Introduction
Sunflower, Helianthus annuus L., is among the top global oilseed crops, with an average production of 40–50 million tonnes per year (FAO, 2017). Genetic self-incompatibility is common in wild sunflowers, creating a dependence on insects, especially bees, to move pollen in order to produce seed (Heiser et al., Reference Heiser, Smith, Clevenger and Martin1969). Though selection in cultivated sunflower has increased self-compatibility (Gandhi et al., Reference Gandhi, Heesacker, Freeman, Argyris, Bradford and Knapp2005), there are at least two reasons bees remain indispensable to the crop. First, because modern sunflowers use a hybrid system based on cytoplasmic male sterility (CMS), hybrid seed production is completely reliant on bees to move pollen from male-fertile to male-sterile parental lines (DeGrandi-Hoffman and Watkins, Reference DeGrandi-Hoffman and Watkins2000; Greenleaf and Kremen, Reference Greenleaf and Kremen2006). Second, even for self-compatible hybrids, stressful conditions during bloom can limit yields (via self-pollination) in the absence of bees (DeGrandi-Hoffman and Chambers, Reference DeGrandi-Hoffman and Chambers2006). Honey bees (Apis mellifera L.) are used as a managed pollinator for production of hybrid seed, but wild bees also contribute directly and indirectly to pollination of sunflowers (DeGrandi-Hoffman and Watkins, Reference DeGrandi-Hoffman and Watkins2000; Greenleaf and Kremen, Reference Greenleaf and Kremen2006; Nderitu et al., Reference Nderitu, Nyamasyo, Kasina and Oronje2008).
Bees often show distinct preferences for certain sunflower inbreds and hybrids (Tepedino and Parker, Reference Tepedino and Parker1982; Cerrutti and Pontet, Reference Cerrutti and Pontet2016; Mallinger and Prasifka, Reference Mallinger and Prasifka2018). Pollinator preferences in cultivated sunflowers have been associated with several traits, including pollen availability (Tepedino and Parker, Reference Tepedino and Parker1982; Mallinger and Prasifka, Reference Mallinger and Prasifka2017), nectar content or composition (Neff and Simpson, Reference Neff and Simpson1990; Pham-Delegue et al., Reference Pham-Delegue, Etievant, Guichard, Marilleau, Douault, Chauffaille and Masson1990), floral odor (Pham-Delegue et al., Reference Pham-Delegue, Etievant, Guichard, Marilleau, Douault, Chauffaille and Masson1990) and floret size (or corolla depth; du Toit and Coetzer, Reference du Toit and Coetzer1991; Mallinger and Prasifka, Reference Mallinger and Prasifka2017). All of these traits may be important in some instances, and differences among pollinators add to the complexity of determining how traits enhance pollinator visitation. For example, Tepedino and Parker (Reference Tepedino and Parker1982) noted that while honey bees preferred CMS inbred lines with little or no pollen, wild bees preferred male-fertile sunflowers. This difference in pollen preference also was observed by Mallinger and Prasifka (Reference Mallinger and Prasifka2017), who excluded other possible causes by using pairs of sunflower isolines with or without CMS. Though the contrast between honey bees and wild bees likely reflects a basic difference in life-histories (i.e. social, and long-lived, honey bees often stop collecting pollen periodically based on need [Camazine, Reference Camazine1993]), it underscores the fact that not all pollinator species will have the same responses to variation in plant traits.
Floret size may be the most attractive trait for further research because it should be relatively easy to measure, may affect other traits important to pollinators (e.g. by physically limiting access to nectar) and is supported by observations in other plant–pollinator systems (Inouye, Reference Inouye1980; Peat et al., Reference Peat, Tucker and Goulson2005). As part of an ongoing effort to explore the potential for improved sunflower–pollinator interactions, floret samples and field observations were used to assess (1) variation in floret size among female inbred sunflower lines, (2) the relationship between overall floret size and corolla depth, (3) possible environmental effects on floret size and (4) the effect of floret size differences on preferences of wild and managed bees.
Materials and methods
Variation in floret size and relationship to corolla depth
To assess variation in the size of sunflower florets, samples were taken from 100 public inbred lines in the USDA-ARS breeding nursery in Glyndon, Minnesota, in 2016. At this location, 8 ha of sunflowers (inbreds, hybrids and entries from segregating populations) were grown in single-row plots (6 m) with 0.76 m between rows and an average of 0.30 m between plants in a plot. Floret sampling focused on single-headed maintainer lines (designated ‘HA’), representing the female heterotic group in sunflowers (as opposed to branched male ‘RHA’ lines). On the first or second day of anthesis (i.e. pollen shed) for heads in the selected inbred lines, a small wedge (or sector) was cut from each of up to five heads per plot, and the wedges were stored frozen (−20 °C) until flowering of all lines was complete. Wedges were processed using forceps to pluck five open florets (with corolla open and anther tube exserted) and five closed (pre-anthesis) florets, which were placed into microcentrifuge tubes and returned to the freezer prior to imaging and analysis. Images for all of the closed florets for each line were obtained using a flatbed scanner with the lid removed but covered by a box to contrast lighter florets with a uniformly dark background. Each TIFF image (600 dpi) was subsequently processed using a macro in ImageJ (Schneider et al., Reference Schneider, Rasband and Eliceiri2012) to automatically measure the total length (called Feret diameter in ImageJ) of individual florets. Examples of scanned florets and images after processing are shown in Fig. 1.
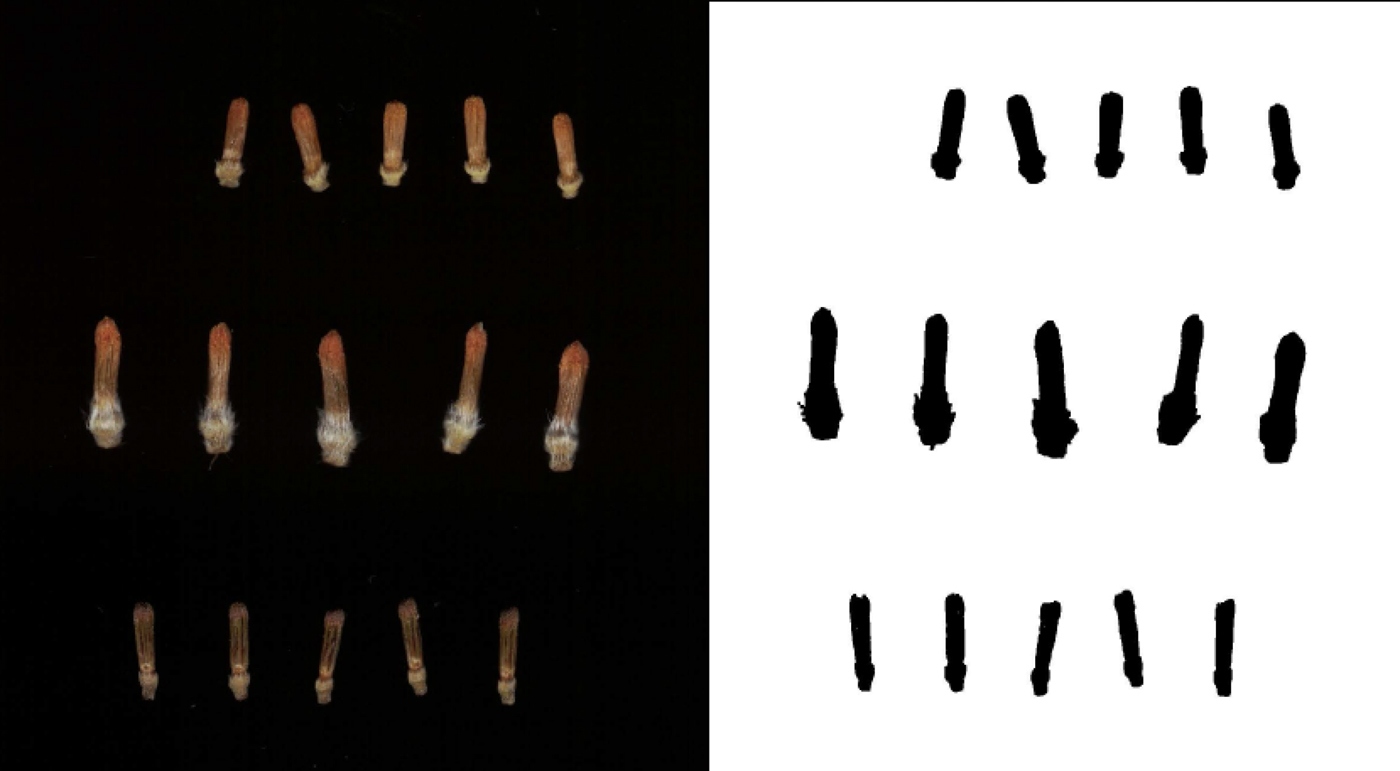
Fig. 1. Examples of scanned florets and processed images used for floret size measurements. Florets shown include the shortest inbred line measured (HA 341, at top), the longest (HA 286, middle) and a locally-collected wild sunflower (bottom).
The approach of measuring florets described above uses total floret length as a proxy for corolla depth, which more accurately indicates the proboscis (‘tongue’) length needed for a pollinator to access nectar from the base of the corolla. The proxy was used because without removing the anther tube of each open floret by hand, there appeared to be no simple way to obtain accurate, automated measures of corolla depth. Consequently, to determine the relationship between floret length and corolla depth, open florets from a random subsample of the lines (n = 30) were also scanned and corolla depth measured by hand in ImageJ.
Floret size and interactions with environment and pollinator visitation
Based on floret size data from the 2016 planting, groups of inbred lines with relatively short or long florets (n = 15 HA lines for each group) and similar maturity (i.e. days from planting to anthesis) were selected for planting at the USDA-ARS sunflower entomology plot on the NDSU Agronomy Seed Farm in Casselton, North Dakota, in 2017. This location comprised 0.4 ha of single-row plots similar to those in the 2016 breeding nursery. The 30 lines were planted in two randomized complete blocks approximately 60 m away from four commercial honey bee hives. To assess possible environmental effects on floret size, samples were taken from the first replicate for each of the lines, which were processed and analysed for total floret length as in 2016. Additionally, to test for effects of floret size on pollinator visits, observations were made during bloom in each of the 60 plots. After the number of blooming plants per plot was counted, once or twice each day a 1-min pollinator observation was made in each plot with >3 blooming plants. Observations were typically made the late morning (11 : 00 AM) or early afternoon (1 : 00 PM), and continued over ten days. Separate counts of honey bees, bumble bees, and other wild bees were recorded for each plot.
Statistical analyses
All analyses were performed using SAS (SAS Institute Inc., 2016). An analysis of variance (ANOVA; PROC GLM) was used to assess whether mean floret length differed significantly among sunflower maintainer lines sampled in 2016. Post-ANOVA means separation used Dunnett's test to compare 99 other inbred lines with HA 441 (PI 639164; Miller and Gulya, Reference Miller and Gulya2006), which had the shortest corollas among 10 maintainer lines measured by Mallinger and Prasifka (Reference Mallinger and Prasifka2017). A simple linear regression was used (PROC REG) with 2016 data to determine whether total (closed) floret length measured automatically from scanned images is a suitable proxy for corolla depth (on open florets) measured by hand. Similarly, possible effects of the year or site variation were assessed by regressing (PROC REG) average floret length of inbred lines for 2017 onto values for the same lines in 2016. Because pollinator counts are influenced by the number of plants observed, total visits of bees to each inbred line were adjusted for the total number of blooming plants for each line overall observations. This adjustment summarized pollinator attraction to each inbred line as a number of bees per 100 plant observations. Though the pollinator visitation experiment was intended to compare two discrete groups (short and long florets), observed floret size data from 2017 were more continuously distributed. As a result, the effect of floret size on pollinator visitation was assessed using simple linear regression (PROC REG).
Results
Sunflower maintainer lines sampled in 2016 showed significant variation in floret length (F = 30.11; df = 99, 387; P < 0.001), but a near-continuous distribution of values from 6.8–9.9 mm and little plant-to-plant variation or measurement error (i.e. low coefficient of variation [CV]; see online Supplementary Table S1). Comparisons to HA 441, previously assessed as having shallow corollas, indicated one-third of the lines have similar (P > 0.050) mean floret length. Though whole floret lengths measured by an ImageJ macro are necessarily greater than corolla depths measured by hand, regression analysis indicates floret length is an adequate, simple proxy (corolla depth = [0.70 × floret length] + 0.28; n = 30; R 2 = 0.78; see online Supplementary Fig. S2).
