Ageing, defined as a multifactorial phenomenon characterised by a time-dependent decline in physiological function, is related to many metabolic alterations, such as dyslipidaemia, atherosclerosis, obesity, type 2 diabetes, arthritis and neurodegenerative disease (Knight, Reference Knight2001). In particular, aged mammals, including man, show increasing lipid concentrations in the serum and liver, and develop CVD directly related to lipid metabolism, such as hypertriacylglycerolaemia and hypercholesterolaemia (Ferrari et al. Reference Ferrari, Radaelli and Centola2003). Moreover, the ageing process involves the accumulation of oxidative damage in cells and tissues. Oxidative stress, such as via the increased production of free radicals and other reactive oxygen species and decreased antioxidative defences, is known to occur during ageing (Beckman & Ames, Reference Beckman and Ames1998; Wickens, Reference Wickens2001; Yu & Chung, Reference Yu and Chung2006). Numerous studies have shown that oxidative stress causes structural and functional alterations in molecular, cellular, tissue and organ systems (Yu & Chung, Reference Yu and Chung2001; Kim et al. Reference Kim, Park, Chung, Zou, Jung, Sung, Yu and Chung2004; Poon et al. Reference Poon, Calabrese, Scapagnini and Butterfield2004). Furthermore, recent molecular studies on oxidative stress have shown altered gene regulation during ageing (Kim et al. Reference Kim, Yu and Chung2002; Yoon et al. Reference Yoon, Yun and Chung2002). Therefore, functional foods which possess both a hypolipidaemic effect and antioxidant activity may be effective and useful for reducing the risk of age-associated pathological damage.
Amla (Emblica officinalis Gaertn.) is a member of the small genus of Emblica (Euphorbiaceae). It grows in tropical and subtropical parts of China, India, Indonesia, and the Malay Peninsula. It is an important dietary source of vitamin C, minerals, and amino acids and also contains phenolic compounds, tannins, phyllembelic acid, phyllemblin, rutin, curcuminoides and emblicol. All parts of the plant are used for medicinal purposes. Especially, the fruit has been used in Ayurveda as a potent rasayana (Thakur, Reference Thakur1985) and traditional medicine for the treatment of diarrhoea, jaundice and inflammation (Deokar, Reference Deokar1998). In addition, the pulp of the fruit is smeared on the head to alleviate headache and dizziness (Perry, Reference Perry1980). Recently, amla extract has been tested for various pharmacological activities. The fruit extract was reported to have hypolipidaemic (Anila & Vijayalakshmi, Reference Anila and Vijayalakshmi2002), antidiabetic (Sabu & Kuttan, Reference Sabu and Kuttan2002) and anti-inflammatory activities (Asmawi et al. Reference Asmawi, Kankaanranta, Moilanen and Vapaatalo1993) and inhibit retroviruses such as HIV-1 (El-Mekkawy et al. Reference El-Mekkawy, Meselhy, Kusumoto, Kadota, Hattori and Namba1995), tumour development (Jose et al. Reference Jose, Kuttan and Kuttan2001) and gastric ulcer (Bandyopadhyay et al. Reference Bandyopadhyay, Pakrashi and Pakrashi2000). Moreover, amla extract exhibited antioxidant properties (Bhattacharya et al. Reference Bhattacharya, Chatterjee, Ghosal and Bhattacharya1999; Anila & Vijayalakshmi, Reference Anila and Vijayalakshmi2003), and it has been reported that the aqueous extract of amla is a potent inhibitor of lipid peroxide formation and a scavenger of hydroxyl and superoxide radicals in vitro (Jose & Kuttan, Reference Jose and Kuttan1995). In a previous study, we also demonstrated the antioxidative property of amla using Cu2+-induced oxidised human LDL (Kim et al. Reference Kim, Yokozawa, Kim, Tohda, Rao and Juneja2005). Taken together, this evidence suggests that amla may be an effective modulator against oxidative stress-related alterations under ageing.
In the present study, we investigated the effects of amla on lipid metabolism and oxidative stress in the ageing process and also determined protein expression involved in age-related changes.
