Introduction
Despite the historical mild or uncommon disease perception, influenza virus infection remains an important cause of medically attended respiratory illness and a significant cause of morbidity and mortality worldwide. Annually, there is an Influenza estimated attack rate of 5–10% in adults while, 20–30% of children are globally affected, causing significant levels of illness severity and death through the world [1]. Seasonal influenza epidemics cause approximately 1 million influenza-associated hospitalisations among children less than 5 years [Reference Nair2]. A recent study conducted from 1999 to 2015, has estimated that between 291 243 and 645 832 influenza-associated respiratory deaths annually (4.0–8.8/100 000 individuals) with the highest mortality rate occurring in sub-Saharan Africa [Reference Iuliano3]. Moreover, outpatient visits attributable to influenza among children under 5-years-old of age were documented to be 10–250 times more than in other age groups [Reference Henkle4]. Altogether these reports indicate that seasonal influenza is a substantial public health problem and a major economic burden that increases heavy healthcare costs, absenteeism and reduced productivity [Reference Chan5]. Notably, studies conducted in developed countries (France, Germany and USA) have estimated the total annual cost of influenza between U$1 million and U$6 million/ 100 000 inhabitants [6]. Based on 2003 US population, the annual direct medical seasonal influenza cost resulted in an average $10.4 billion; while indirect costs, which include estimated lost earnings due to both illness and death, average $16.3 billion [Reference Molinari7]. However, very few information about the incidence of influenza is available in low-income tropical countries [Reference Simmerman and Uyeki8, Reference Leo, Lye and Chow9] even for countries with a long history of influenza sentinel surveillance, such as Senegal [Reference Niang10]. The influenza burden assessment in these areas faces many challenges. Indeed, in addition to the frequent influenza-like illness (ILI) case definition confusions, the presence of other respiratory viruses causing similar symptoms, difficulties to have complete data points for rigorous surveillance, the fact that persons with ILI do not present themselves present to healthcare centres in remote areas. As a consequence, patients with ILI are often treated empirically without respiratory sampling [Reference Nair2]. This lack of data on the real burden of influenza in resource-limited settings is among the main obstacles that have significantly impeded the implementation of effective preventive measures such as immunisation. Therefore, understanding the burden of influenza-related disease in tropical countries is crucial for determining the risk of morbidity and mortality in different segments of the population, also in vaccination programs guidance, evaluating the use of diagnostic tests and antiviral drugs and planning for seasonal epidemics and future pandemics.
The present study is a first step in increasing the knowledge on the real impact of influenza in Senegal. So, using the 4S network surveillance data, we try to estimate the burden of flu-association ILI visits on total clinic outpatient visits during three consecutive annual influenza seasons in Senegal, 2013–2015.
Methods
Study design and setting
Senegal, a sub-Saharan Africa country, has a long-standing influenza surveillance system, which has been steadily improved over the years with the Institut Pasteur de Dakar (IPD) operating as the national Influenza centre (NIC). Fully focused on a virological surveillance in early years, the influenza sentinel surveillance was reviewed through the establishment of a new surveillance network, based on syndromic approach, called Senegalese Syndromic Sentinel Surveillance Network or 4S network [Reference Dia11]. This network was set up through collaboration between the Ministry of Health (MOH), the WHO country office and the IPD after the influenza A/H1N1pdm09 pandemic episode. The 4S network is actually composed of 15 ILI surveillance sentinel sites located in 10 out of 14 Senegal administrative regions. The 4S network provides data on influenza epidemiology, seasonality and also circulating strains over the country. In addition to a better understanding of the seasonal distribution of influenza disease, analysis of the data accumulated over the years has allowed us to have a better perception on ILI cases distribution in the different age groups and also to have molecular data on the different influenza strains that have circulated within Senegal [Reference Dia11]. Despite notable progress, there are still gaps which need to be further investigated to gain insight into the influenza burden in Senegal.
Sentinel sites
This study was carried out between 2013 and 2015. