstones. Additionally, thin intraformational clay conglomerate beds containing shells and bivalves are preserved within the paleosols. The present micromammals were recovered from these clay conglomerates by bulk sampling and by a maceration process as described below.

Figure 1. (1) General geological map of the Siwaliks Jammu sub-Himalaya (modified after Gupta and Verma, Reference Gupta and Verma1988; Basu, Reference Basu2004). (2) Enlarged map of the Siwalik Group surrounding Ramnagar showing the Dehari locality discussed in the text (yellow circle) and other fossil localities. (3) Simplified stratigraphic section of the study locality (Dehari).
Materials and methods
The micromammal teeth were recovered by macerating ~200 kg of sediments from Dehari in the Biostratigraphy Lab at the Wadia Institute of Himalayan Geology (WIHG), Dehradun (India), originally collected by R.K. Sehgal (Fig. 1) during the 2017 and 2019 field seasons. The sediments were broken into fragments and then soaked in plastic tubs with buffered acetic acid and water. The loose material was wet sieved through 20, 40, and 60 mesh sieves (ISTM). Material collected in the sieves was dried in the sun and microvertebrates were sorted using a fine brush under a binocular microscope housed at WIHG. The specimens described in this paper are housed in the WIHG, and bear the acronym WIMF/A (Wadia Institute Micro Fossil Series A).
In order to facilitate the study of these small micromammal teeth, three-dimensional (3D) imaging was obtained using high-resolution micro-CT (μCT), housed in the Molecular Imaging Center of the Keck School of Medicine of the University of Southern California (Los Angeles, CA, USA). Each fossil tooth was scanned individually within a plastic sample holder (i.e., centrifuge tube), with the specimen held securely in place using foam and soft cotton to prevent movement artefacts during the scan. Scans were obtained with a GE Phoenix Nanotom M system (GE Inspection Technologies, Lewistown, PA, USA) with the following parameters: voltage = 120; current = 70; filter = 2.5 mm Al + Al 0.5 mm; averaging = 2; magnification = 27.778–33.335; isometric voxel dimensions = 0.00299–0.00359 mm. 3D surface renderings of each specimen were created in Amira 3D v.2021.1 software (Thermo Fisher Scientific, Inc., Waltham, MA, USA) from image stacks of 16-bit unsigned DICOM images after undergoing a median (2 pixel) filter. These 3D surfaces, as shown in Figures 2, 7, and 8, are available to download from MorphoSource (www.morphosource.org) as part of the project “Siwalik Fossils from Ramnagar (Jammu and Kashmir), India.”
Following the methods of Selig et al. (Reference Selig, Sargis and Silcox2019a, Reference Selig, Sargis and Silcoxb, Reference Selig, Sargis, Chester and Silcox2020), 3D geometric morphometric (3DGM) analyses were conducted using data from the new treeshrew specimen (WIMF/A 4699) in relation to a large sample of extant and fossil treeshrew m2s, where available. The same 18 landmarks taken by Selig et al. (Reference Selig, Sargis, Chester and Silcox2020) were collected on WIMF/A 4699 in the Avizo v.8.1.1 software (Thermo Fisher Scientific, Inc., Waltham, MA) using the landmark editor function, then the landmark data for this specimen were added to the accessible sample (N = 46) from the Selig et al. (Reference Selig, Sargis, Chester and Silcox2020) dataset (i.e., two specimens, Ptilocercus lowii Gray, Reference Gray1848 [YPM MAM 10179] and Dendrogale murina Schlegel and Müller, Reference Schlegel and Müller1843, UAM: Mamm 103000 had to be excluded; see Supporting Information [SI] Table 1 and SI Dataset 1 for list of included specimens). A Generalized Procrustes Analysis (GPA) was performed to scale, rotate, and translate the landmark data, followed by a Principal Components Analysis (PCA) in the software package Morphologika2 (O'Higgins and Jones, Reference O'Higgins and Jones2006) using wireframes to visualize differences in shape along the resulting PC axes. For visualization, the Procrustes coordinates were also submitted to a PCA in the software package PAST v.4.03 (Hammer et al., Reference Hammer, Harper and Ryan2001) along with a UPGMA cluster analysis based on the generic-level averages of the first five PC scores among the included taxa to assess phenetic affinities.
Table 1. Comparative dental measurements (mm) of extant and fossil treeshrews. Max Width = maximum width; Max Length = maximum length. Comparative measurements from Chopra and Vasishat (Reference Chopra and Vasishat1979), Chopra et al. (Reference Chopra, Kaul and Vasishat1979), Jacobs (Reference Jacobs and Luckett1980), Qiu (Reference Qiu1986), Mein and Ginsburg (Reference Mein and Ginsburg1997), Ni and Qiu (Reference Ni and Qiu2012), and the current study. Tupaia glis (Diard, Reference Diard1820); Tupaia minor Günther, Reference Günther1876; Tupaia montana Thomas, Reference Thomas1892; and Tupaia miocenica Mein and Ginsburg, Reference Mein and Ginsburg1997,

In order to make inferences of dietary adaptations about the fossil treeshrew, Dirichlet normal energy (DNE), 3D orientation patch count rotated (3D-OPCR), and relief index (RFI) were measured in WIMF/A 4699 relative to a large sample of other extant and fossil treeshrews previously measured by Selig et al. (Reference Selig, Sargis, Chester and Silcox2020) (see SI Table 1). These methods are among a suite of dental topographic metrics that quantify functional aspects of the occlusal surface of teeth that can be tied directly to dietary adaptations (Ungar and M'Kirera, Reference Ungar and M'Kirera2003; Evans et al., Reference Evans, Wilson, Fortelius and Jernvall2007; Boyer, Reference Boyer2008; Bunn et al., Reference Bunn, Boyer, Lipman, St Clair, Jernvall and Daubechies2011, Winchester, Reference Winchester2016). For example, DNE quantifies occlusal curvature, so teeth with sharper crests and cusps have higher DNE values. High DNE values relate to more mechanically challenging diets, such as insects, whereas lower values relate to the processing of soft foods, such as fruit (Bunn et al., Reference Bunn, Boyer, Lipman, St Clair, Jernvall and Daubechies2011; Winchester, Reference Winchester2016). 3D-OPCR is a measure of surface complexity, so teeth with more crests, cusps, and ridges have higher values. High 3D-OPCR values also relate to insectivory, whereas low values relate to the consumption of softer foods, such as fruits (Evans et al., Reference Evans, Wilson, Fortelius and Jernvall2007; Winchester, Reference Winchester2016). Finally, RFI is a measure of relative crown height—teeth with relatively taller cusps or relatively taller teeth overall have higher RFI values. High RFI values relate to the consumption of insects, whereas low values relate to the consumption of softer foods (Ungar and M'Kirera, Reference Ungar and M'Kirera2003; Boyer, Reference Boyer2008).
Prior to analyzing WIMF/A 4699 for topographic analyses, the 3D surface was simplified to 10,000 faces and smoothed to 100 iterations with a lambda at 0.6 following the protocol of Selig et al. (Reference Selig, Sargis and Silcox2019b, Reference Selig, Sargis, Chester and Silcox2020). All three topographic metrics were measured using MorphoTester v.1.1.1 software (Winchester, Reference Winchester2016) using the default settings for DNE and the patch count set at 5 for 3D-OPCR. A second PCA was performed on the covariance matrix including the species means of these three topographic metrics, thus allowing visualization of overall variation in dental topography across species in our sample. All 3DGM and dental topographic data were collected by a single observer (KRS). See Selig et al. (Reference Selig, Sargis, Chester and Silcox2020) for additional methodological details.
The dental terminologies used here follow Jacobs (Reference Jacobs1978) for murids; Ziegler (Reference Ziegler1990) for erinaceids; Jacobs (Reference Jacobs1978), Flynn (Reference Flynn1982a), and López-Antoñanzas et al. (Reference López-Antoñanzas, Flynn and Knoll2013) for rhizomyines; Baskin (Reference Baskin1996) and López-Antoñanzas and Knoll (Reference López-Antoñanzas and Knoll2011) for ctenodactylines; and Jacobs (Reference Jacobs and Luckett1980) for tupaiids.
Repositories and institutional abbreviations
American Museum of Natural History, New York, NY (AMNH); Geological Survey of India (GSI); Howard University-Geological Survey of Pakistan (H-GSP); Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China (IVPP); Thailand Li Mae Long (T Li); Panjab University Anthropology (PUA); University of Alaska Museum of the North, Fairbanks, AK (UAM); Wadia Institute Micro Fossil Series A (WIMF/A); University of Michigan Museum of Zoology (UMMZ); Pakistan Museum of Natural History (PMNH); Vertebrate Palaeontology Laboratory/Jammu University/Lower Siwalik Mammals (VPL/JU/LSM); Vertebrate Palaeontology Laboratory/Rajeev Patnaik-Haritalyangar Micromammal (VPL/RP-HM); Yale Peabody Museum of Natural History, Yale University, New Haven, CT (YPM); Yale-Geological Survey of Pakistan (YGSP).
Systematic paleontology
Scandentia Wagner, Reference Wagner1855
Tupaiidae Gray, Reference Gray1825
Genus Sivatupaia new genus
Type species
Sivatupaia ramnagarensis n. gen. n. sp., only known species, from the site of Dehari near Ramnagar, Udhampur district, Jammu and Kashmir, India.
Diagnosis
As for type species (see below).
Etymology
The genus name Sivatupaia is derived from a combination of Siva, from being found in the Siwaliks (=Sivaliks), and Tupaia for treeshrew.
Holotype and only known specimen
WIMF/A 4699 (Wadia Institute Micro Fossil Series A); moderately worn, left lower m1 or m2 (Fig. 2).
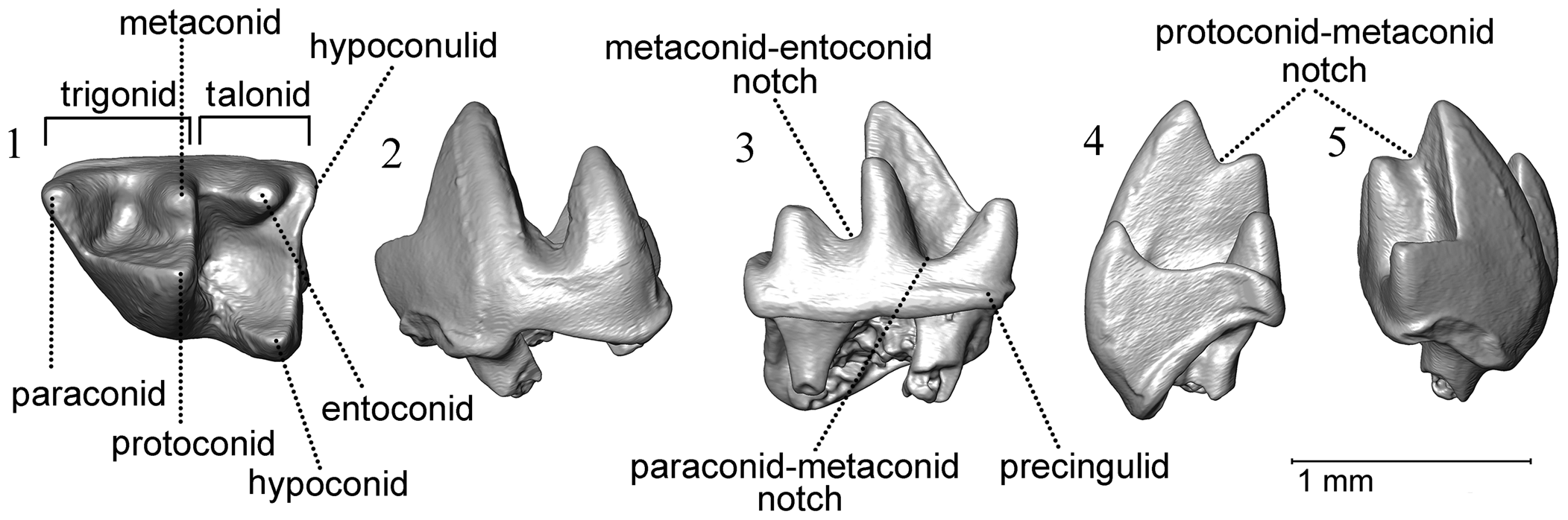
Figure 2. Sivatupaia ramnagarensis n. gen. n. sp., WIMF/A 4699 (holotype). 3D surface renderings of tooth in: (1) occlusal; (2) buccal; (3) lingual; (4) posterior; (5) anterior views.
Diagnosis
Sivatupaia ramnagarensis n. gen. n. sp. differs from known extant tupaiid genera Anathana and Dendrogale in lower molar features, having a well-developed metaconid and entoconid, as well as a relatively vertical hypoconulid. It differs from Prodendrogale by having a smaller hypoconulid and a narrower notch separating the entoconid and hypoconulid. It is also much smaller than Prodendrogale yunnanica Qiu, Reference Qiu1986 (see measurements in Table 1). Sivatupaia ramnagarensis n. gen. n. sp. demonstrates some similarities to the lower molars of Tupaia due to its more distinct entoconid, hypoconulid, and vertical hypoconulid. However, it is distinct from all other treeshrew specimens in shape, displaying a combination of a relatively narrow crown, a mesially shifted paraconid, a tall and more lingually shifted protoconid resulting in a narrow trigonid, and a tall trigonid compared to the talonid (See Figs. 3.1, 4.1, and supplementary material SI Dataset 1). Sivatupaia ramnagarensis n. gen. n. sp. generally exhibits lower topographic values compared to other extant and fossil treeshrew genera as well (Fig. 5; see also SI Table S1).

Figure 3. (1) Scatterplot of tupaiid m2 specimens illustrating the ratio of tooth width/length vs. talonid width/trigonid width. (2) Scatterplot of Galerix P4 shape (BL/MD) vs. size (square root of BL*MD).