Materials and methods
Materials
The following reagents were purchased from Wako Pure Chemical Industries, Ltd (Osaka, Japan): 4,6-dihydroxy-2-mercaptopyrimidine (2-thiobarbituric acid; TBA), bovine serum albumin, 2-amino-2-hydroxymethyl-1,3-propanediol (tri(hydroxymethyl)-aminomethane), Tween 20, phenylmethyl sulfonyl fluoride (PMSF), protease inhibitor cocktail and skimmed milk powder. Precision plus protein standards and the Bio-Rad protein assay kit were purchased from Bio-Rad Laboratories, Japan. Rabbit anti-human PPARα, rabbit anti-human bax, mouse anti-mouse bcl-2, rabbit anti-human NF-κB p65 polyclonal antibody, rabbit anti-human inhibitor binding protein κB-α (IκB-α) polyclonal antibody, mouse anti-mouse NOS2 monoclonal antibody (primary antibody for inducible NO synthesis (iNOS)), mouse anti-human cyclo-oxygenase-2 (COX-2) monoclonal antibody, goat anti-rabbit IgG horseradish peroxidase conjugated secondary antibody, and goat anti-mouse IgG horseradish peroxidase conjugated secondary antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-mouse β-actin antibody was purchased from Sigma-Aldrich (St Louis, MO, USA). ECL™ Western blotting detection reagents were purchased from Amersham Bioscience (Piscataway, NJ, USA).
Preparation of amla extract
Two grades of amla extract have been used. The first one is a commercial product of amla, an enzymic amla fruit juice extract called SunAmla (Taiyo Kagaku Co. Ltd, Yokkaichi, Japan), and the other one is a polyphenol-rich fraction of amla fruits derived from ethyl acetate (EtOAc) extraction. The EtOAc extract was prepared by extracting the air-dried amla fruit pieces in water–EtOAc (1:4) at room temperature for 24 h. The extract was evaporated under reduced pressure followed by lyophilisation. The yield was 12 % of the dried fruits. The composition was 81·2 % moisture, 14·1 % carbohydrate, 3·4 % fibre, 0·63 % minerals, 0·5 % protein, 0·1 % fat, 0·05 % Ca and 0·02 % P.
Total polyphenols and vitamin C contents of amla extracts
Total polyphenol contents of the SunAmla and EtOAc extract of amla were measured by a colorimetric method using gallic acid as a standard. The vitamin C content was measured using HPLC.
Animals and treatments
The ‘Guidelines for Animal Experimentation’ approved by the University of Toyama were followed during these experiments. Male Wistar strain rats (Japan SLC Inc., Hamamatsu, Japan), aged 2 and 10 months, were used in the experiment. Young rats were 2 months old and weighed 125 (se 3) g at the beginning of the experiment. Aged rats were 10 months old and weighed 476 (se 15) g. Rats were kept in a wire-bottomed cage under a conventional light regimen with a dark night at room temperature (about 23°C) and humidity (about 60 %). Animals were given laboratory pellet chow (CE-2, comprising 24·0 % protein, 3·5 % lipid and 60·5 % carbohydrate; CLEA Japan Inc., Tokyo, Japan) and water ad libitum. Food consumption was measured daily, and body weight was recorded weekly. Twenty-one of the rats were divided into three groups, matched for their serum total cholesterol and body weight. The rats were fed a basal diet (CE-2) and treated with SunAmla extract or EtOAc extract of amla. Amla extract was dissolved in water and given orally to rats at a dose of SunAmla or EtOAc extract of amla of 40 or 10 mg/kg body weight per d for 100 d using a stomach tube. The oral dose was determined by the effective dose in our previous study (Kim et al. Reference Kim, Yokozawa, Kim, Tohda, Rao and Juneja2005). Control rats were given access to water alone. At 6 h after the last dose, the rats were decapitated, their blood was drawn, and serum was collected by centrifuging the blood at 1000 g for 15 min at 4°C. Each liver was removed, dried on tissue paper, weighed and stored at − 80°C until analysis.
Measurement of serum cholesterol and triacylglycerol levels
Serum total and non-esterified cholesterol levels were determined using commercial kits (Cholesterol E-Test Wako and Free Cholesterol E-Test Wako, respectively; Wako Pure Chemical Industries Ltd, Osaka, Japan). Serum esterified cholesterol levels were calculated by subtracting the non-esterified cholesterol levels from the total cholesterol levels. Serum TAG levels were determined using commercial kits (Triglyceride E-Test Wako; Wako Pure Chemical Industries Ltd).
