Introduction
Systemic racism is deeply embedded in biomedical and health research in the US. It dates to performance of experimental gynecologic surgery on unanesthetized enslaved Black women in the pre-Civil War era, with prominent 20th Century examples including the Tuskegee syphilis study conducted by the US Public Health Service and the exploitation of Henrietta Lacks in the development of the HeLa cell line [Reference Gamble1]. It remains a feature of the broader medical culture, as evidenced by the recent revelation of coerced gynecologic operations performed on immigrant women at a US detention center [Reference Washington and Olivares2] and a 2016 study documenting false beliefs among white physician trainees about Blacks feeling less pain than whites [Reference Hoffman, Trawalter, Axt and Oliver3]. Structural racism also finds expression in the insufficient engagement of patient and community stakeholders, particularly those from Black, Indigenous, and People of Color (BIPOC) communities, as full partners in the conduct of clinical and translational science [Reference George, Duran and Norris4]. Lack of community engagement not only diminishes the social accountability of research, but also undermines the quality of the science [Reference Wallerstein and Duran5–Reference Jagosh, Macaulay and Pluye7]. This is particularly true for clinical trials, which often fail to enroll participants representative of the nation’s demographic diversity, take account of the feasibility and acceptability of adopting study interventions in real world practice, or address research questions and interventions that community members consider most salient [Reference Jagosh, Macaulay and Pluye7–Reference Woolf10]. Examples of efforts to move community engagement in research from marginalized to mainstream include the NIH Clinical and Translational Science Awards (CTSAs), which require a community engagement program, and the Patient Centered Outcomes Research Institute (PCORI), which mandates that all sponsored studies include stakeholder engagement.
The COVID-19 pandemic has exposed how little these efforts have changed the dominant culture of clinical research. When faced with the urgent need to generate knowledge about prevention and treatment of the novel coronavirus, researchers and the organizations that sponsor their research largely neglected to involve community stakeholders early in the research process. The result is predictable and repeats past failings. BIPOC populations bear a disproportionate toll of COVID-19 cases and deaths, driven by manifestations of structural racism such as living in crowded housing, being incarcerated and having a low paying job as an essential front line worker [Reference Cooper and Crews11,Reference Webb Hooper, Nápoles and Pérez-Stable12]. Between January and October 2020, the number of excess deaths relative to mortality rates in 2015–19 were 54% higher among Latinx, 37% higher among Asians, 33% higher among Black persons, and 29% higher among American Indians and Alaska Natives, in comparison to 12% higher among whites [Reference Rossen, Branum, Ahmad, Sutton and Anderson13]. Although it would be logical to focus COVID-19 research on the populations most heavily impacted by COVID-19, BIPOC populations are underrepresented in COVID-19 clinical trials [Reference Borno, Zhang and Gomez14,Reference Chastain, Osae, Henao-Martinez, Franco-Paredes, Chastain and Young15]. Despite high levels of interest in medical science among BIPOC populations, their underrepresentation in clinical research reflects entrenched norms of study design, recruitment, and retention that have historically excluded BIPOC populations, as well as deficits in the trustworthiness of researchers and research institutions [Reference George, Duran and Norris4,Reference Cooper and Crews11,Reference Milani, Swain, Otufowora, Cottler and Striley16–Reference Wilkins18].
Too Little, Too Late?
Noting the concerning lack of diversity among participants in COVID-19 clinical trials, in September, 2020 the NIH launched the Community Engagement Alliance (CEAL) Against COVID-19 Disparities program, allocating $11 million to fund academic-community partnership consortia in 11 states to “promote and facilitate the inclusion and participation of these [underrepresented] groups in vaccine and therapeutic clinical trials” and conduct COVID-19 outreach and education [19]. Most of these CEAL consortia include members of CTSA community engagement programs. The recognition of the importance of community engagement in COVID-19 clinical research is welcome. But is it too little, too late?