Figure 4. Results of PCA and UPGMA cluster analyses resulting from a 3DGM analysis of m2 shape in extant and fossil treeshrews. Wireframe outlines in occlusal and buccal views representing the extreme shape loadings at the ends of each PC axis are provided for visual comparison. (1) PC 1 vs. PC 2; (2) PC 2 vs. PC 3; (3) PC 3 vs. PC 4; (4) dendrogram resulting from UPGMA cluster analysis of genus/species averages for the first 5 PCs. The cophenetic correlation (cc) is high, indicating that the dendrogram is an accurate representation of the pairwise distances among taxa. Note that WIMF/A 4699 is phenetically distant from both extant and fossil tupaiids and ptilocercids. Numbers below branches represent bootstrap support values based on 10,000 replicates.

Figure 5. Box plots of DNE, 3D-OPCR, and RFI for species of Ptilocercus, Dendrogale, and Tupaia, along with Ptilocercus kylin and the new taxon (Sivatupaia ramnagarensis n. gen. n. sp.).
Occurrence
Dehari locality; ~0.5 km northeast of Dehari village and ~5 km southwest of the town of Ramnagar, Udhapur District, Jammu and Kashmir, India (Fig. 1); Middle Miocene of Lower Siwaliks.
Description
WIMF/A 4699 is a moderately worn, left lower m1 or m2 (Fig. 2). As is typical of primitive placentals, it has three cusps in the trigonid (paraconid, protoconid, and metaconid) and three in the talonid (hypoconid, entoconid, and hypoconulid). The trigonid basin is narrow and open lingually, with a strong and lingually positioned paraconid that is shorter than the metaconid and protoconid. The metaconid is smaller than the protoconid, but the protoconid is shifted toward the midline of the crown so that it is positioned close to metaconid. The talonid basin is large and closed, and it is ~10–15% wider than the trigonid at its maximum distal breadth (Table 1). The hypoconid is the largest cusp on the talonid, positioned at the distobuccal corner of the crown. The hypoconulid is small and shifted lingually, being located directly posterior to the relatively large entoconid at the distolingual corner of the tooth. The cristid obliqua is relatively mesiodistally oriented, extending from the hypoconid to meet the trigonid at the base of the protoconid, slightly buccal to this cusp. The notch separating the paraconid and metaconid is similar in depth to, or slightly deeper than, the notch separating the metaconid and entoconid. A moderately developed precingulid is present on the mesial end of the tooth and extends around to the lingual part of the trigonid, but no buccal cingulid is present. The specimen preserves the remnants of two roots.
Etymology
Species name ramnagarensis is in reference to the Ramnagar area (Jammu region, India), where the type specimen was found.
Remarks
WIMF/A 4699 was identified as a tupaiid treeshrew after multiple comparisons with other micromammal taxa. Shrews and most microchiropteran bats generally have a buccal cingulum as well as a paraconid and metaconid that are more deeply separated than the present specimen. The adapisoricids display a hypoconulid on the lower molars that is oriented in a median or slightly lingual position (Krishtalka, Reference Krishtalka1976a), whereas the hypoconulid of WIMF/A 4699 is more strongly displaced lingually directly behind the entoconid. Early Tertiary erinaceids are characterized by having greatly reduced hypoconulids (Krishtalka, Reference Krishtalka1976a). The hypoconulid in the present specimen is not greatly reduced.
Within treeshrews, WIMF/A 4699 differs from ptilocercids by the absence of a buccal cingulum and a trigonid that is much higher than the talonid (Li and Ni, Reference Li and Ni2016). WIMF/A 4699 is most similar to tupaiids on account of the lingual position of the hypoconulid (close to and directly behind the entoconid), the absence of a buccal cingulum, and the lack of a deep notch between the paraconid and metaconid (Jacobs, Reference Jacobs and Luckett1980). Because WIMF/A 4699 displays a well-developed metaconid and entoconid, as well as a relatively vertical hypoconulid, it differs from the genera Anathana (Lyon, Reference Lyon1913) and Dendrogale (Jacobs, Reference Jacobs and Luckett1980). WIMF/A 4699 compares well with Prodendrogale yunnanica Qiu, Reference Qiu1986, from the late Miocene of Lufeng, Yunnan, China, but differs by having a smaller hypoconulid and a narrower notch separating the entoconid and hypoconulid. In addition, the present molar (length = 1.17 mm, trigonid width = 0.76 mm, talonid width = 0.87 mm; see Table 1) is only half the size of the Prodendrogale yunnanica m1 (Table 1; original length = 2.45 mm, trigonid width = 1.40 mm, talonid width = 1.60 mm; see Ni and Qiu, Reference Ni and Qiu2012, table 1). It is similar to the lower molars that tentatively were attributed to Tupaia from Late Miocene deposits of Pakistan (Jacobs, Reference Jacobs and Luckett1980) and Haritalyangar, Middle Siwaliks, India (Chopra et al., Reference Chopra, Kaul and Vasishat1979) due to its more distinct entoconid, hypoconulid, and vertical hypoconulid. The wider talonid relative to the trigonid in WIMF/A 4699 (Fig. 3.1) is more typical of a tupaiid m1, but m2s sometimes can have a wider talonid to trigonid breadth ratio among tupaiids (see Butler, Reference Butler and Luckett1980; Table 1). In addition, among living Tupaia, a precingulum is never found on m1, but often can be found on m2 or m3 (Steele, Reference Steele1973). Thus, the specimen is either an m1 or an m2, although the mesially restricted cingulum perhaps makes m2 more likely.
Given the similarity in shape and morphological features seen in isolated m1s and m2s among tupaiids (as evinced by the difficulty in distinguishing them), WIMF/A 4699 was included in a large 3D dataset of extant and fossil treeshrew m2s for morphometric overall shape and dental topographic analyses. A PCA of Procrustes-fitted landmarks demonstrates that WIMF/A 4699 is morphologically distinct from other treeshrew specimens, plotting outside of the extant and fossil treeshrew convex hulls (tupaiid and ptilocercid) at the positive end of PC 1 (Fig. 4.1). PC 1 is related to relative crown breadth, the position of the paraconid, the relative height of the protoconid/trigonid, and the position of the protoconid/relative breadth of the trigonid, with taxa possessing narrower crowns, more mesially shifted paraconids, taller protoconids/trigonids compared to the talonid, and more lingually shifted protoconids resulting in narrower trigonids at the positive end of PC 1. PC 2 seems most closely associated with features such as crown height, paraconid position, protoconid position/trigonid breadth, and hypoconulid/postcristid position, with lower crowns, mesially shifted paraconids, buccally shifted protoconids/wider trigonids, and mesially shifted hypoconulid/postcristids found at the positive end of PC 2. WIMF/A 4699 has an intermediate value on PC 2 and plots within the tupaiid range but outside of the ptilocercid range (Fig. 4.1). Finally, a UPGMA cluster analysis of the first 5 PCs representing ~66.7% of the variation strongly suggests that WIMF/A 4699 is phenetically distinct from all known extant and fossil treeshrews in terms of its overall shape (Fig. 4.4). Thus, in combination with its very small size relative to all known fossil and extant treeshrews, WIMF/A 4699 represents a new genus and species, Sivatupaia ramnagarensis, which we place within Tupaiidae for now given its lack of a buccal cingulum (distinguishing it from ptilocercids) combined with other general aspects of shape that are more similar to tupaiids (e.g., relatively tall trigonids, see PC2 and PC3 in Fig. 4.2).
Dietary analyses also demonstrate the distinctiveness of WIMF/A 4699 relative to other scandentians. Dental topographic analyses (DTA) suggest that WIMF/A 4699 generally displays lower topographic values compared to the comparative extant and fossil sample (Fig. 5; see also SI Table 1). Furthermore, a PCA based on the DTA again places WIMF/A 4699 outside of the known range of variation among extant and fossil treeshrews on PC 1, confirming its unique morphology and likely dietary adaptations (Fig. 6). Thus, it appears that this newly discovered treeshrew was better adapted to a more frugivorous or less mechanically challenging diet with considerably less shearing than seen in many other scandentian taxa. Whether this is a primitive retention or represents a derived adaptation is currently unknown and will depend on future clarification of the scandentian fossil record and Euarchontan relationships, more broadly.

Figure 6. Top: Reconstructed meshes showing the topographic maps of DNE, 3D-OPCR, and RFI for WIMF/A 4699. Bottom: Scatterplot depicting PC 1 and PC 2 of species means for all three topographic variables. The scatterplot indicates how curvature (DNE), complexity (3D-OPCR), and relief (RFI) load along the axes. Taxa inferred to be more insectivorous sit on the right side of the plot whereas taxa inferred to be more frugivorous sit on the left side.
Eulipotyphla Waddell, Okada, and Hasegawa, Reference Waddell, Okada and Hasegawa1999
Erinaceidae Fischer, Reference Fischer1814
Genus Galerix Pomel, Reference Pomel1848
Occurrence
Dehari locality at Ramnagar (Udhampur District, Jammu and Kashmir, India), and K2 Kulwanta locality at Ramnagar (Parmar et al., Reference Parmar, Norboo and Magotra2022).

Figure 7. 3D surface renderings of Galerix sp. P4s. (1–5) WIMF/A 4697, left P4 in: (1) occlusal; (2) buccal; (3) lingual; (4) posterior; and (5) anterior views; (6–10) WIMF/A 4698, right P4 in: (6) occlusal; (7) buccal; (8) lingual; (9) posterior; (10) anterior views. Scales = 1 mm.
Description
The outline of WIMF/A 4697's crown is trapezoidal, as is distinctive of Galerix P4s. The crown is relatively broad labiolingually and short mesiodistally (max breadth/max length ratio = 0.99). There is a low rounded ridge that curves mesially at the paracone margin, forming a small parastyle. The paracone is the largest cusp. A crest moves downwards posteriorly from the paracone, then turns sharply in a buccal direction (almost 90°), ascending upwards towards the metacone in the distobuccal corner of the crown, forming a sharply demarcated distobuccal notch and flange in occlusal outline. On the lingual side of the crown, the protocone and hypocone are relatively low cusps, and the protocone is slightly larger than the hypocone. The protocone is slightly mesial relative to the paracone, and it is slightly worn on its occlusal surface. The hypocone is distinct and situated just distal to the protocone, directly across from the distinctive buccal notch and mesial to the metacone, such that the protocone and hypocone are spaced much more closely together than the paracone and metacone. A small crest is present between the protocone and hypocone. A posterior cingulum runs along the distal margin of the tooth, from the metacone to the hypocone, with a slight extension at the distolingual corner of the tooth. Three roots appear to have been present, of which the lingual one has the largest diameter.
WIMF/A 4698 is similar in outline and morphology to WIMF/A 4697, but slightly larger and significantly more worn, with damage to both the mesial and distal borders of the crown.
Materials
WIMF/A 4697 left P4, WIMF/A 4698 right P4.
Remarks
The present P4 specimens can be referred to the genus Galerix on the basis of a large paracone, being broad labiolingually and short mesiodistally, the presence of a small parastyle curving around the paracone margin, a well-developed distal cingulum, and a clear notch on the buccal margin between the metacone and paracone resulting in a distinctive buccal flange at the distobuccal margin of the tooth (Zijlstra and Flynn, Reference Zijlstra and Flynn2015). In Pakistan, two species of Galerix, G. rutlandae and G. wesselsae, are distinguished mainly on the basis of size, with smaller specimens belonging to G. rutlandae and larger ones referred to G. wesselsae (Zijlstra and Flynn, Reference Zijlstra and Flynn2015). The present specimens are larger than all measured G. rutlandae P4s and are more similar to G. wesselsae in size, and the shape of the occlusal crown (width/length ratio) is very similar to the shape of the only complete G. wesselsae P4 known (see Table 2; Fig. 3.2). Some caution is needed given that only two P4s are currently known from Dehari and the previously documented sample sizes of G. rutlandae and G. wesselsae P4s are small, making the full range of variation in both taxa unclear for this tooth position. Furthermore, Parmar et al. (Reference Parmar, Norboo and Magotra2022) recently described an M2 from their K2 Kulwanta locality at Ramnagar and assigned it to G. rutlandae, raising the possibility that the two Dehari P4s may represent large P4s of that taxon. In fact, the M2 from Kulwanta is also relatively large (see measurements in Parmar et al., Reference Parmar, Norboo and Magotra2022)—larger in overall area than the sample of G. rutlandae specimens measured by Zjilstra and Flynn (Reference Zijlstra and Flynn2015)—and within the range of G. wesselsae in terms of overall size (SI Table S2; SI Fig. S1). Despite the larger overall size of the specimen, Parmar et al. (Reference Parmar, Norboo and Magotra2022) attributed the Kulwanta M2 to G. rutlandae based on occlusal features, including a strong connection between the protocone and hypocone as well as a connection between the protocone and metaconule.
Table 2. Comparative dental measurements (mm) of Galerix rutlandae and Galerix wesselsae P4s from Pakistan and India. Max Width = maximum width; Max Length = maximum length; Area = Max Width × Max Length. Comparative measurements from Zijlstra and Flynn (Reference Zijlstra and Flynn2015) and the current study.
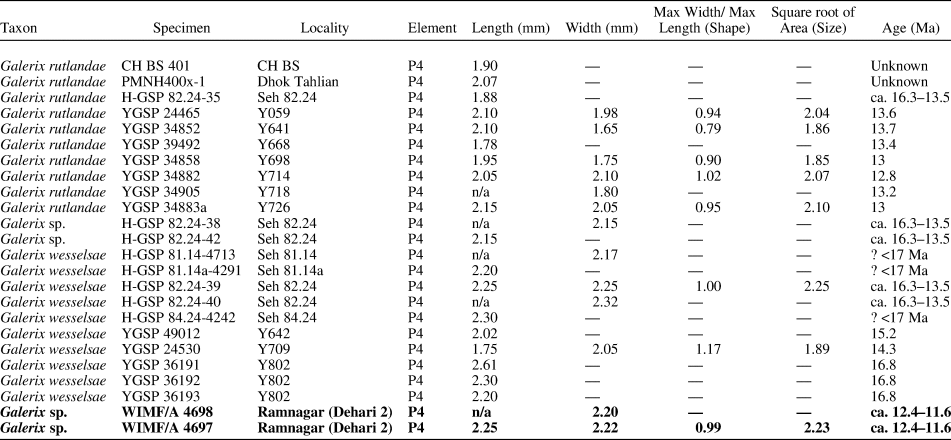
Taken together, it seems that the Dehari Galerix specimens and the Kulwanta specimen are all larger than typical G. rutlandae (more similar in size to G. wesselsae), and yet the more diagnostic M2 displays features argued to be more consistent with G. rutlandae. The consistency in size across all three Ramnagar teeth suggests to us that it is most parsimonious to assume all of the Ramnagar Galerix specimens belong to a single species, but the unique mix of M2 features and relatively large size does not fit perfectly with G. rutlandae or G. wesselsae as defined from the Potwar Plateau. Therefore, we have determined that it is most prudent to formally assign all of these specimens to Galerix sp. indet., pending additional data.
Rodentia Bowdich, Reference Bowdich1821
Spalacidae Gray, Reference Gray1821
Rhizomyinae Winge, Reference Winge1887
Genus Kanisamys Wood, Reference Wood1937
Type species
Kanisamys indicus Wood, Reference Wood1937.
Kanisamys indicus Wood, Reference Wood1937
Figure 8.1
Holotype
YPM 13810, partial right dentary with m1–m3, comes from the Chinji Zone, south of Chinji Village, Pakistan (Wood, Reference Wood1937, pl. 68, fig. 7).

Figure 8. 3D surface renderings in occlusal view of (1) WIMF/A 4689 Kanisamys indicus M2; (2) WIMF/A 4695 Sayimys sivalensis M2 or M3; (3) WIMF/A 4693 Murinae indet. m1; (4) WIMF/A 4692 Murinae indet. m2; (5) WIMF/A 4696 Murinae indet. M2. Scales = 1 mm.
Occurrence
Dehari locality at Ramnagar (Udhampur District, Jammu and Kashmir, India).
Description
WIMF/A 4689 is longer than wide with moderate lophodonty and a well-preserved, though worn, protocone, hypocone, paracone, and metacone. The lingual cusps (protocone and hypocone) are taller than the buccal cusps (paracone and metacone), and the remains of three roots can be observed on the inferior surface of the crown. The protocone is slightly smaller than the hypocone and directed posterolingually. The specimen displays an anteroloph, protoloph, mesoloph, metaloph, and posteroloph, but no anterolingual flexus is present. The protoloph is posterobuccally oriented from the protocone-mure junction, and the mesoloph is relatively long. A small mesostyle is present buccal to the mesoloph. The metaloph and posteroloph join at the buccal margin of the crown, forming a small posterosinus. The lingual re-entrant is short and curves anteriorly. The anterosinus separates the anteroloph from the protoloph, and the mesosinus separates the protoloph from the mesoloph in addition to separating the mesoloph from the metaloph. No ectoloph is present.
Material
WIMF/A 4689, left M2.
Remarks
WIMF/A 4689 is more similar to K. indicus than to other species of Kanisamys, including K. potwarensis, K. sivalensis Wood, Reference Wood1937, and K. nagrii Prasad, Reference Prasad1968, because of its smaller size (length = 1.95 mm, width = 1.86 mm; see Table 3), shape (width/length; see Fig. 9.1), and low crown height (0.56 mm). Besides the crown height, K. indicus has a crown that is longer than wide, a short lingual re-entrant, a strong mesoloph, and a distinct metaloph from posteroloph, whereas K. potwarensis is characterized as having a width greater than the length, a slender mesoloph, and a fused metaloph with posteroloph (Flynn, Reference Flynn1982b). M2s of K. nagrii and K. sivalensis are highly lophodont, unlike WIMF/A 4689. Earlier, Parmar et al. (Reference Parmar, Prasad and Norboo2018) reported K. indicus from Dehari, and those specimens share similar characters with the present specimen. Therefore, WIMF/A 4689 is assigned here to K. indicus on account of occlusal crown features, overall shape, and height.

Figure 9. (1) Scatterplot of M2 shape (BL/MD) vs. size (square root of BL*MD) of Siwalik Kanisamys specimens. (2) Scatterplot of M2-M3 crown shape (BL/MD) vs. size (BL*MD) of Siwalik Sayimys specimens.
Table 3. Comparative dental measurements (mm) of WIMF/A 4689 and previously described Kanisamys specimens. Max Width = maximum width; Max Length = maximum length; Area = Max Width × Max Length. Comparative measurements from Black (Reference Black1972), Flynn (Reference Flynn1981, Reference Flynn1982a), Wessels and de Bruijn (Reference Wessels and de Bruijn2001), Parmar et al. (Reference Parmar, Prasad and Norboo2018), and this study.

Ctenodactylidae Zittel, Reference Zittel1893
Ctenodactylinae Hinton, Reference Hinton1933
Genus Sayimys Wood, Reference Wood1937
Type species
Sayimys perplexus Wood, Reference Wood1937.
Sayimys sivalensis (Hinton, Reference Hinton1933)
Figure 8.2
Holotype
GSI D284, left partial dentary with m2–m3 from the Middle Miocene Chinji Formation, Pakistan (Hinton, Reference Hinton1933).
Occurrence
Dehari locality at Ramnagar (Udhampur District, Jammu and Kashmir, India).
Description
WIMF/A 4695 is a left M2 (or possibly M3) that is broken distally. An anteroloph is absent. The anterior cusps (protocone and paracone) are larger than the posterior cusps (hypocone and metacone). The paraflexus is absent. The mesoflexus and hypoflexus are about equal in depth and terminate opposite to each other. The metaflexus is present, but short and shallow. The posteroloph is shorter than the metaloph. The specimen exhibits three roots.
Materials
WIMF/A 4695, left partial M2.
Remarks
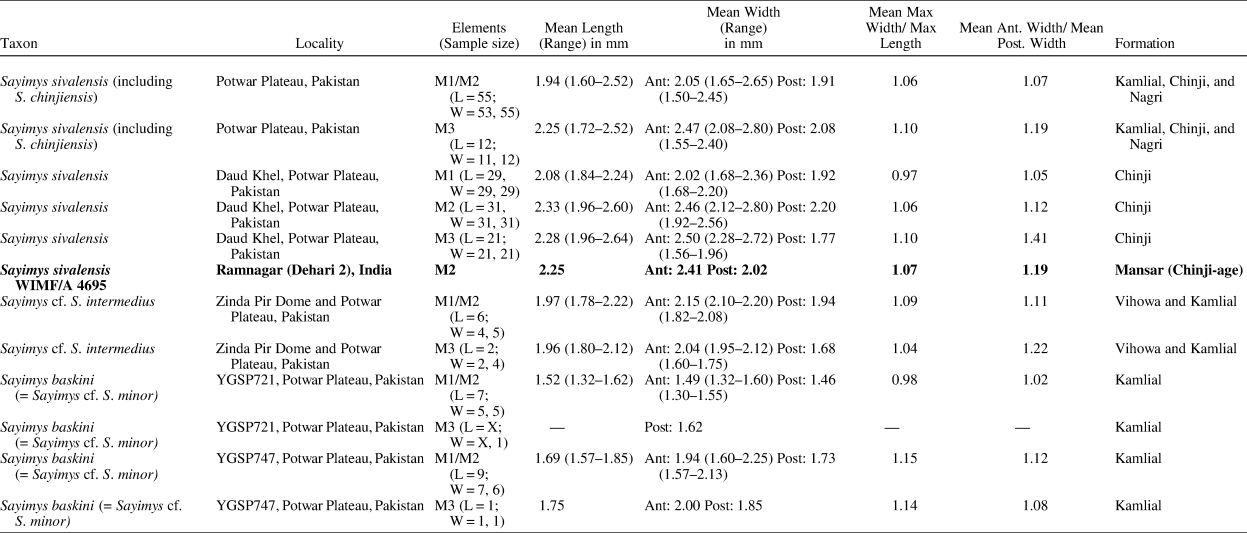
Five ctenodactylid species belonging to two genera, Prosayimys and Sayimys, are currently recognized in the Indian subcontinent during the Neogene (López-Antoñanzas and Sen, Reference López-Antoñanzas and Sen2003; López-Antoñanzas and Knoll, Reference López-Antoñanzas and Knoll2011). Prosayimys flynni Baskin, Reference Baskin1996, is found in the Early Miocene Chitarwata Formation of Zinda Pir Dome, Pakistan, and at least four species of Sayimys are recognized at many Neogene sites across India and Pakistan (López-Antoñanzas and Sen, Reference López-Antoñanzas and Sen2003; López-Antoñanzas and Knoll, Reference López-Antoñanzas and Knoll2011). The M3 of S. sivalensis is slightly larger than the M2–M3 of S. baskini López-Antoñanzas and Sen, Reference López-Antoñanzas and Sen2003, and S. intermedius Sen and Thomas, Reference Sen and Thomas1979 (López-Antoñanzas and Sen, Reference López-Antoñanzas and Sen2003). In addition to its occlusal features, WIMF/A 4695 can be referred to S. sivalensis on the basis of its overall size and shape (length = 2.25 mm, anterior width = 2.41 mm, posterior width = 2.02 mm), which fall clearly within the range of S. sivalensis M2s and M3s (Table 4; Fig. 9.2).
Table 4. Comparative dental measurements (mm) of WIMF/A 4695 and other Sayimys specimens. Max = maximum; Ant = anterior; Post = Posterior. Comparative measurements from Munthe (Reference Munthe1980), Baskin (Reference Baskin1996), López-Antoñanzas and Sen (Reference López-Antoñanzas and Sen2003), and the current study.
Muridae Illiger, Reference Illiger1811
Murinae Illiger, Reference Illiger1811
Murinae indet.
Figure 8.3–8.5
Occurrence
Dehari locality at Ramnagar (Udhampur District, Jammu and Kashmir, India).
Description
WIMF/A 4696 (M2) has a somewhat trapezoidal occlusal outline. On the anterior margin, an anterostyle (t1) and labial anterocone (t3) are present, t1 being larger than t3. The enterostyle (t4) is triangular, longitudinally elongated, isolated from and positioned posteriorly to the protocone (t5). The paracone (t6) is fairly large and strongly connected to t5. Both these cusps (t5 and t6) lie at the same height. The hypocone (t8) is large and is connected to the smaller metacone (t9), almost lying at the same height. The groove between the anterostyle (t1) and enterostyle (t4) is shallow and similar in depth to the groove between the enterostyle (t4) and hypocone (t8). None of the cusps shows longitudinal (i.e., mesiodistal) connections. The posterior cingulum is ridge-like, connected to t8, and separated from t9 by a small and narrow groove. The tooth has three roots.
The WIMF/A 4693 (m1) prelobe comprises a posteriorly placed labial anteroconid that is slightly smaller than the more anteriorly placed lingual anteroconid. The anteroconids and the cusps of the second lobe (metaconid-protoconid) have a rather asymmetrical, centrally positioned “X” shaped longitudinal connection; the metaconid and protoconid are also weakly connected to each other. A medial anteroconid is absent. The cusps of the third lobe, the hypoconid, and entoconid are transversely connected. There is no central mure present, and thus a continuous central sinusoid is present in front of the third lobe. The labial cingulum is well developed and a prominent C1 is present. The posterior cingulum is elongated and augen- (lentil-) shaped.
WIMF/A 4692 (m2) exhibits a rectangular occlusal outline. A labial anteroconid is present, and the second (metaconid-protoconid) and third (entoconid-hypoconid) chevrons are slightly oblique. The cusps are gently inclined mesiodistally. A weak labial cingulum is present with an isolated cuspid C1. The posterior cingulum is elongate and lens-/lentil-shaped.
Materials
WIMF/A 4696 right M2, WIMF/A 4693 left m1, WIMF/A 4692 right m2.
Remarks
The present M2 is similar to that of Antemus chinjiensis reported from the Lower Siwalik of Potwar, Pakistan (Jacobs, Reference Jacobs1978) and Ramnagar, India (Sehgal and Patnaik, Reference Sehgal and Patnaik2012) in terms of overall size and having an enterostyle (t4) that is isolated from the protocone (t5). However, WIMF/A 4696 (length = 1.30 mm, width = 1.00 mm; see SI Table S3) is slightly elongated and narrower in shape (see Fig. 10.1) compared to those of Antemus chinjiensis (original range of length = 1.00–1.28 mm and original range of width = 1.02–1.20 mm) described by Jacobs et al. (Reference Jacobs, Flynn and Downs1989) and Sehgal and Patnaik (Reference Sehgal and Patnaik2012). The present m1 (length = 1.99 mm, width = 1.17 mm; see SI Table S3) is also distinct from those of Antemus chinjiensis in having a strong asymmetrical “X” shaped longitudinal connection between the first two lobes and being larger in overall size. In size and shape (see Fig. 10.2), it is a good match for the ca. 12.4 Ma sample from site Y496 (Potwar, Pakistan) that was originally described as “near Progonomys” (Jacobs and Flynn, Reference Jacobs, Flynn, Lieberman, Smith and Kelley2005; Kimura et al., Reference Kimura, Jacobs and Flynn2013), but more recently referred to “post-Antemus” by Kimura et al. (Reference Kimura, Flynn and Jacobs2021). The second lower molar (length = 1.25 mm, width = 1.00 mm; see SI Table S3) falls within the size of Antemus chinjiensis (see Fig. 10.3), but differs in having gently inclined cusps as well as having a weaker cingulum.

Figure 10. Scatterplot of molar shape (BL/MD) vs. size (square root of BL*MD) in Siwalik murid specimens (see SI Table 3) including Antemus chinjiensis Jacobs, Reference Jacobs1977; Karnimata fejfari Kimura et al., Reference Kimura, Flynn and Jacobs2017; Karnimata darwini Jacobs, Reference Jacobs1978; Progonomys morganae Kimura et al., Reference Kimura, Flynn and Jacobs2017; Progonomys debruijni Jacobs, Reference Jacobs1978; and Progonomys hussaini Cheema et al., Reference Cheema, Raza, Flynn, Rajpar and Tomida2000. (1) M2; (2) m1; (3) m2.