Regression of mean floret lengths from 2017 to 2016 data showed concordance between the two data sets (2017 = [1.13 × 2016] – 0.93; n = 30; R 2 = 0.88); in terms of year-to-year differences, the relative change in floret length estimates was about 3.50% across the 30 inbred lines (Fig. 2). Pollinator observations in the 2017 plots included a total of 7290 bees, with very few honey bees (<1%) and bumble bees (2%), but other abundant wild bees, with Melissodes trinodis Robertson and Andrena helianthi Robertson among the most common species. With all bee observations pooled for each line, floret length data from 2017 explained a majority of the variation in bee visitation to inbred lines, with a reduction of floret length of 2 mm more than doubling pollinator activity (bee visits = [−34.55 × floret length] + 368.70; n = 30; R 2 = 0.52; Fig. 3).

Fig. 2. Regression of mean floret lengths from 2017 to 2016 for n = 30 inbred lines. Estimates for each year represent five replicates (plants) with five subsamples (florets) per plant.

Fig. 3. Regression of bee visitation data onto floret length for n = 30 inbred lines in 2017. Bee observations are adjusted for the total number of plants observed for each inbred line over ten days.
Discussion
Traits related to floral size, appearance, or floral rewards are associated with the number or duration of pollinator visits in many non-crop systems (Galen and Stanton, Reference Galen and Stanton1989; Manetas and Petropoulou, Reference Manetas and Petropoulou2000; Kaczorowski et al., Reference Kaczorowski, Seliger, Gaskett, Wigsten and Raguso2012). Recently, more attention has been paid to floral traits in cultivated crops, which represent an opportunity to improve both crop production and resources for pollinators. In some cropping systems, there is a realization that pollinators provide greater benefits than generally acknowledged. For example, while generally not considered pollinator-dependent, soybean (Glycine max [L.] Merr.) yields are increased by pollinators (Erickson, Reference Erickson1975a) and cultivars vary in floral traits that favour pollination (Erickson, Reference Erickson1975b); these and other similar observations have led Palmer et al. (Reference Palmer, Perez, Ortiz-Perez, Maalouf and José Suso2009) to advocate for pollinator-friendly breeding of legumes. Bailes et al. (Reference Bailes, Ollerton, Pattrick and Glover2015) more generally promote breeding for enhanced plant–pollinator interactions to improve yields and food security. Other recent examples of efforts to understand and enhance crop–pollinator interactions include Courcelles et al. (Reference Courcelles, Button and Elle2013), who noted effects of flower morphology on honey bee pollination of cultivated blueberries (Vaccinium spp.); Soto et al. (Reference Soto, Maldonado, Gil, Peralta, Silva and Galmarini2013), who found differences in nectar sugar content in onion (Allium cepa L.) and positive correlations between nectary volume and honey bee visitation; and Carruthers et al. (Reference Carruthers, Cook, Wright, Osborne, Clark, Swain and Haughton2017), who document variation in floral traits within and between oilseed rape (Brassica napus L.) breeding systems and suggest enhancement of nectar as a target for breeding programmes.
Floret size has often been considered an important part of sunflower–pollinator interactions (Cirnu et al., Reference Cirnu, Dumitrache and Hociota1974; Shein et al., Reference Shein, Sargent and Miko1980; Sammataro et al., Reference Sammataro, Erickson and Garment1983; du Toit and Coetzer, Reference du Toit and Coetzer1991). However, much of the previous research has not been peer-reviewed, lacked useful data, included few genotypes or used exclusively private germplasm, all of which limit the accessibility or value of the work, especially for subsequent efforts in breeding and genetics. Our data, which included observations on large numbers of public inbred lines, were intended to remedy some of the liabilities in previous work in sunflower pollination; results showed that floret lengths differed significantly between inbred lines, were consistent between years, and explained a majority of variation in visitation by wild pollinators.