One risk is that corrective efforts may emphasize opportunistic recruitment of more diverse study participants without appreciating the importance of long-term investment in trusting and empowering community partnerships. The fall-out from researchers rushing onto the COVID-19 field without first getting community members on board is evident to many of us working in community engagement programs at research institutions across the US. Investigators struggling in mid-study to recruit the number of participants needed for their studies are seeking assistance from our community engagement programs. Many clinical trialists are discovering that their research teams are ill-equipped to succeed in recruitment when the majority of people in their community infected by SARS CoV2 are BIPOC. Research groups conducting COVID-19 cohort and population studies find their studies being populated with participants from more socially advantaged populations rather than populations at highest risk of COVID-19. Research teams belatedly realize that they lack the cultural and linguistic competency necessary for successfully connecting with individuals from the populations most affected by COVID-19 – expertise held by members of the targeted communities and the local organizations serving those communities. Researchers often fail to include in their grant budgets appropriate levels of funding for community partners [Reference Freeman, Seifer, Stupak and Martinez20].
Approaching community members for help late in the process engenders an understandable backlash [Reference Natafgi, Tafari, Chauhan, Bekelman and Mullins21]. Many community members express frustration about being asked to rescue studies in midstream instead of being invited early on to collaborate in designing the project and have ongoing involvement as respected partners. They take note of multi-million-dollar budgets for clinical trials and the small fraction of expenses allocated to community partners, and see tokenism rather than genuine commitment. Deep wounds of distrust from a history of exploitation in research are reopened and deepened among BIPOC [Reference Cooper and Crews11,Reference Wilkins18].
A prime example of this predicament is occurring with COVID-19 vaccine trials. At trial inception, there was neither an explicitly articulated goal by Pharma or an NIH mandate to recruit participants in proportion to the distribution of COVID-19 cases among different racial-ethnic groups, or even proportionate to their share of the population. Many vaccine trials reached the halfway mark in participant recruitment and had their sponsors and investigators wake up, partly at the prodding of the NIH and community activists, to the need to address the underrepresentation of people of color in the trials [Reference Nania22]. Although the CEAL networks are now focusing on this issue, many of our community partners react with alarm at terms in the informed consent process. For example, informed consent documents for some vaccine trials place extreme limits on financial liability for adverse vaccine events. Community members question why the trials do not assure provision of free health care to participants who become infected with SARS CoV2 during the trial when many of the very people they wish to include lack sufficient access to health care services. When community partners or their trusted ambassadors were not invited to be at the table when study protocols were created, should we be surprised if they lack enthusiasm to promote trial participation among their constituents?
Why Community Engagement Matters
Lack of engagement compromises the scientific validity and relevance of clinical research [Reference Hoffman, Trawalter, Axt and Oliver3,Reference George, Duran and Norris4,Reference De las Nueces, Hacker, DiGirolamo and Hicks9]. Cohort studies that do not recruit populations at high risk of COVID-19 will not give an accurate picture of disease patterns and trends. Therapeutic and vaccine trials may produce misleading findings about efficacy if they fail to include participants from diverse social contexts that influence vulnerability to or resilience against infection. Engaged stakeholders are a powerful force for dissemination of research findings, especially to lay communities, facilitating translation of discovery into practices to improve health. Reduced public confidence in clinical science undermines translational benefit when therapeutics, vaccines, and other interventions become publicly available. For example, Black Americans, compared to whites, report less confidence in medical scientists acting in the public interest, which contributes to Blacks being less likely than whites to say that they would definitely or probably get a COVID-19 vaccine when available to the public [Reference Gramlich and Funk23,Reference Altman24].
Community engagement is also essential for social accountability. In addition to being a necessary component of restorative truth and reconciliation to address a shameful history of exploitation of BIPOC in research, engagement shifts the paradigm to a “for us, by us” model that promotes sharing of power to dismantle racial and class inequities. Community engagement demonstrates respect to communities by recognizing community values and interests that matter to community members as people. This model privileges community voice about who benefits from and is impacted by research and the potential positive and negative consequences of a study. Social justice requires that research be at a minimum bi-directionally transactional, and ideally relational [Reference Wallerstein and Duran5,Reference Michener, Cook, Ahmed, Yonas, Coyne-Beasley and Aguilar-Gaxiola6,Reference Cooper and Crews11,Reference Wilkins18].
