Bipolar disorder is a chronic, progressive disease that presents severe challenges for long-term treatment because each episode seems to increase the risk for another episode, and subsequent episodes appear to be increasingly treatment resistant. Reference Mclntyre1 Pharmacological treatment options are limited, and mounting evidence suggests that exposure to antidepressants increases long-term mood instability in patients with bipolar disorder. Reference Strejilevich, Martino, Marengo, Igoa, Fassi and Whitham2,Reference Ghaemi3 Considering those challenges, novel treatment options are needed and are being investigated. A limited number of clinical trials have explored the use of atypical antipsychotics to treat depression in bipolar disorder. Quetiapine demonstrated efficacy when used as monotherapy for treatment of bipolar depression in two placebo-controlled trials of patients with bipolar I or bipolar II disorder experiencing a major depressive episode. Reference Calabrese, Keck, Macfadden, Minkwitz, Ketter and Weisler4,Reference Thase, Macfadden, Weisler, Chang, Paulsson and Khan5 In three placebo-controlled clinical trials, aripiprazole did not show efficacy in the treatment of acute bipolar depression or in the prevention of depressive relapse. Reference Quante, Zeugmann, Luborzewski, Schommer, Langosch and Born6,Reference Thase, Jonas, Khan, Bowden, Wu and McQuade7 Similarly, a placebo-controlled trial of ziprasidone did not show efficacy for the treatment of depression in patients with bipolar I disorder. Reference Sachs, Ice, Chappell, Schwartz, Gurtovaya and Vanderburg8 The efficacy and safety of a second-generation atypical antipsychotic, olanzapine, and the combination of olanzapine and fluoxetine in the treatment of depressive episodes in patients with bipolar disorder were examined in a single-protocol study that was divided into two identical, 8-week studies. Analyses of the whole sample showed that both olanzapine and combination therapy with olanzapine and fluoxetine were significantly more effective than placebo. Reference Tohen, Vieta, Calabrese, Ketter, Sachs and Bowden9 However, when the two identical studies were analysed separately, the olanzapine/fluoxetine combination remained significantly more effective than placebo in both studies, but olanzapine alone was significantly more effective than placebo in only one study (details available from the author on request). Significantly more patients in the olanzapine and olanzapine/fluoxetine groups reported somnolence, weight gain, increased appetite, dry mouth and asthaenia compared with the placebo group (P⩽0.03). Reference Tohen, Vieta, Calabrese, Ketter, Sachs and Bowden9 These treatment-emergent adverse events are similar to those observed during treatment with olanzapine/fluoxetine combination in a 6-month study comparing olanzapine/fluoxetine combination with lamotrigine in patients with bipolar I disorder depression Reference Brown, Dunner, McElroy, Keck, Adams and Degenhardt10 as well as those seen during a 24-week open-label extension study comparing olanzapine/fluoxetine combination and olanzapine monotherapy in the treatment of bipolar depression. Reference Corya, Perlis, Keck, Lin, Case and Williamson11
The current study was intended to evaluate whether olanzapine is superior to placebo in the treatment of patients with diagnosed bipolar I disorder with an acute major depressive episode. In addition, we aimed to examine the extent to which these findings can be applied to a primarily East Asian population.
Method
The primary objective of the current study was to assess whether olanzapine (5–20 mg/day) is superior to placebo in patients with bipolar I disorder depression on the basis of symptom improvement as measured by the least-squares mean baseline to end-point change in Montgomery–Åsberg Depression Rating Scale (MADRS) Reference Montgomery and Åsberg12 total score. Secondary objectives were to assess additional measures of efficacy across the range of bipolar mood states and to examine rates of response, remission, recovery and study discontinuation and the emergence of mania. The safety of olanzapine treatment in this population was also assessed through evaluation of treatment-emergent adverse events; changes in vital signs, laboratory analytes and electrocardiograms (ECGs); emergence of suicidality or serious suicidal risk; and the incidence and severity of extrapyramidal symptoms.
The protocol was reviewed and approved by the appropriate institutional review boards before study initiation. The study was conducted in conformity with the USA Food and Drug Administration Code of Federal Regulations (21 CFR, Part 50), and the Declaration of Helsinki and its amendments. Study procedures and execution were consistent with Good Clinical Practice guidelines and all applicable regulatory requirements. The trial was registered with ClinicalTrials.gov (NCT00510146).
Patients
Male and female patients aged ⩾18 years and <65 years who met diagnostic criteria for bipolar I disorder and a major depressive episode as defined by DSM-IV-TR 13 were recruited. Patients were required to have been in a depressive episode for ⩽90 days at the time of randomisation, to have a 17-item Hamilton Rating Scale for Depression (HRSD-17) Reference Hamilton14 total score ⩾18 and to have a history of ⩾1 manic or mixed episode in the previous 6 years without currently being in a manic episode (Young Mania Rating Scale (YMRS) Reference Young, Biggs, Ziegler and Meyer15 total score <8 at randomisation). Excluded were patients with an unstable medical illness (where death was anticipated within 1 year, or intensive care unit hospitalisation for the disease was anticipated within 6 months of screening); a history of diabetes or a haemoglobin A1c ⩾6.5% or blood glucose level indicative of diabetes; recent substance dependence; a history of serious psychiatric illness other than bipolar disorder; recent use of clozapine, depot antipsychotics or central nervous system medications other than mood stabilisers; and current rapid-cycling mood disturbance. Patients were recruited in Japan, China, Taiwan, Korea and the USA in both in-patient and out-patient settings. After complete description of the study to the patients, written informed consent was obtained.
Study design
This was a 6-week, randomised, double-blind, placebo-controlled, phase 3 trial of olanzapine as monotherapy for treatment of patients with bipolar disorder in a depressive state. A screening visit occurred 2–28 days before the double-blind acute treatment to determine patient eligibility. Patients who met all inclusion criteria and no exclusion criteria were enrolled in the study and randomly assigned in a 2:1 ratio to olanzapine and placebo once daily at bedtime. Patients were stratified by region and randomised at the study level. Assignment to treatment groups was determined by a computer-generated random sequence using an interactive voice response system. The response system was used to assign blister packs containing double-blind study drug to each patient. Patient numbers were assigned at visit 1, and patients who met all criteria for enrollment were randomly allocated to double-blind treatment at visit 2 by the interactive voice response system.
Starting at visit 2, patients assigned to the olanzapine group received the study drug 5 mg/day once daily; after 3–7 days (visit 3 or 4) at this dose, patients underwent a forced titration to 10 mg/day olanzapine once daily. On the basis of the investigator's judgement, the single daily olanzapine dose could be increased to 20 mg or decreased to 5 mg at subsequent once-weekly study visits.
Efficacy evaluation
Efficacy of the study medication was evaluated with changes from baseline to 6 weeks on the MADRS total score; the Clinical Global Impression – Bipolar Version (CGI-BP), Reference Spearing, Post, Leverich, Brendt and Nolan16 including the depression, mania and bipolar subscale scores; the HRSD-17 total score; and the YMRS total score. Post hoc analysed were MADRS item-by-item scores and the MADRS-6 subscale, which focuses on the ‘core’ symptoms of depression (as assessed by the MADRS items: Apparent Sadness, Reported Sadness, Inner Tension, Lassitude, Inability to Feel, and Pessimistic Thoughts). Reference Thase, Bowden, Nashat, Eudicone, Marcus and McQuade17,Reference Bech, Tanghoj, Andersen and Overo18 Response was defined as a ⩾50% reduction in MADRS at end-point. Remission was a priori defined as a MADRS total score ⩽12 (called partial remission in this paper) and was defined post hoc as a MADRS total score ⩽8 (as recommended by the International Society for Bipolar Disorders, Reference Tohen, Frank, Bowden, Colom, Ghaemi and Yatham19 called full remission in this paper). Recovery assessment was based on a MADRS total score ⩽12 for ⩾4 weeks of treatment and completion of the 6-week acute phase.
Safety evaluation
To assess the safety of the study medication, spontaneously reported treatment-emergent adverse events were collected at every visit. Laboratory analytes and ECGs were assessed at baseline and at end-point, with clinical chemistry, lipid panel, electrolyte and hematology measures also being collected at visit 5. Vital signs and weight were assessed at each study visit. Additionally, the Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS) Reference Inada20 and the Mini International Neuropsychiatric Interview (MINI) Reference Sheehan, Lecrubier, Harnett-Sheehan, Amorim, Janavs and Weiller21 to assess suicidality were administered at every study visit.
Statistical analysis
Calculations determined that a total of 510 patients (olanzapine, n = 340; placebo, n = 170) would have 90% power (type II error = 0.1) to detect a treatment difference of 3.9 of MADRS total score change from baseline. Because this study included patients from geographical regions in which olanzapine in bipolar depression has not been studied, a conservative standard deviation of 12.38 was used; this corresponds to an effect size of 0.315. The sample size for each treatment group was based on the use of a two-sided t-test of significance, with alpha (type I error) = 0.05.
All analyses were based on the intention-to-treat set of patients. All tests of treatment effect were conducted at a two-sided significance level of 0.05, and no adjustments for multiple comparisons were made. Continuous data were assessed using analysis of covariance models with type III sums of squares with terms for treatment and geographical region and with the baseline measurement value included as a covariate. For continuous data where a baseline measurement was not applicable, analysis of variance models were used, with terms for treatment and geographical region. The primary analysis of change from baseline to end-point in MADRS total score was based on last observation carried forward (LOCF) least-squares mean change. Additionally, observed case visit-wise analyses and mixed-effects repeated measures analysis of variance were used to assess changes in MADRS total score. For analysis of proportions, the Cochran–Mantel–Haenszel test was used to adjust for geographical region in efficacy assessments; the Fisher exact test was used when geographical region was not adjusted, mainly for safety assessments. Times to discontinuation, to response and to remission were evaluated using the log rank test from Kaplan–Meier survival analyses.
Results
Patient disposition
The first patient enrolled into the study on 27 August 2007, and the last patient completed the study on 9 July 2010. A total of 514 patients were randomly assigned to treatment. The acute phase of the study was completed by 267 of the 343 patients assigned to the olanzapine group (77.8%) and by 122 of the 171 patients in the placebo group (71.3%; P = 0.126) (Fig. 1). A total of 30 patients receiving olanzapine (8.7%) and 13 patients receiving placebo (7.6%) discontinued (P = 0.737) because of adverse events.
Baseline demographics
Treatment groups were similar in gender, ethnicity, age, age at onset of bipolar disorder and history of prior depressive, manic and mixed episodes as well as illness severity demonstrated by baseline MADRS total, HRSD-17 total, and YMRS total scores, and CGI-BP mania, depression and bipolar subscales (Table 1).
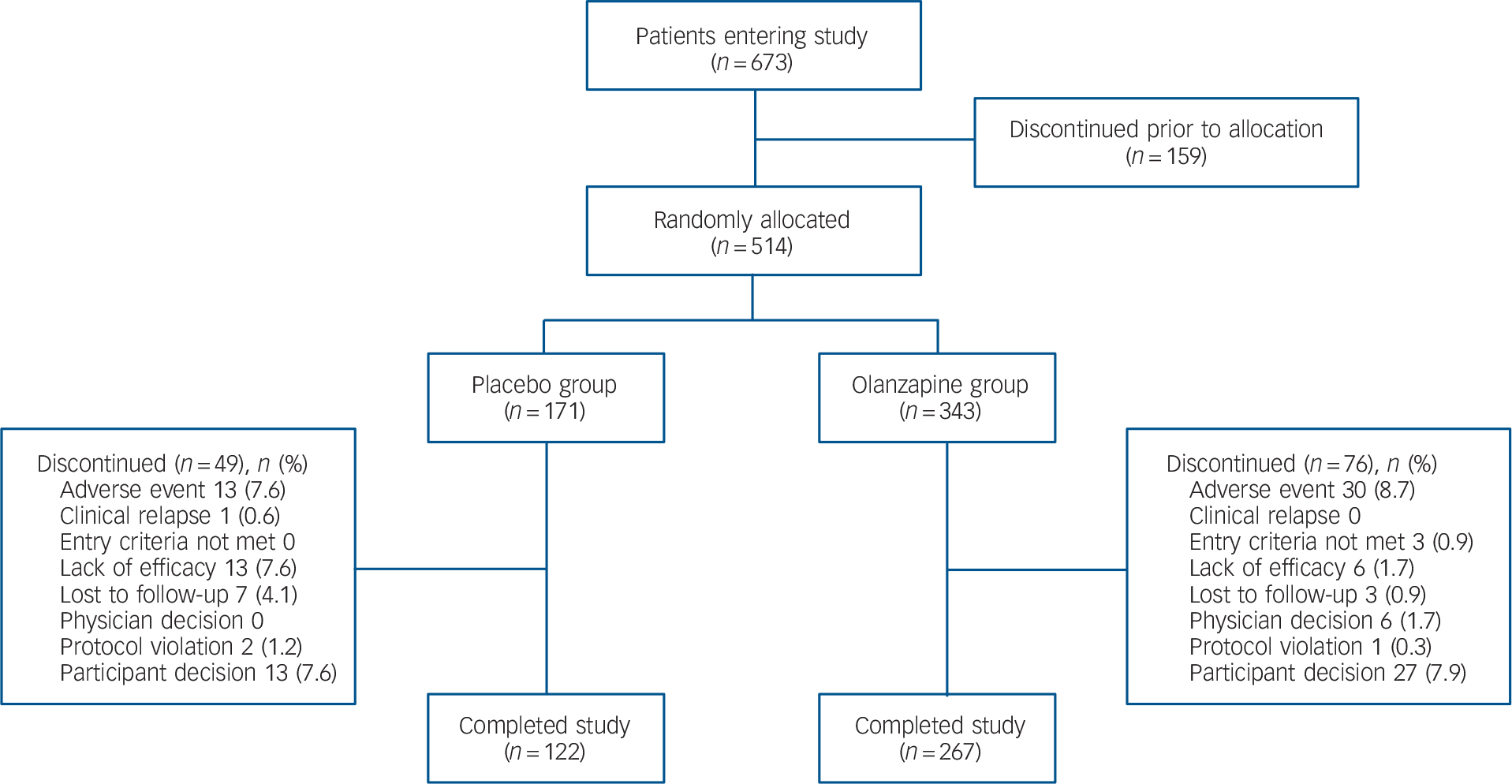
FIG. 1 Flow diagram of patient disposition during the acute phase.
Primary efficacy outcome
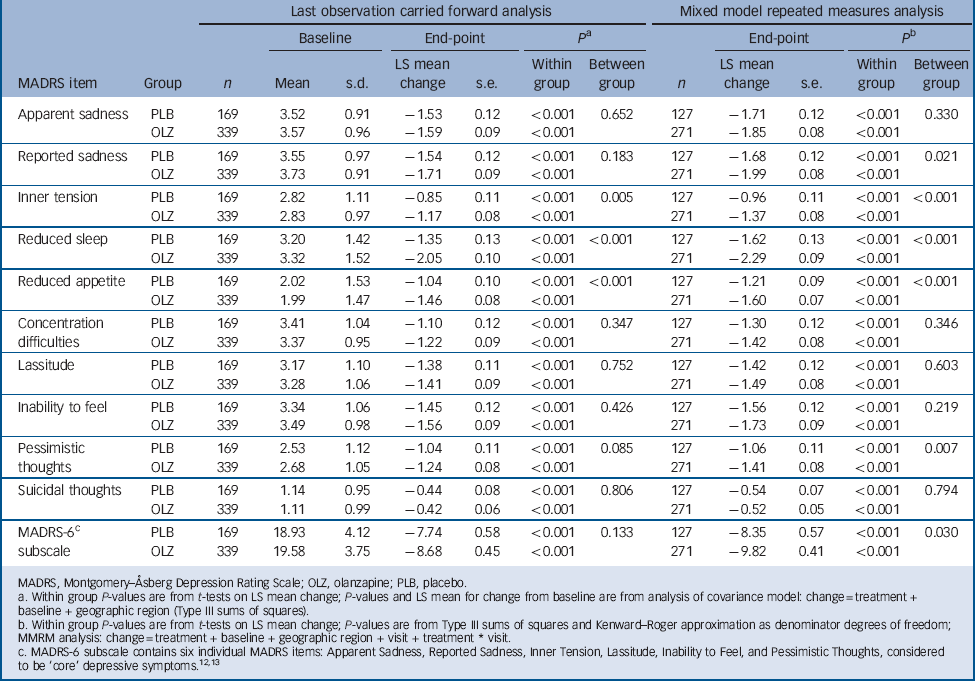
The baseline to end-point decrease (improvement) in least-squares mean MADRS total score was significantly greater in the olanzapine group than in the placebo group after 6 weeks of double-blind treatment (–13.82 v. –11.67; P = 0.018), with an effect size of 0.22. At all study visits, except at week 1, the olanzapine group had significantly greater reductions in least-squares mean MADRS total score than the placebo group (LOCF analysis, Fig. 2). Sensitivity analyses using observed case (week 2: –11.10 v. –9.17, P = 0.007; week 6: –15.61 v. –13.02, P = 0.006) and mixed-effects repeated measures (week 2: –11.05 v. –9.26, P = 0.013; week 6: –15.65 v. –13.02, P = 0.007) methodologies yielded similar results. Baseline to end-point score changes on individual MADRS items and the MADRS-6 subscale are presented in Table 2 (LOCF and mixed-effects repeated measures analyses).
Secondary efficacy outcomes
The olanzapine group had a greater baseline to end-point improvement in CGI-BP depression, CGI-BP mania and CGI-BP bipolar subscales scores, as well as the HRSD-17 total score, compared with the placebo group (Fig. 3 and Table 3). Patients demonstrated a low level of mania symptoms at baseline (mean YMRS 2.08 (s.d. = 2.13)), and patients treated with olanzapine had statistically greater least-squares mean decreases from baseline to end-point in YMRS total score compared with patients treated with placebo (Table 3 and online Fig. DS1).
Patients in the olanzapine group had significantly higher rates of response than those treated with placebo (P = 0.0498) (Table 4 and online Fig. DS2). In addition, Kaplan–Meier survival analysis showed a significant difference (P = 0.038) between treatment group curves, with an estimate of median time to response for the olanzapine group of 32 days v. 45 days in the placebo group. Partial remission rates for the olanzapine group and the placebo group did not differ significantly (P = 0.367) when remission was defined a priori as MADRS total score ⩽12 (Table 4 and Fig. DS2). However, when remission was defined post hoc as MADRS total score ⩽8, significantly more olanzapine-treated patients went into remission compared with those receiving placebo (P = 0.038) (Table 4 and Fig. DS2). No significant between-group differences were observed for recovery (P = 0.156) (Table 4).
TABLE 1 Baseline demographics and illness characteristics

| Placebo (n = 171) | Olanzapine (n = 343) | |||
|---|---|---|---|---|
| Female, n (%) | 95 | 55.6 | 205 | 59.8 |
| Ethnicity, n (%) | ||||
| White | 22 | 12.9 | 36 | 10.5 |
| African | 4 | 2.3 | 18 | 5.2 |
| Hispanic | 3 | 1.8 | 6 | 1.7 |
| East Asian | 141 | 82.5 | 282 | 82.2 |
| Native American | 1 | 0.6 | 1 | 0.3 |
| Age, years: mean (s.d.) | 35.0 | 11.0 | 36.0 | 11.1 |
| Age at onset of bipolar disorder, years: mean (s.d.) | 26.1 | 9.9 | 27.6 | 11.0 |
| Prior episodes, mean (s.d.) | ||||
| Manic | 3.6 | 4.1 | 3.4 | 4.8 |
| Depressive | 5.3 | 9.0 | 5.0 | 7.8 |
| Mixed | 0.9 | 2.6 | 0.8 | 4.7 |
| Illness severity scores, mean (s.d.) | ||||
| MADRS total | 28.7 | 6.3 | 29.3 | 5.7 |
| YMRS total | 2.0 | 2.2 | 2.1 | 2.1 |
| HRSD-17 total | 22.3 | 3.5 | 22.6 | 3.5 |
| CGI-BP depression | 4.5 | 0.7 | 4.5 | 0.7 |
| CGI-BP bipolar | 4.4 | 0.8 | 4.5 | 0.8 |
| CGI-BP mania | 1.1 | 0.3 | 1.1 | 0.3 |
CGI-BP, Clinical Global Impression - Bipolar Version; HRSD-17, 17-item Hamilton Rating Scale for Depression; MADRS, Montgomery-Åsberg Depression Rating Scale; YMRS, Young Mania Rating Scale.
Safety results
Overall, safety data from the acute phase of this study were consistent with the known safety profile of olanzapine. Significantly more patients in the olanzapine group (n = 239, 69.7%) experienced ⩾1 treatment-emergent adverse events compared with patients in the placebo group (n = 93, 54.4%; P<0.001). The most commonly reported adverse events for olanzapine-treated patients were somnolence, increased weight, increased appetite and sedation (online Table DS1). Using the American Diabetes Association criteria to assess abnormal blood glucose levels (www.diabetes.org/diabetes-basics/prevention/pre-diabetes/diagnosis.html) and the National Cholesterol Education Program criteria 22 to assess lipid changes, we identified categorical changes with potential clinical significance occurring more often in the olanzapine group than in the placebo group (online Table DS2). Compared with patients in the placebo group, a higher percentage of the olanzapine group experienced a categorical shift in fasting cholesterol from normal to borderline (P<0.001) and from borderline to high (P = 0.005), in fasting triglycerides from normal to high (P = 0.022), and in fasting glucose from impaired to high (P = 0.048) and from normal/impaired to high (P = 0.019) (Table DS2). The percentages of patients experiencing categorical shifts in fasting low-density lipoprotien from normal to borderline, normal to high, and borderline to high, and in fasting high-density lipoprotein from normal to low were not significantly different between treatment groups (Table DS2). After 6 weeks of treatment, patients in the olanzapine group had significantly greater weight gain than patients in the placebo group (P<0.001) (online Table DS3), and weight gain ⩾7% above baseline occurred more commonly in the olanzapine group (P<0.001) (Table DS2). Mean increases in fasting cholesterol (P<0.001), low-density lipoprotien cholesterol (P<0.001) and fasting triglycerides (P = 0.003) were also higher in the olanzapine group (Table DS3).
Regarding laboratory values other than glucose and lipids, a higher proportion of patients receiving olanzapine had treatment-emergent abnormal high values for alanine aminotransferase (P<0.001), aspartate aminotransferase (P<0.001), gamma-glutamyl transpeptidase (P = 0.005) and prolactin (P<0.001) and abnormal low values for neutrophils (P = 0.012) compared with patients receiving placebo. A higher proportion of the placebo group had treatment-emergent abnormal high values for hematocrit (P = 0.033) and mean cell volume (P = 0.029) and treatment-emergent abnormal low values for potassium (P<0.001) compared with the olanzapine group.
No significant differences between treatment groups were observed in the proportion of patients with potentially clinically significant changes in ECG intervals and heart rate. No between-group differences were observed in extrapyramidal symptoms measured through DIEPSS total score mean change from baseline to end-point, and no significant differences between the groups were detected for changes from baseline to end-point in suicidality total scores.
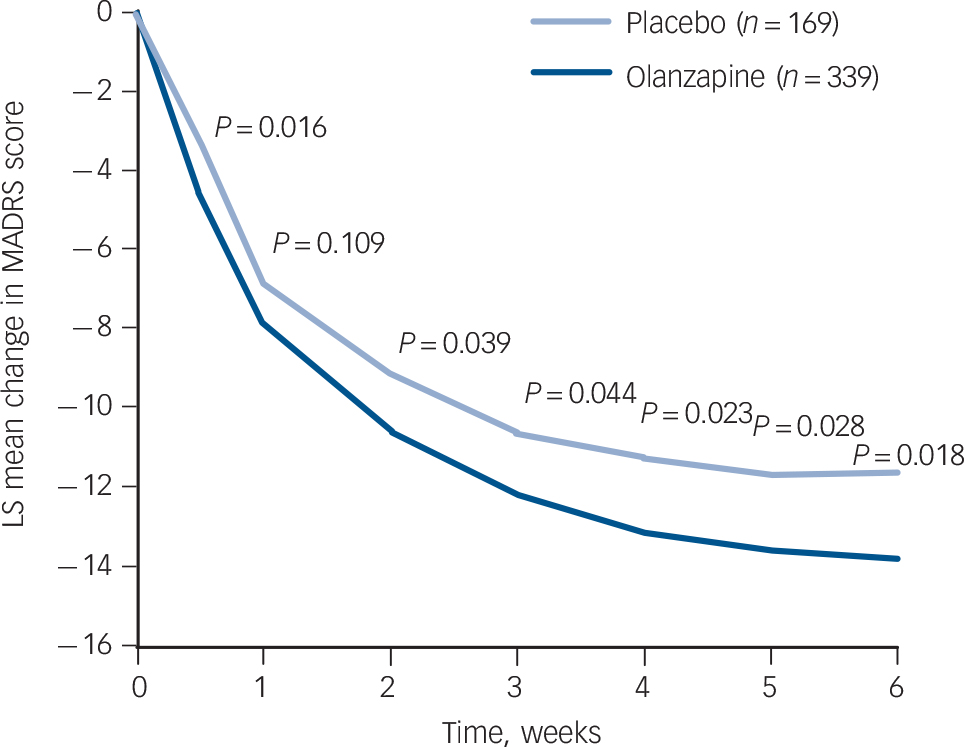
FIG. 2 Last observation carried forward analysis of visit-wise change from baseline in least-squares (LS) mean Montgomery–Åsberg Depression Rating Scale (MADRS) total score.
TABLE 2 Baseline to end-point least-squares (LS) mean changes on individual MADRS items and MADRS-6 subscale
| Last observation carried forward analysis | Mixed model repeated measures analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | End-point | P Footnote a | End-point | P Footnote b | |||||||||
| MADRS item | Group | n | Mean | s.d. | LS mean change |
s.e. | Within group |
Between group |
n | LS mean change |
s.e. | Within group |
Between group |
| Apparent sadness | PLB | 169 | 3.52 | 0.91 | –1.53 | 0.12 | <0.001 | 0.652 | 127 | –1.71 | 0.12 | <0.001 | 0.330 |
| OLZ | 339 | 3.57 | 0.96 | –1.59 | 0.09 | <0.001 | 271 | –1.85 | 0.08 | <0.001 | |||
| Reported sadness | PLB | 169 | 3.55 | 0.97 | –1.54 | 0.12 | <0.001 | 0.183 | 127 | –1.68 | 0.12 | <0.001 | 0.021 |
| OLZ | 339 | 3.73 | 0.91 | –1.71 | 0.09 | <0.001 | 271 | –1.99 | 0.08 | <0.001 | |||
| Inner tension | PLB | 169 | 2.82 | 1.11 | –0.85 | 0.11 | <0.001 | 0.005 | 127 | –0.96 | 0.11 | <0.001 | <0.001 |
| OLZ | 339 | 2.83 | 0.97 | –1.17 | 0.08 | <0.001 | 271 | –1.37 | 0.08 | <0.001 | |||
| Reduced sleep | PLB | 169 | 3.20 | 1.42 | –1.35 | 0.13 | <0.001 | <0.001 | 127 | –1.62 | 0.13 | <0.001 | <0.001 |
| OLZ | 339 | 3.32 | 1.52 | –2.05 | 0.10 | <0.001 | 271 | –2.29 | 0.09 | <0.001 | |||
| Reduced appetite | PLB | 169 | 2.02 | 1.53 | –1.04 | 0.10 | <0.001 | <0.001 | 127 | –1.21 | 0.09 | <0.001 | <0.001 |
| OLZ | 339 | 1.99 | 1.47 | –1.46 | 0.08 | <0.001 | 271 | –1.60 | 0.07 | <0.001 | |||
| Concentration | PLB | 169 | 3.41 | 1.04 | –1.10 | 0.12 | <0.001 | 0.347 | 127 | –1.30 | 0.12 | <0.001 | 0.346 |
| difficulties | OLZ | 339 | 3.37 | 0.95 | –1.22 | 0.09 | <0.001 | 271 | –1.42 | 0.08 | <0.001 | ||
| Lassitude | PLB | 169 | 3.17 | 1.10 | –1.38 | 0.11 | <0.001 | 0.752 | 127 | –1.42 | 0.12 | <0.001 | 0.603 |
| OLZ | 339 | 3.28 | 1.06 | –1.41 | 0.09 | <0.001 | 271 | –1.49 | 0.08 | <0.001 | |||
| Inability to feel | PLB | 169 | 3.34 | 1.06 | –1.45 | 0.12 | <0.001 | 0.426 | 127 | –1.56 | 0.12 | <0.001 | 0.219 |
| OLZ | 339 | 3.49 | 0.98 | –1.56 | 0.09 | <0.001 | 271 | –1.73 | 0.09 | <0.001 | |||
| Pessimistic | PLB | 169 | 2.53 | 1.12 | –1.04 | 0.11 | <0.001 | 0.085 | 127 | –1.06 | 0.11 | <0.001 | 0.007 |
| thoughts | OLZ | 339 | 2.68 | 1.05 | –1.24 | 0.08 | <0.001 | 271 | –1.41 | 0.08 | <0.001 | ||
| Suicidal thoughts | PLB | 169 | 1.14 | 0.95 | –0.44 | 0.08 | <0.001 | 0.806 | 127 | –0.54 | 0.07 | <0.001 | 0.794 |
| OLZ | 339 | 1.11 | 0.99 | –0.42 | 0.06 | <0.001 | 271 | –0.52 | 0.05 | <0.001 | |||
| MADRS-6Footnote c | PLB | 169 | 18.93 | 4.12 | –7.74 | 0.58 | <0.001 | 0.133 | 127 | –8.35 | 0.57 | <0.001 | 0.030 |
| subscale | OLZ | 339 | 19.58 | 3.75 | –8.68 | 0.45 | <0.001 | 271 | –9.82 | 0.41 | <0.001 | ||
MADRS, Montgomery-Åsberg Depression Rating Scale; OLZ, olanzapine; PLB, placebo.
a Within group P-values are from t-tests on LS mean change; P-values and LS mean for change from baseline are from analysis of covariance model: change = treatment + baseline + geographic region (Type III sums of squares).
b Within group P-values are from t-tests on LS mean change; P-values are from Type III sums of squares and Kenward-Roger approximation as denominator degrees of freedom; MMRM analysis: change = treatment + baseline + geographic region + visit + treatment * visit.
c MADRS-6 subscale contains six individual MADRS items: Apparent Sadness, Reported Sadness, Inner Tension, Lassitude, Inability to Feel, and Pessimistic Thoughts, considered to be ‘core’ depressive symptoms.12, 13
Discussion
Main findings
Results presented here are consistent with those from a prior study in which olanzapine monotherapy proved superior to placebo in reducing depressive symptoms in patients with bipolar disorder experiencing an acute depressive episode. Reference Tohen, Vieta, Calabrese, Ketter, Sachs and Bowden9 Here, we used a study population largely of East Asian ethnicity, whereas the previous study examined a largely non-Asian population. Reference Tohen, Vieta, Calabrese, Ketter, Sachs and Bowden9
Improvements in depressive symptoms were noted using multiple assessment tools (MADRS, HRSD-17 and CGI-BP) and criteria for response. These assessment tools are well established in the literature and frequently used in clinical practice to examine depressive symptoms in a variety of patient populations. Reference Komossa, Depping, Gaudchau, Kissling and Leucht23–Reference Vieta, Blasco-Colmenares, Figueira, Langosch, Moreno-Manzanaro and Medina25 Improvement of core symptoms of depression as assessed by the MADRS-6 subscale (containing the following MADRS items: Apparent Sadness, Reported Sadness, Inner Tension, Lassitude, Inability to Feel, and Pessimistic Thoughts) was significantly greater during treatment with olanzapine compared with placebo when analysed with mixed-effects repeated measures; baseline to end-point changes on the MADRS-6 subscale were numerically, but not statistically significantly (P = 0.133) greater during treatment with olanzapine compared with placebo when analysed with LOCF. These results suggest that olanzapine has an effect on the core symptoms of depression.
The effect size demonstrated by olanzapine monotherapy in this study was modest. Although this could be a sign of a modest effect of the active drug treatment, it may also have been influenced by a relatively high response to placebo. Increasing placebo response in recent years is a phenomenon which has been shown in different areas of psychopharmacology. Reference Kemp, Schooler, Kalali, Alphs, Anand and Awad26,Reference Walsh, Seidman, Sysko and Gould27 Nevertheless, the consistency of the results across different assessment tools, as well as response rate and the post hoc remission rate (no significant between-group differences were observed for recovery and a priori remission rates), suggest that it is a clinically meaningful effect. It is important to consider that bipolar disorder is a particularly difficult to treat condition and that there are very few regulatory approved treatments for bipolar depression. Therefore, even a modest effect size may be of clinical relevance to patients, although treatment-emergent adverse events need to be taken into consideration as well. Future studies are warranted to explore long-term effectiveness and safety of olanzapine monotherapy in patients with bipolar depression. This seems to be especially important considering recent findings that indicate that early improvement in patients with bipolar disorder does not seem to be a reliable predictor of response or remission, while the absence of early improvement appears to be a highly reliable predictor of non-response. Reference Kemp, Ganocy, Brecher, Carlson, Edwards and Eudicone28,Reference Tohen, Case, Trivedi, Thase, Burke and Durell29 Additional potential limitations for long-term efficacy lie in the observation that a significant number of patients discontinue olanzapine owing to adverse events Reference Gao, Kemp, Fein, Wang, Fang and Ganocy30 and that olanzapine, like other second-generation antipsychotics, is frequently not used at the recommended dose levels in patients with bipolar disorder. Reference Rascati, Richards, Ott, Goddard, Stafkey-Mailey and Alvir31
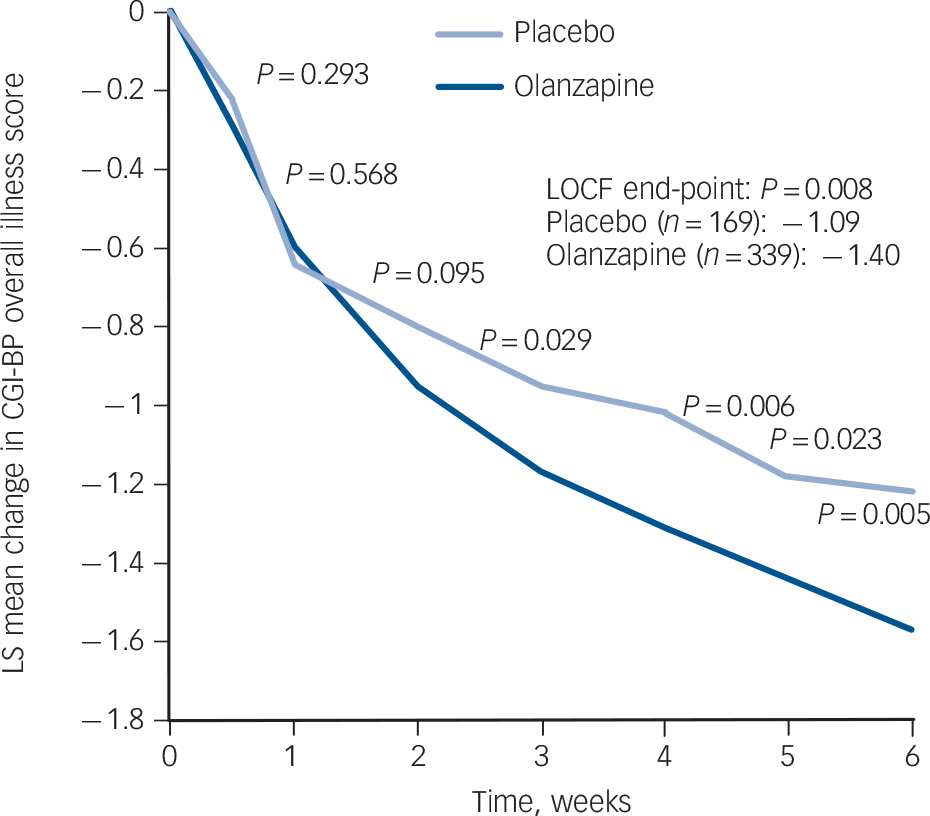
FIG. 3 Observed case analysis of visit-wise change from baseline in least-squares (LS) mean Clinical Global Impression – Bipolar Version (CGI-BP) overall illness score. LOCF, last observation carried forward.
TABLE 3 Baseline to end-point least-squares mean changes on CGI-BP, HRSD-17 and YMRS
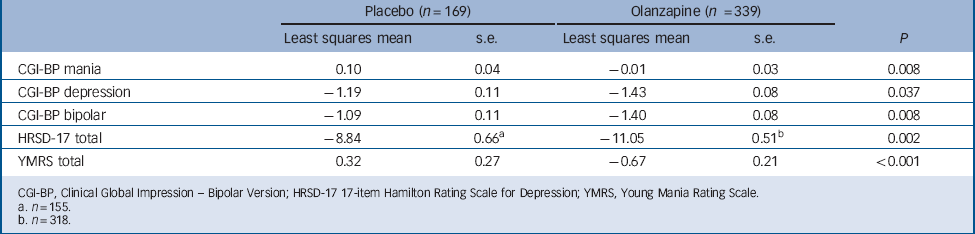
| Placebo (n = 169) | Olanzapine (n = 339) | ||||
|---|---|---|---|---|---|
| Least squares mean | s.e. | Least squares mean | s.e. | P | |
| CGI-BP mania | 0.10 | 0.04 | –0.01 | 0.03 | 0.008 |
| CGI-BP depression | –1.19 | 0.11 | –1.43 | 0.08 | 0.037 |
| CGI-BP bipolar | –1.09 | 0.11 | –1.40 | 0.08 | 0.008 |
| HRSD-17 total | –8.84 | 0.66Footnote a | –11.05 | 0.51Footnote b | 0.002 |
| YMRS total | 0.32 | 0.27 | –0.67 | 0.21 | <0.001 |
CGI-BP, Clinical Global Impression - Bipolar Version; HRSD-17 17-item Hamilton Rating Scale for Depression; YMRS, Young Mania Rating Scale.
a n = 155.
b n = 318.
TABLE 4 Response, remission and recovery rates
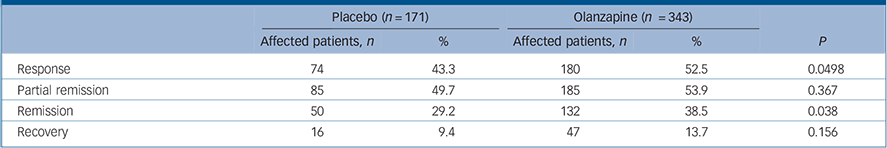
| Placebo (n = 171) | Olanzapine (n = 343) | ||||
|---|---|---|---|---|---|
| Affected patients, n | % | Affected patients, n | % | P | |
| Response | 74 | 43.3 | 180 | 52.5 | 0.0498 |
| Partial remission | 85 | 49.7 | 185 | 53.9 | 0.367 |
| Remission | 50 | 29.2 | 132 | 38.5 | 0.038 |
| Recovery | 16 | 9.4 | 47 | 13.7 | 0.156 |
In the olanzapine group, no increase of manic symptoms occurred during our study, but YMRS scores improved. This finding has clinical implications because improvement of manic symptoms in patients with bipolar disorder has been shown to improve treatment outcomes. Reference Benazzi, Berk, Frye, Wang, Barraco and Tohen32 Additionally, this finding suggests that treatment of patients with bipolar disorder during a depressive episode with olanzapine monotherapy can produce a mood-stabilising effect without increasing the risk that the patient will cycle into a manic episode. Consistent with previous studies, patients treated with olanzapine had significantly (P<0.01) greater mean increases in weight, fasting cholesterol and triglycerides compared with the placebo group, and a higher percentage of the olanzapine group experienced categorical shifts in fasting cholesterol, fasting triglycerides and fasting glucose. Additionally, significantly more (P<0.001) patients receiving olanzapine gained ⩾7% body weight. Overall, the safety profile of olanzapine during this 6-week trial was similar to that seen in previous studies of olanzapine therapy and consistent with the known safety profile of olanzapine treatment, indicating that olanzapine's safety profile in patients with bipolar disorder appears to be consistent across populations with different ethnicities.
Limitations
The interpretation of the data is limited by the fact that the remission analysis was post hoc; however, the criteria used in the remission analysis were very stringent and were recommended by the International Society for Bipolar Disorders. Additionally, our criteria used to define recovery (MADRS ⩽12 for ⩾4 weeks plus treatment completion) might have been too stringent considering the total study length was only 6 weeks. Finally, the results presented here are limited to: patients at least 18 years old and younger than 65 years; patients who had been in a depressive episode for ⩽90 days at the time of randomisation; patients without serious comorbid psychiatric illness; and patients who are not rapidly cycling. Future studies should consider including broader patient populations.
Clinical implications
In summary, olanzapine monotherapy has shown efficacy in the acute treatment of bipolar depression. Although the observed safety profile was similar to that seen during previous studies of olanzapine therapy, clinicians need to monitor safety carefully. In making treatment decisions for individual patients, clinicians should consider carefully the risks and benefits of olanzapine treatment for each individual patient.
Funding
This study was funded by Eli Lilly and Company.
Acknowledgements
The study findings presented here are derived from a collaboration of investigators in several Eastern Asian countries (China, Korea, Japan and Taiwan) with investigators in the USA to create a unique patient sample with predominantly East Asian ethnicity. This collaboration was the initiator that led to the formation of the East Asian Bipolar Forum (EABF; www.isbd.org/edcenter/chapters/affiliates.asp). The EABF contains experts in bipolar disorder from Korea, Japan, China and Taiwan, and aims to enhance international collaborations for improving treatments for bipolar disorders. The authors thank Dr Alexandra Heinloth and Ms Laura Altobelli, both full-time employees of PharmaNet/i3, part of inVentiv Health Company, for assistance in the preparation of this manuscript.










eLetters
No eLetters have been published for this article.