In contrast to relatively minor differences in floret length of inbred lines between 2016 and 2017, Atlagić et al. (Reference Atlagić, Joksimović, Sakač, Miklič and Dušanić2003) found that year (i.e. environment) effects appeared to explain more variance in sunflower corolla length than genotype. Though a small number (6) of the 30 inbred lines measured in both 2016 and 2017 showed differences in measured floret length of more than 5%, these changes were not consistently directional, with some lines increasing in size and others decreasing. Because of the effect that changes in plant density have to sunflower head size (Holt and Zentner, Reference Holt and Zentner1985), it is possible that variation in plant spacing explains the year-to-year changes, but insufficient data were collected to test this hypothesis. Another difference with previous research (Fell, Reference Fell1986; Dag et al., Reference Dag, Lior and Afik2002; Greenleaf and Kremen, Reference Greenleaf and Kremen2006; Nderitu et al., Reference Nderitu, Nyamasyo, Kasina and Oronje2008) is the near absence of honey bees foraging in the inbred lines in 2017, despite the location of hives 60 m away. Because of abundant pollen in the selected sunflower lines, limited honey bee foraging in sunflowers may reflect an avoidance of pollen, caused by a negative feedback mechanism within the colonies (Camazine, Reference Camazine1993). Also, the presence of alternate sources of pollen and nectar may determine honey bee visitation; abundant non-crop bee forage is a key attribute that makes North Dakota a favoured summering location for honey bees (Otto et al., Reference Otto, Roth, Carlson and Smart2016), and pollen-collecting honey bees appear to prefer other plants as pollen sources (Prasifka and Mallinger, unpublished data). Whatever the cause, previous results from the same location have found honey bee visitation to be inconsistent between years (Mallinger and Prasifka, Reference Mallinger and Prasifka2017).
Understanding the importance of floret size to wild pollinators of sunflower and identifying highly attractive, short floret lines suggest potential to improve both the efficiency of hybrid seed production and the consistent, high yields of the resulting hybrids grown by sunflower producers. However, several potential limitations remain. First, because floret size differences of 1 mm or less may affect pollinator behaviour, it may be tedious to select for shorter florets without the use of genetic markers. Second, it is not clear to what degree pleiotropic effects on other traits may lead to undesirable trade-offs. For example, correlations among some sunflower seed traits are already known (Wills et al., Reference Wills, Abdel-Haleem, Knapp and Burke2010); if floret size and seed size are highly correlated, selection for shortened florets could be impractical when seed size is important (e.g. confection sunflowers) or limiting to oil yields if seed-to-hull ratios are affected. Third, the relationship between wild bee visitation and floret size provides only a partial explanation of behaviour and does not include information on how the honey bee, a key managed pollinator in sunflower hybrid seed production, responds to floret size. There is some variation in proboscis length in honey bees (Waddington and Herbst, Reference Waddington and Herbst1987), but data on body size and proboscis length from many bee species (Cariveau et al., Reference Cariveau, Nayak, Bartomeus, Zientek, Ascher, Gibbs and WInfree2016) suggests limitations to honey bee foraging imposed by sunflowers with deep corollas may be similar to some common wild bees in sunflower (M. trinodis and Melissodes agilis Cresson; both long-tongued species) and less strong than others (A. helianthi; a short-tongued bee).
The most efficient way to improve pollinator–sunflower interactions appears to be directly addressing the limitations noted above. Examining trait correlations and obtaining genetic markers should be cost-efficient given available sunflower genetic resources (Mandel et al., Reference Mandel, Nambeesan, Bowers, Marek, Ebert, Rieseberg, Knapp and Burke2013) and success in understanding control of flower size in other plants (Krizek and Anderson, Reference Krizek and Anderson2013). It also may be worthwhile to determine the most important pollinator species, a value that can logically be inferred by data on species’ per-visit efficacy (successful pollinations per visit; Parker, Reference Parker1981) and abundance in key growing areas; if relatively large bees are common and efficient pollinators, selection for minor reductions in floret size could provide a substantial improvement in pollination (i.e. there would be diminishing returns from further reducing floret size). Lastly, though quantifying other floral traits such as pollen and nectar content is slow and difficult, these traits may help explain residual variation in bee visitation (Somme et al., Reference Somme, Vanderplanck, Michez, Lombaerde, Moerman, Wathelet, Wattiez, Lognay and Jacquemart2015) and contribute to improved sunflower pollination.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262118000072
Acknowledgements
The authors thank Lisa Brown (USDA-ARS) for help in collecting floret size data and taking bee observations, Brady Koehler (USDA-ARS) for establishing and maintaining the 2016 field trials, and the North Dakota State University Agronomy Seed farm for providing land for field trials in 2017. Qing-Ming Gao (USDA-ARS) provided a constructive review of an earlier version of this manuscript. This publication is supported by the USDA Agricultural Research Service Long-Term Agroecosystem Research (LTAR) Network.