Discussion
Historically, treeshrews have been intensively studied to understand their evolutionary significance and phylogenetic position relative to other members of Euarchontoglires, in particular primates and dermopterans (McKenna, Reference McKenna1963, Reference McKenna1966; Jacobs, Reference Jacobs and Luckett1980; Sargis, Reference Sargis2001, Reference Sargis2002a, Reference Sargisb, Reference Sargis2004). Currently, treeshrews are regarded as close relatives of dermopterans and primates within the Superorder Euarchonta (e.g., see Murphy et al., Reference Murphy, Eizirik, Johnson, Zhang, Ryder and O'Brien2001; Helgen, Reference Helgen, Wilson and Reeder2005; Janečka et al., Reference Janečka, Miller, Pringle, Wiens, Zitzmann, Helgen, Springer and Murphy2007; O'Leary et al., Reference O'Leary, Bloch, Flynn, Gaudin and Giallombardo2013; Sargis et al., Reference Sargis, Woodman, Morningstar, Reese and Olson2013; Zhang et al., Reference Zhang, Ameca, Cowlishaw, Pettorelli, Foden and Mace2019). However, their evolutionary history and phylogenetic position relative to other extant and fossil euarchontans are still debated in part due to the lack of a detailed fossil record (Sargis, Reference Sargis2004). Scandentia, the mammalian order including treeshrews, is classified into two families: Tupaiidae and Ptilocercidae (Helgen, Reference Helgen, Wilson and Reeder2005). Previously, fossil taxa such as Entomolestes granger, E. nitrens, and Tupaiodon morrisi were considered to be tupaiids originating at the beginning of the Cenozoic (Matthew, Reference Matthew1909; Gregory, Reference Gregory1913; Matthew and Granger, Reference Matthew and Granger1924). However, many of these species were later demonstrated to belong to different families such as Erinaceidae, Adapisoricidae, and Nyctitheriidae (Simpson, Reference Simpson1931; Krishtalka, Reference Krishtalka1976b; Krishtalka and West, Reference Krishtalka and West1977). Anagale gobiensis Simpson, Reference Simpson1931, from the Oligocene Ulan Gochu Formation of Mongolia and the Paleocene genus Adapisoriculus were once considered to be close relatives of the Tupaiidae (Simpson, Reference Simpson1931; Van Valen, Reference Van Valen1965), but their affinities to living treeshrews were later disputed (Chopra and Vasishat, Reference Chopra and Vasishat1979; Jacobs, Reference Jacobs and Luckett1980). As such, there is currently no fossil record of Paleogene treeshrews except for Ptilocercus kylin Li and Ni, Reference Li and Ni2016, from the early Oligocene (ca. 34 Ma) of China, representing the oldest definitive fossil ptilocercid (Li and Ni, Reference Li and Ni2016).
Undoubted Miocene tupaiid fossils have been discovered in Pakistan (Jacobs, Reference Jacobs and Luckett1980), India (Dutta, Reference Dutta1975; Chopra and Vasishat, Reference Chopra and Vasishat1979), Thailand (Mein and Ginsburg, Reference Mein and Ginsburg1997), and China (Qiu, Reference Qiu1986; Ni and Qiu, Reference Ni and Qiu2002, Reference Ni and Qiu2012). Jacobs (Reference Jacobs and Luckett1980) reported a large treeshrew rostrum from ca. 10 Ma Miocene deposits in Pakistan, but he did not identify this specimen to the genus level. In India, Dutta (Reference Dutta1975) reported a possible Tupaia rib cage from the Tatrot beds of the Upper Siwaliks. Chopra and Vasishat (Reference Chopra and Vasishat1979) reported a new species of tupaiid, Palaeotupaia sivalicus, from the Middle Siwaliks of Haritalyangar, which is now widely regarded as representing the extant genus Tupaia. Based on an M2 specimen, Mein and Ginsburg (Reference Mein and Ginsburg1997) named Tupaia miocenica from Thailand, and Qiu (Reference Qiu1986) described a new genus and species, Prodendrogale yunnanica, based on isolated teeth and a jaw fragment from Lufeng, China. Finally, Ni and Qiu (Reference Ni and Qiu2012) reported two new species, Tupaia storchi Ni and Qiu, Reference Ni and Qiu2012, and Prodendrogale engesseri Ni and Qiu, Reference Ni and Qiu2012, from isolated teeth at the late Miocene Yuanmou Lufengpithecus locality in Yunnan Province, China.
Sivatupaia ramnagarensis n. gen. n. sp., dating to the middle Miocene, is significant in extending the temporal and morphological range of tupaiid evolution. Specifically, the occurrence of WIMF/A 4699 at Dehari (Ramnagar), likely correlating to the upper half of the Chinji Formation, extends the time range of fossil tupaiids downward in the Siwalik fossil record. Furthermore, S. ramnagarensis n. gen. n. sp. is distinct in its morphology and dietary adaptations compared to other living and fossil treeshrews. It further expands the known dental ecospace of treeshrews and suggests that tupaiids may have been more ecologically diverse in the past (Selig et al., Reference Selig, Sargis, Chester and Silcox2020). Its small size and distinctive shape make it difficult to place definitively within the extant treeshrew families, and the lower molar topographic values surface suggests a more frugivorous and/or less challenging diet compared to other known treeshrew taxa.
Galericines (hedgehogs) are poorly known from Southeast and East Asia, with small samples from Pakistan (Zijlstra and Flynn, Reference Zijlstra and Flynn2015), China (Li et al., Reference Li, Lin, Gu, Hou, Wu and Qiu1983; Storch and Qiu, Reference Storch and Qiu1991), and Thailand (Mein and Ginsburg, Reference Mein and Ginsburg1997; Cailleux et al., Reference Cailleux, Chaimanee, Jaeger and Chavasseau2020). In Pakistan, Galerix is represented by two taxa, G. rutlandae Munthe and West, Reference Munthe and West1980, and the older and larger G. wesselsae Zijlstra and Flynn, Reference Zijlstra and Flynn2015. Galerix wesselsae first appears around 19 Ma, and was apparently replaced by G. rutlandae during the Middle Miocene, ca. 14.3–14.1 Ma (Zijlstra and Flynn, Reference Zijlstra and Flynn2015). Galerix rutlandae then persists in Pakistan until ca. 11.6 Ma (Zjilstra and Flynn, Reference Zijlstra and Flynn2015). In Thailand, the tribe Galericini is represented by two genera, Galerix (G. rutlandae) and Lantanotherium (L. anthrace Cailleux et al., Reference Cailleux, Chaimanee, Jaeger and Chavasseau2020, and Lantanotherium sp.) from the Middle Miocene (13.4–13.2 Ma) of the Mae Moh Basin (Cailleux et al., Reference Cailleux, Chaimanee, Jaeger and Chavasseau2020).