Mid-Course Corrections and Best Practices
Community engagement requires investment of time as well as money. In a research culture that considers community engagement optional rather than essential, it may be understandable that the pressure for “warp speed” progress on COVID-19 clinical research contributed to investigators neglecting due diligence in engaging stakeholders early on in research projects. As noted above, through efforts such as the CEAL program, the NIH is attempting to make up for some lost ground. The NIH’s COVID-19 Prevention Network (CoVPN) is building on established networks among BIPOC leaders and communities to develop and disseminate tools and resources targeted to these populations. The CoVPN has endorsed guidelines developed by one of its member groups, the HIV Vaccine Trials Network, to “effectively involve communities as key players in research;” the guidelines recommend establishing Community Advisory Boards for all trial sites [25]. The NIH has also earmarked a portion of the funding for its recent Rapid Acceleration of Diagnostics (RADx) COVID-19 testing initiative to community engaged research.
Many COVID-19 community-based participatory research projects across the nation are demonstrating the power of authentic partnerships with BIPOC communities (Table 1). For example, Howard University, a Historically Black University, and the University of California, San Francisco (UCSF) have taken similar approaches to collaborating with local agencies and coalitions. Both partnered with the local health department and community-based groups to open COVID-19 testing sites in underserved communities. In San Francisco, community coalitions are using the findings from testing studies to advocate with local government to target new resources to meet unmet needs for prevention and social services [Reference Kerkhoff, Sachdev and Mizany26]. Howard faculty partnered with a community led coalition to develop community-level responses to COVID-19 disparities. Community engagement programs at both institutions have developed COVID-19-focused community advisory councils. Howard University’s community advisory board provides guidance on local COVID-19 vaccine trial implementation, ensuring funding for community partner participation and multi-lingual research resources. The UCSF COVID-19 Patient and Community Advisory Board proactively reaches out to UCSF investigators to provide consultation on community engagement strategies for a variety of COVID-19 studies, and has consulted on more than 20 studies to date. CTSA-funded institutes at both universities have dedicated pilot grant funding for new community-engaged COVID-19 studies and social justice research projects. In Colorado, the Partnership of Academicians and Communities for Translation council worked with the COVID-19 equity advisory board to draft a document that Governor Polis signed and sent to researchers to increase awareness of the need for community involvement in COVID-19 vaccine trial decision-making. Many other members of the Association for Clinical and Translational Science Partners for the Advancement of Community Engaged Research (PACER) group (https://www.actscience.org/About/Special-Interest-Groups) are helping to lead similar efforts in their communities.
Table 1. Types of community engaged COVID-19 research activities conducted by PACER members

PACER, Partners for the Advancement of Community Engaged Research group; Association for Clinical and Translational Science; CE, community engagement; SES, socieconomic status.
Making Community Engagement Obligatory, Not Optional
COVID-19 has been a wake-up call on many fronts – it was a pandemic on top of multiple epidemics, including racism. It is requiring new ways of thinking about disaster preparedness and control, working and delivering care remotely, and health and social inequities. It makes evident the need for a transformational shift in culture, policy, and structure across the research enterprise among both funders and research institutions to support and value community leadership in research. This engagement must center members of BIPOC communities that repeatedly bear the brunt of health disparities, whether from COVID-19 or non-communicable diseases, and acknowledge the need for reparation of the injustices that have contributed to these inequities. It should not have required a pandemic to make community engagement in research leadership a priority. Community engagement must be integrated as an integral operating principle for all types of research and throughout the research enterprise. It should be as essential to the research enterprise as Institutional Review Boards (IRBs).
We call on research funders and institutions to take the following actions (Table 2):
Table 2. Recommendations for action

Funders
PCORI has established a strong precedent for institutionalizing stakeholder engagement as a core operating principle. PCORI includes patient and stakeholder engagement as one of its six merit review criteria, with scoring elements for this criterion including extent and authenticity of engagement and adequacy of resources budgeted for engagement [27,Reference Sheridan, Schrandt, Forsythe, Hilliard and Paez28]. Moreover, PCORI merit review committees include patients and stakeholders in addition to scientists. Evaluations have demonstrated the feasibility and benefits of PCORI’s approach, with stakeholder participation in the review process complementing rather than detracting from consideration of a proposal’s scientific merit [Reference Natafgi, Tafari, Chauhan, Bekelman and Mullins21,Reference Forsythe, Frank and Tafari29,Reference Forsythe, Frank and Hemphill30]. The NIH, CDC, AHRQ, SAMSA, and other federal agencies funding research should follow PCORI’s precedent. We urge these agencies to:
-
1. Require all proposals for research awards involving human participants to include a section addressing community and stakeholder engagement. Review criteria could be modeled after those used by PCORI and include assessments of the quality of the community engagement plan and adequacy of the budget allocated to community partners for their planned contributions. A first step would be for the NIH to immediately implement this policy for all new COVID-19 clinical research funding opportunities.
-
2. Include community stakeholders in agency governance and proposal reviews. Community advisory boards at the agency and/or institute level should be invited to provide guidance on prioritization and design of initiatives and funding opportunities. Indeed, the NCATS Advisory Council Working Group on the IOM Report called in 2014 for “full and effective integration of all stakeholders at all levels of governance” of the CTSA program [31]. For proposal review committees, community member participation would bring valuable perspectives and insights complementing those of traditional committee members.
-
3. Increase investment in the local infrastructure for community engaged research. A starting point would be for NCATS to conduct a systematic assessment of the community engagement programs among CTSA awardees, including an inventory of the amount of award dollars invested by recipients in these programs. An early assessment by the Resources Workgroup of the community engagement Key Function Committee a decade ago revealed inadequate support for CE cores before the Institute of Medicine in its review of the CTSA initiative recommended expanding community engagement responsibilities to encompass the full spectrum of clinical and translational research [32,Reference Michener, Scutchfield and Aguilar-Gaxiola33]. Such an assessment should determine whether the budgets for community engagement programs are sufficient for the desired aims. NCATS could use the findings from this evaluation to decide if the institute should specify a minimum proportion of awards to be budgeted to the community engagement cores.
Because many clinical trials are sponsored by pharmaceutical companies and not government agencies, the federal government should also consider how its regulatory powers might influence industry sponsored clinical research to promote greater community engagement. For example, the FDA might place more emphasis in the vaccine, drug, and device review process on the representativeness of study participants and inclusion of community advisory boards in clinical trials.
Research Institutions
Wilkins and Alberti have previously called on academic health centers to “establish the necessary infrastructure to support long-term community partnerships, adapt policies to support and reward engaged scholarship and teaching, and consider new ways of integrating community members in roles as advisors and collaborators [Reference Wilkins and Alberti34].” In addition to endorsing these and other recommendations [Reference George, Duran and Norris4], we urge all research institutions to:
-
1. Develop and implement a strategic plan at each research institution to achieve greater racial and ethnic diversity among the institution’s research workforce. Many studies have demonstrated the important role BIPOC investigators and research staff play in earning community trust and strengthening community collaboration and participation in research studies [Reference George, Duran and Norris4,Reference Oh, Galanter and Thakur8,Reference Sierra-Mercado and Lázaro-Muñoz35]. In response to the profound underrepresentation of BIPOC individuals among research faculty, agencies such as the NIH have launched initiatives in partnership with research and educations institutions to promote careers in research among BIPOC populations [36]. Although a comprehensive review of all the barriers and facilitators to diversity in the research workforce [37,Reference Kaplan, Raj, Carr, Terrin, Breeze and Freund38] is beyond the scope of this commentary, we emphasize two priorities. First, every institution should have an explicit strategic plan for research workforce diversity, with measurable objectives and timelines and clearly articulated tactics to achieve the objectives. Community members should participate in the develop and implementation of these plans. Second, the plans should focus not only on doctoral-degree investigators, but also on front-line staff such as clinical research coordinators and research nurses who are usually the members of the research team who have the most interaction with study participants and community members and have a key role in building trusting relationships. Diversity can be further enhanced through exposure and mentorship into many of the other roles within the research enterprise such as informaticians, data analysts, research administration, and regulatory professionals.
-
2. Modify IRB policies to require that protocol submissions for all human participant research and research on community health include a section addressing the study’s community engagement plan. The intent would not be punitive and lack of a robust community engagement plan would not necessarily jeopardize IRB approval of a protocol. Rather, this policy would serve to call attention to the importance of community engagement and provide an opportunity for the IRB to refer investigators to helpful resources such as an institution’s CTSA community engagement program.
-
3. Make a course in community engaged research a required rather than elective course for all clinical research training programs. For example, a community engaged research course should be required for all Masters of Clinical Research training programs. Moreover, institutions should include community partners in prominent roles as educators for such courses and recognize their contributions with formal “community faculty” titles. Basic training must then be reinforced for researchers at all stages of their careers through ongoing experiential learning with ready opportunities for consultation, such as that provided by community engagement consultation services.
-
4. Establish standing community advisory boards for institutional offices of research and major research institutes such as those funded by CTSA awards.
Conclusion
Myriad inequities require that the nation’s research enterprise overcome its past failures and become a more trustworthy partner. In seeking to increase diversity among clinical trial participants, what would an approach informed by social justice look like? While social justice is undeniably promoted by policy at the national and state levels, it finds its ultimate expression at the local, community level where individuals interact on a daily basis. Absent a thriving, continuous dialogue between events on the ground and regional and national policy, efforts to promote social justice risk being at best tone deaf and, at worse, re-enacting systemic racism and reifying social, political, and institutional inequities. A social justice framework calls for researchers to focus both on individuals and the community and move beyond transactional interactions to transformational relationships. Where bridges and relationships between academic health centers and communities have been built with investments of time and money, they foster trust and mutual understanding to ensure not only the success of vaccine trials, but more importantly, the success of subsequent efforts to reach the most vulnerable communities with COVID-19 vaccines and other effective preventive and therapeutic interventions. Funders and research institutions must take decisive action to make community engagement obligatory, not optional, in clinical and translational research.
Acknowledgements
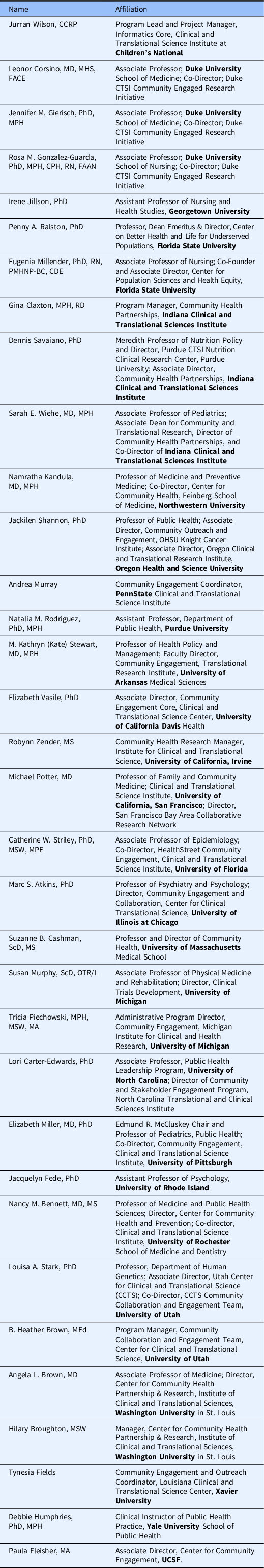
The following members of the PACER group who did not participate in the writing of this commentary endorse the content of this article:
The authors acknowledge funding from the following sources: National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number #UL1 TR001872 to the UCSF CTSI; #UL1 TR002494 to UMN CTSI; #UL1 TR001427 to UF/FSU CTSI; #UL1 TR002535 to Colorado CTSI; #UL1 TR001422 to Northwestern CATS; and #UL1 TR001409 to Georgetown-Howard CCTS.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Disclosures
The authors have no conflicts of interest to declare.