Measurement of hepatic cholesterol and triacylglycerol amounts
The liver of each rat was homogenised, total lipids of the liver homogenates were extracted with a mixture of chloroform and methanol (2:1, v/v) according to the method of Folch et al. (Reference Folch, Lees and Sloane Stanley1957), and the amounts of total cholesterol, non-esterified cholesterol and TAG were determined using the Wako kit described earlier. Esterified cholesterol amounts were calculated by subtracting the non-esterified cholesterol amounts from the total cholesterol amounts.
Measurement of serum 2-thiobarbituric acid-reactive substance levels
Serum TBA-reactive substance levels were determined using the method of Naito & Yamanaka (Reference Naito and Yamanaka1978).
Isolation of hepatic mitochondria and measurement of 2-thiobarbituric acid-reactive substance levels
Mitochondria were prepared from liver homogenates by differential centrifugation (800 g and 12 000 g; 4°C; 15 min) according to the methods of Johnson & Lardy (Reference Johnson and Lardy1967) and Jung & Pergande (Reference Jung and Pergande1985), respectively, with slight modifications. Each pellet was re-suspended in preparation medium and the TBA-reactive substance concentration was determined by the method of Buege & Aust (Reference Buege and Aust1978). Briefly, 250 μl of each re-suspended pellet or working standard was added to 750 μl TBA–TCA–HCl solution (0·4 % of TBA, 15 % of TCA, 2·5 % HCl) and it was heated at 95–100°C for 20 min and cooled in an ice-bath. Then, samples were centrifuged at 1000 g at room temperature for 10 min to transfer supernatant fractions from the denatured protein precipitate. TBA-reactive substance was determined by measuring absorbance at 532 nm. The value of TBA-reactive substance was expressed in nmol malondialdehyde/mg protein by a calibration curve constructed from malondialdehyde (0–25 nmol/ml) in 1,1,3,3-tetramethoxypropane. The protein level was evaluated by the method of Itzhaki & Gill (Reference Itzhaki and Gill1964) with bovine serum albumin as the standard.
Homogenisation, isolation of cytosol, and nuclear extracts
Livers were homogenised (Choi et al. Reference Choi, Lee, Nguyen, Jang, Lee, Wu, Takano, Maki, Henkard and Trepel1997) at 4°C in homogenisation buffer (25 mm-tri(hydroxymethyl)-aminomethane-Cl (pH 7·5), 250 mm-NaCl, 5 mm-EDTA, 1 mm-PMSF and 1 mm-dithiothreitol), and supplemented with protease inhibitor cocktail that consisted of 100 mm-4-(2-aminoethyl) benzenesulfonyl fluoride, 0·08 mm-aprotinin, 2 mm-leupeptin, 5 mm-bestatin, 1 mm-pepstatin A and 1·5 mm-E-64. Homogenates were incubated for 15 min on ice, added to 10 % Nonidet P-40, and centrifuged at 4000 g, at 4°C, for 5 min. For Western blot analysis, each sample (20 μg protein/lane) was denatured by boiling in Laemmli sample buffer and stored at − 80°C until the assay.
Nuclear extracts were isolated using the method of Sakurai et al. (Reference Sakurai, Hisada, Ueno, Sugiura, Kawashima and Sugita1996). Briefly, liver tissue was weighed and homogenised by a Potter Elvehjem homogeniser (Shintokagaku, Tokyo, Japan) in 4 vol. (w/v) of buffer A containing 10 mm-HEPES (pH 7·9), 10 mm-KCl, 0·1 mm-EDTA, 1 mm-dithiothreitol, 0·5 mm-PMSF, and protease inhibitor. Homogenates were incubated for 15 min on ice, added to 10 % Nonidet P-40, and centrifuged at 4000 g, at 4°C, for 5 min. Supernatant fractions were discarded and pellets were re-suspended in 2 vol. buffer B containing 20 mm-HEPES (pH 7·9), 0·4 m-NaCl, 1 mm-EDTA, 1 mm-dithiothreitol, 1 mm-PMSF, and protease inhibitors. Homogenates were kept for 15 min at 4°C and then centrifuged at 14000 g for 5 min at 4°C. Nuclear extracts were collected in microfuge tubes and stored in samples at − 80°C. The protein concentration of the nuclear extracts was determined by the Bio-Rad protein assay.
Western blot analysis
Homogenates (20 μg for bax, bcl-2, iNOS and COX-2), cytosol extract (IκB-α) and crude nuclear extract (20 μg for PPARα and NF-κB) from the liver were subjected to SDS-PAGE (10 %, w/v). Proteins were then transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA) and blocked overnight at 4°C with 5 % non-fat dry milk in 25 mm-tri(hydroxymethyl)-aminomethane-Cl (pH 8·3), 140 mm-NaCl, 2 mm-KCl and 0·1 % Tween 20. Membranes were then incubated for 3 h at 4°C with the primary polyclonal antibody raised against PPARα (dilution 1:1000), bax (dilution 1:1000), bcl-2 (dilution 1:1000), NF-κB (dilution 1:1000), IκB-α (dilution 1:1000), iNOS (dilution 1:1000), COX-2 (dilution 1:1000) and β-actin (1:5000). Western blotting with rabbit polyclonal antibody (dilution 1:1000) or mouse monoclonal antibody (dilution 1:1000) was also performed. Detection was achieved using the ECL chemiluminescence kit for horseradish peroxidase. The density was quantified in a Phosphor Imager (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
Results are expressed as mean values with their standard errors. The effect on each parameter was examined using one-way ANOVA. Individual differences between groups were evaluated using the Dunnett's test and those at P < 0·05 were considered significant.
Results
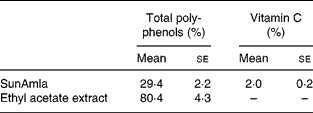
Total polyphenol and vitamin C contents of amla extract
The total polyphenol content of SunAmla was about one-third that of the EtOAc extract. However, SunAmla contained vitamin C at 2 %. Vitamin C was not present in the EtOAc extract (Table 1).
Table 1 Total polyphenol and vitamin C contents of amla extracts (Mean values with their standard errors for ten replicate samples)
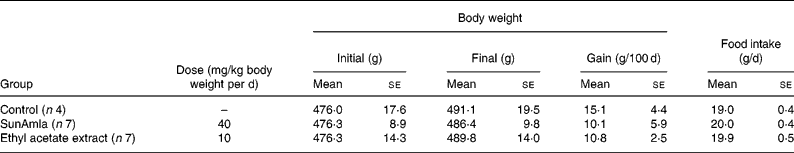
Body weight and food intake
Table 2 shows the changes in body weight and food intake during the oral administration of amla to aged rats for 100 d. Body-weight gain was slightly decreased compared with aged control rats after the oral administration of SunAmla or EtOAc extract of amla, but it was not significantly different among the groups. Also, there were no differences in food intake among the groups.
Table 2 Body weight and food intake of ageing rats (Mean values with their standard errors)
Serum and hepatic cholesterol levels
The serum and hepatic cholesterol profiles of the aged rats fed SunAmla or EtOAc extract of amla are summarised in Table 3. The serum total and non-esterified cholesterol levels in aged control rats significantly increased compared with young rats, whereas the levels were decreased by the amla extract, especially in the EtOAc extract of amla at 10 mg/kg body weight per d. However, esterified cholesterol levels of the rats were not significantly different among the groups. The hepatic total, non-esterified and esterified cholesterol levels in the aged control rats were significantly increased compared with the young rats (P < 0·001). However, the hepatic total cholesterol levels were significantly reduced by 18 or 26 % by the administration of SunAmla extract at 40 or EtOAc extract of amla at 10 mg/kg body weight per d compared with aged control rats, respectively (P < 0·001). The hepatic non-esterified cholesterol levels were also significantly decreased by the administration of amla extracts compared with aged control rats (P < 0·001). In addition, the esterified cholesterol level in the livers of rats fed SunAmla extract at 40 or EtOAc extract of amla at 10 mg/kg body weight per d was significantly reduced by 19 or 29 % compared with aged control rats, respectively (P < 0·001).
Table 3 Serum and hepatic cholesterol profiles (Mean values with their standard errors)

Mean value was significantly different from that of the young rats: *P < 0·05, **P < 0·01, ***P < 0·001.
Mean value was significantly different from that of the ageing control rats: †P < 0·05, ‡P < 0·001.
Serum and hepatic triacylglycerol levels
Table 4 shows the serum and hepatic TAG levels of aged rats given SunAmla or EtOAc extract of amla. The serum TAG level in aged control rats markedly increased by 2·7-fold compared with young rats (P < 0·001). However, the administration of SunAmla or EtOAc extract of amla significantly reduced the serum TAG level by 30 or 24 %, respectively. Hepatic TAG levels in aged control rats also increased by 2·7-fold compared with young rats (P < 0·001). However, the hepatic TAG levels significantly declined from 38·0 to 32·7 or 30·9 mg/g by the administration of SunAmla or EtOAc extract of amla, respectively (P < 0·001).
Table 4 Serum and hepatic triacylglycerols (Mean values with their standard errors)
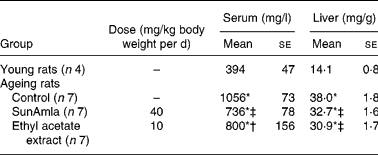
*Mean value was significantly different from that of the young rats (P < 0·001).
Mean value was significantly different from that of the ageing control rats: †P < 0·01, ‡P < 0·001.
Expression of peroxisome proliferator-activated receptor-α protein
To investigate the effects of amla extract on lipid metabolism in aged rats, hepatic PPARα protein levels were determined using Western blot analysis (Fig. 1). The PPARα protein levels in aged control rats were markedly decreased by 60 % compared with young rats (P < 0·001), whereas the oral administration of SunAmla or EtOAc extract of amla significantly increased the levels to 48 % compared with aged control rats, respectively (P < 0·001).

Fig. 1 PPARα protein in hepatic nucleus. Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that of the young rats (P < 0·001). † Mean value was significantly different from that of the ageing control rats (P < 0·001). EtOAc; ethyl acetate.
2-Thiobarbituric acid-reactive substance levels of serum and hepatic mitochondria
Table 5 represents the inhibitory effect of amla extract on lipid peroxidation induced by age-related oxidative stress. The TBA-reactive substance levels of serum and hepatic mitochondria in aged control rats were significantly increased compared with young rats. However, the administration of SunAmla or EtOAc extract of amla significantly reduced the serum TBA-reactive substance levels by 22 or 36 % compared with aged control rats, respectively. Moreover, the TBA-reactive substance levels of hepatic mitochondria were also significantly lower in rats given amla extract than in aged control rats.
Table 5 2-Thiobarbituric acid-reactive substance levels of serum and hepatic mitochondria (Mean values with their standard errors)
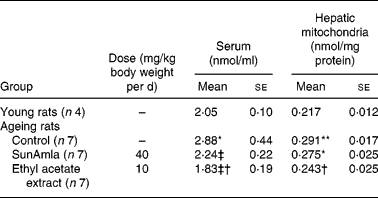
Mean value was significantly different from that of the young rats: *P < 0·01, **P < 0·001.
Mean value was significantly different from that of the ageing control rats: †P < 0·05, ‡P < 0·01, ‡†P < 0·001.
Expressions of bax and bcl-2 proteins
We also investigated the effects of amla extract on apoptosis in the liver of aged rats (Fig. 2). As compared with young rats, the bax protein levels in aged control rats were significantly increased by 46 % (P < 0·001). However, oral administration of amla extract significantly decreased the level of bax protein (P < 0·001). Bcl-2 protein levels in aged control rats did not show significant differences compared with those of young rats, while those of rats given SunAmla extract were significantly increased (P < 0·001).

Fig. 2 Bax (A) and bcl-2 (B) proteins in hepatic homogenate. * Mean value was significantly different from that of the young rats (P < 0·001). † Mean value was significantly different from that of the ageing control rats (P < 0·001). EtOAc; ethyl acetate.
Expressions of nuclear factor-κB and inhibitor binding protein κB-α proteins
As shown in Fig. 3, NF-κB protein levels in the livers of aged control rats were higher than those of young rats. However, the oral administration of amla extract decreased the NF-κB protein levels compared with aged control rats, although the IκB-α protein level did not show any significant difference among all groups.

Fig. 3 NF-κB (A) and inhibitor binding protein κB-α (IκB-α) (B) proteins in hepatic nucleus and cytoplasm. Mean value was significantly different from that of the young rats: * P < 0·05, ** P < 0·001. † Mean value was significantly different from that of the ageing control rats (P < 0·001). EtOAc; ethyl acetate.
Expressions of inducible nitric oxide synthase and cyclo-oxygenase-2 proteins
Fig. 4 shows the effects of amla on levels of iNOS and COX-2 protein. The iNOS protein levels in aged control rats markedly increased by 4·9-fold compared with young rats (P < 0·001), whereas the oral administration of SunAmla or EtOAc extract of amla led to a significant decrease to 15 or 46 % compared with aged control rats, respectively (P < 0·001). In addition, COX-2 protein levels in aged control rats were slightly increased compared with young rats (P < 0·01), while those of rats given SunAmla at 40 mg/kg body weight per d or EtOAc extract of amla at 10 mg/kg body weight per d were significantly lower by 25 or 27 % than those of aged control rats, respectively (P < 0·001).

Fig. 4 Inducible NO synthase (iNOS) (A) and cyclo-oxygenase-2 (COX-2) (B) proteins in hepatic homogenate. Mean value was significantly different from that of the young rats: * P < 0·01, ** P < 0·001. † Mean value was significantly different from that of the ageing control rats (P < 0·001). EtOAc; ethyl acetate.
Discussion
Old age is associated with the appearance of dyslipidaemia, atherosclerosis, obesity and type 2 diabetes mellitus (Knight, Reference Knight2001). These risk factors steadily increase the incidence of CVD among elder individuals. In addition, aged mammals including man show increasing plasma concentrations of lipids and reduced fatty acid oxidation (Parini et al. Reference Parini, Angelin and Rudling1999). Recently, several studies showed that the significant increase of lipid levels in the ageing process is associated with a reduction in the expression and activity of PPARα, a nuclear transcription factor, in the liver of rats (Lacko & Davis, Reference Lacko and Davis1979; Sanguino et al. Reference Sanguino, Ramón, Michalik, Wahli, Alegret, Sánchez, Vázquez-Carrera and Laguna2004a). Therefore, great effort has been focused on a PPARα activator for patients with dyslipidaemia requiring lipid-lowering therapy.
In the present study, we investigated the effect of amla extract on lipid profiles and oxidative stress in the ageing process. The present results also showed that the total cholesterol and TAG levels in the serum of aged control rats (13 months) were higher than those of young rats (2 months) and accumulated higher total, non-esterified and esterified cholesterol, and TAG levels in the liver (Tables 3 and 4). However, the oral administration of SunAmla or EtOAc extract of amla for 100 d to aged rats partially prevented age-related increases in cholesterol and TAG levels in the serum and liver. A small fraction of the cholesterol in the liver is incorporated into the membranes of hepatocytes, but most is exported. Esterified cholesterol is one of the exported forms. It is formed in the liver through the action of acyl-CoA-cholesterol acyl transferase, which catalyses the conversion of cholesterol into a more hydrophobic form that is transported in secreted lipoprotein particles to other tissues that use cholesterol or is stored in the liver. Thus, the reduction of esterified cholesterol level indicates that the cholesterol was used for the synthesis of vital molecules in tissues, including the liver.