In the Indian Siwaliks, a galericine M2 was recently described from the K2 locality at Kulwanta, Ramangar, as G. rutlandae based on a number of subtle dental features shared with G. rutlandae specimens on the Potwar Plateau (Parmar et al., Reference Parmar, Norboo and Magotra2022). However, the Kulwanta M2 is larger in terms of measured area (mesiodistal length × buccolingual breadth) than known G. rutlandae M2s from Pakistan, and instead is more similar to G. wesselsae in size (SI Table S2; SI Fig. S1). The Galerix P4 specimens from Dehari are also larger than the few G. rutlandae P4s that have been described from Pakistan, and on this basis the P4s from Dehari and the M2 from Kulwanta are interpreted most parsimoniously as the same species. Because the more diagnostic M2 from Kulwanta appears more similar to G. rutlandae in known dental features, and yet all of the Ramnagar specimens appear larger and more similar to G. wesselsae in size, it is difficult to confidently assign the Ramnagar Galerix specimens to any known species at this time. Instead, we prefer to recognize them as Galerix sp. indet. until additional specimens are found to confirm the presence of G. rutlandae or further suggest that a distinct, larger hedgehog species is present at Ramnagar. In any case, the newly described specimens from Kulwanta and Dehari represent the first erinaceid fossils from the entire Siwalik deposits of India.
Previous to the current micromammal collection, Parmar et al. (Reference Parmar, Prasad, Kumar, Malik and Norboo2015, Reference Parmar, Prasad and Norboo2018) recorded Megacricetodon daamsi, Kanisamys indicus, Sayimys sivalensis, Myomimus sp., and Tamias urialis from Dehari. In combination with Kanisamys cf. K. potwarensis (Parmar and Prasad, Reference Parmar and Prasad2006), they estimated a ca. 13.6–13.2 Ma age range for Dehari, projected from Potwar biostratigraphy (Parmar et al., Reference Parmar, Prasad and Norboo2018). In our present collection from Dehari, the murine specimens possess an interesting mix of features seen in both Antemus and later “pre-Progonomys” or Progonomys (see Kimura et al., Reference Kimura, Flynn and Jacobs2021). The m1 specimen WIMF/A 4693, in particular, is most similar in size and shape to the population most recently referred to as “post-Antemus” from a ca. 12.4 Ma site on the Potwar Plateau, Pakistan (Fig. 10.2; Kimura et al., Reference Kimura, Flynn and Jacobs2021). Although the m2 (WIMF/A 4692) is more similar to Antemus chinjiensis in size and shape (Fig. 10.3), both lower molars described here are also more robust and express weaker cingulae, similar to Progonomys in these features. The upper M2 WIMF/A 4696 displays features present in Antemus (e.g., an isolated enterostyle), but also features reminiscent of later pre-Progonomys and Progonomys, such as its overall longer and narrower shape (Fig. 10.1). It is currently unclear whether these specimens document a single variable species that is transitional between Antemus and Progonomys, perhaps similar to the “post-Antemus” population on the Potwar Plateau, or whether there are two lineages present at Dehari: Antemus/“post-Antemus” and pre-Progonomys/Progonomys.
In either case, this mix of murine dental features begins to appear around 12.4 Ma at site Y496 on the Potwar Plateau and would be most consistent with an age range at Dehari between ca. 12.7–11.6 Ma (i.e., between the last appearance date of Antemus samples and the first appearance date of the more derived pre-Progonomys samples). An age for Dehari at the older end of this range also would be consistent with previous reports of Megacricetodon daamsi (LAD ca. 12.5 Ma on the Potwar Plateau; Flynn et al., Reference Flynn, Morgan, Pilbeam, Jacobs and Lindsay1995) from the locality (Parmar et al., Reference Parmar, Prasad, Kumar, Malik and Norboo2015). The only reported rodent taxon known from Dehari that would appear at odds with an age of ca. 12.7–11.6 Ma would be K. cf. K. potwarensis (Parmar and Prasad, Reference Parmar and Prasad2006), which has a LAD of ca. 13.2 Ma on the Potwar Plateau (Flynn et al., Reference Flynn, Morgan, Pilbeam, Jacobs and Lindsay1995). However, we note that the original assignment to this species was only tentative, and the measurements of the m2 and m3 in this small Kanisamys specimen are closer in size to K. indicus, particularly the sample described from the Manchar Formation in Sindh Province (Wessels and de Bruijn, Reference Wessels and de Bruijn2001). Most recently, Bhandari et al. (Reference Bhandari, Bajpai, Flynn, Tiwari and Mandal2021) suggested that this specimen is in fact K. indicus, which has a LAD of ca. 11.4 Ma. Thus, it appears as if the reported rodents from Dehari are all consistent with an age range between ca. 12.7–11.6 Ma. In any case, if confirmed with future specimens, the presence of “post-Antemus”-like murines in combination with other biochronological and geochronological data may help to narrow down the possible age range of Dehari even further, and the Ramnagar region more broadly.
Conclusions
A number of micromammal specimens recently have been discovered at Ramnagar, and the current sample contains hedgehogs, biochronologically informative rodent specimens, and the earliest known treeshrew from the Indian Siwaliks. The treeshrew, which is represented by an isolated m1 or m2, represents a new genus and species, Sivatupaia ramnagarensis n. gen. n. sp. We place S. ramnagarensis n. gen. n. sp. within Tupaiidae, and note its morphological distinctiveness suggesting a less mechanically challenging, and perhaps more frugivorous, diet compared to other extant and fossil tupaiids. Hedgehog specimens described here appear to represent the genus Galerix, but clear identification to the species level is not possible at this time. Finally, murine rodent specimens showing a combination of Antemus-like and Progonomys-like features are most similar to murine specimens known from ca. 12.7–11.6 Ma localities on the Potwar Plateau, which may suggest a similar age for Dehari and have implications for the age of Ramnagar more broadly, roughly equivalent to the middle to upper section of the Chinji Formation.
Acknowledgments
RKS, NPS, and APS thank the Director, Wadia Institute of Himalayan Geology, Dehradun for the research facilities (contribution no. WIHG/201). This research was supported by the Leakey Foundation, the PSC-CUNY faculty award program, Hunter College, and the U.S. National Science Foundation (BCS Award nos. 1945736, 1945743, 1945618). We thank T. Skorka at the University of Southern California's Molecular Imaging Center (MIC) for assisting with μCT scanning; the MIC receives funding from the U.S. National Institutes of Health (NIH S10 RR027665 01). KRS is supported by the Kalbfleisch Fellowship, Richard Gilder Graduate School, AMNH. We thank E. Sargis, E. Seiffert, L. Flynn, and L. Jacobs for helpful advice, directions to important papers, and assistance with specimen identification. L. Flynn and L. Jacobs also provided valuable and constructive comments on an earlier version of this paper. V. Parmar kindly provided information on the geographic location of previously worked Dehari sites.
Data availability statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.8kprr4xqk.
The list of specimens included in the analyses and results of the topographic analysis for Dirichlet normal energy (DNE), three-dimensional orientation patch count rotated (3D-OPCR), and relief index (RFI) are available in Supplemental Data (SI Table S1 and SI Dataset 1). Comparative measurements of Galerix rutlandae and G. wesselsae M2 specimens along with a plot illustrating the large size of the M2 from Kulwanta are found in SI Table S2 and SI Figure S1. Comparative measurements of murine specimens, including the specimens described here, are provided in SI Table S3.

