PPARα, a nuclear transcription factor, is known to regulate the transcription of genes involved in lipid, cholesterol, lipoprotein and glucose energy metabolism, and insulin sensitivity (Ricote & Glass, Reference Ricote and Glass2001; Barbier et al. Reference Barbier, Torra, Duguay, Blanquart, Fruchart, Glineur and Staels2002; Blanquart et al. Reference Blanquart, Barbier, Fruchart, Staels and Glineur2003; Berger et al. Reference Berger, Akiyama and Meinke2005). PPARα is expressed in tissues such as the liver, muscle, kidney and heart, where it stimulates the β-oxidative degradation of fatty acids. Aged mammals show a decreased capacity for fatty acid oxidation (Toth & Tehernof, Reference Toth and Tehernof2000) and this may be an underlying cause of age-related increases in plasma lipid concentrations and dyslipidaemia. In addition, PPARα deficiency is consistently related to increased plasma levels of TAG and cholesterol (Peters et al. Reference Peters, Hennuyer, Staels, Fruchart, Fievet, Gonzalez and Auwerx1997). Therefore, we determined the PPARα expression level in the liver. The present results also demonstrated that the hepatic PPARα protein level in the liver of aged rats was increased by 48 or 47 % with SunAmla at 40 mg/kg body weight per d or EtOAc extract of amla at 10 mg/kg body weight per d, respectively, compared with aged control rats (Fig. 1). Based on these results, we found that serum and hepatic lipids are moderately increased in old animals as a result of the reduction of the hepatic PPARα protein level, while amla extract decreased the elevated levels of lipids in aged rats by activating the PPARα level (Tables 3 and 4; Fig. 1). These results suggest that amla may be an effective PPARα activator and protect against CVD resulting from dyslipidaemia.
It is well known that reactive oxygen species-mediated lipid peroxidation increases with age (Halliwell & Chirico, Reference Halliwell and Chirico1993; Lin & Beal, Reference Lin and Beal2003). In addition, Inoue et al. (Reference Inoue, Noji, Shen, Takahashi and Katayama1997) showed the negative correlation between the liver expression of the PPARα gene and the plasma TBA-reactive substance level. As shown by the present study, TBA-reactive substance levels of serum and hepatic mitochondria in aged rats were also significantly increased, whereas the administration of SunAmla or EtOAc extract of amla significantly decreased the TBA-reactive substance level compared with aged control rats, respectively (Table 5). It is thought that reduction of the TBA-reactive substance level by amla extract may be due to the decrease of age-related dyslipidaemia through the activation of PPARα and the antioxidant effect of amla extract which contains polyphenols and vitamin C (Table 1).
Bcl-2 family proteins, anti-apoptotic proteins, protect against cell death by acute oxidative stress (Chan & Yu, Reference Chan and Yu2004). It has been demonstrated that bcl-2 overexpression preserves viability and diminishes lipid peroxidation in cells exposed to oxidative stress (Hockenbery et al. Reference Hockenbery, Oltvai, Yin, Milliman and Korsmeyer1993). Conversely, some pro-apoptotic proteins such as bax, a mammalian cell-death protein that targets mitochondrial membranes, can induce mitochondrial damage and cell death even when caspases are inactivated (Green & Reed, Reference Green and Reed1998). However, several workers have reported that antioxidants slow down or block the apoptotic process by stabilising mitochondrial functions (Samhan-Arias et al. Reference Samhan-Arias, Martín-Romero and Gutiérrez-Merino2004; Williams et al. Reference Williams, Spencer and Rice-Evans2004). In the present results, the bax protein level was significantly enhanced in the aged control rats (P < 0·001), while bcl-2 protein was slightly reduced compared with young rats but not significant between the aged-rat groups. The oral administration of amla extract has a beneficial effect on these proteins (Fig. 2). These results indicate that amla extract has protective effects against cell death by age-related oxidative stress.
Chronic oxidative stress and inflammatory reactions also lead to many age-associated diseases and the ageing process was found to enhance the activation of NF-κB by down regulating IκB-α (Spencer et al. Reference Spencer, Poynter, Im and Daynes1997; Poynter & Daynes, Reference Poynter and Daynes1998). Under resting conditions, NF-κB exists in the cytoplasm as a dimer bound to the inhibitory protein IκB-α. Inducers of NF-κB, such as inflammatory cytokines, reactive oxygen species, and viral products, activate a dimeric IκB-α kinase (IKK) complex, causing the phosphorylation and ubiquitination of IκB-α and its release from NF-κB. The free NF-κB dimer translocates to the nucleus, where it regulates target gene transcription such as iNOS, COX-2, IL-6, IL-12 and TNFα (Baldwin, Reference Baldwin2001; Li & Verma, Reference Li and Verma2002). Transcription factors that are directly influenced by reactive oxygen species and pro-inflammatory cytokines include NF-κB, AP-1, PPAR, and other members of the nuclear receptor superfamily involved in ageing (Lavrovsky et al. Reference Lavrovsky, Chatterjee, Clark and Roy2000; Sanguino et al. Reference Sanguino, Roglans, Alegret, Sánchez, Vázquez-Carrera and Laguna2004b). In the present study, we also measured NF-κB and IκB-α protein levels during ageing. NF-κB protein in aged control rats increased compared with young rats, whereas the oral administration of amla extract decreased the elevated NF-κB protein level compared with aged control rats. Interestingly, the protein level of IκB-α was not changed in spite of the increased NF-κB protein level with ageing, indicating that there might be another regulation mechanism of the IκB-α protein level during ageing (Fig. 3). These results imply that amla protects against oxidative stress by inhibiting NF-κB activation during ageing.
Recently, PPARα activation has also been demonstrated to play an important clinical role in the control of the cellular redox balance and inflammatory responses such as iNOS and COX-2 by negatively interfering with NF-κB transcriptional activity (Spencer et al. Reference Spencer, Poynter, Im and Daynes1997; Blanquart et al. Reference Blanquart, Barbier, Fruchart, Staels and Glineur2003). Modulation of this process may prevent or at least attenuate age-related changes in lipid metabolism and oxidative stress. Many investigators have shown that antioxidants, PPARα activators and hypolipidaemic drugs slow the ageing process and prevent age-related disease (Poynter & Daynes, Reference Poynter and Daynes1998; Sanguino et al. Reference Sanguino, Roglans, Alegret, Sánchez, Vázquez-Carrera and Laguna2005). Thus, we assessed the contributory roles of iNOS and COX-2 to investigate the effect of amla on oxidative stress in the ageing process. As shown by the present study, old age induced the overexpressions of iNOS and COX-2 proteins, and these age-related changes were inhibited by the oral administration of amla extract (Fig. 4). Moreover, this inhibitory effect was higher in the EtOAc extract of amla, a polyphenol-enriched extract, than SunAmla. Therefore, these findings imply that amla extract can act as an antioxidant to alleviate age-related oxidative stress by the inhibition of both iNOS and COX-2 as a result of the down regulation of NF-κB activation and up regulation of PPARα.
Zhang et al. (Reference Zhang, Tanaka, Yang and Kouno2001) isolated several novel sesquiterpenoids from the roots, new organic acid gallates and polyphenols from the fruit juice, and new ellagitannins and flavonoids from the branches and leaves of Phyllanthus emblica. Among them, corilagin (β-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-d-glucose) and its analogue (1,6-di-O-galloyl-β-d-glucose) from fresh juice or solvent extracts of amla showed very strong antioxidant effects in vitro and in vivo (Ghosal et al. Reference Ghosal, Tripathi and Chauhan1996; Bhattacharya et al. Reference Bhattacharya, Chatterjee, Ghosal and Bhattacharya1999; Zhang et al. Reference Zhang, Tanaka, Yang and Kouno2001). Moreover, anti-inflammatory and anti-ageing mechanisms of polyphenolic compounds found in various plants involve the down regulation of the inflammatory response through inhibiting factors such as iNOS and COX-2 via its inhibitory effects on NF-κB or AP-1 (De la Lastra & Villegas, Reference De la Lastra and Villegas2005). Hypolipidaemic drugs and PPARα activators reduce oxidative stress and inhibit NF-κB activation by activation of PPARα (Inoue et al. Reference Inoue, Itoh and Aoyagi2002; Pahan et al. Reference Pahan, Jana, Liu, Taylor, Wood and Fischer2002). On the basis of this evidence, the hypolipidaemic and PPARα-activating activities of amla extract in the present study can be explained by its antioxidant effect derived from its polyphenolic constituents.
In conclusion, the present results demonstrated that age-related increases of lipids and oxidative stress are mediated by the reduction of hepatic PPARα expression. However, the administration of amla extract to aged rats attenuates age-related dyslipidaemia and oxidative stress by inhibiting NF-κB transcriptional pathways through modulating hepatic PPARα expression, suggesting that amla may be beneficial in preventing age-related atherosclerotic CVD.











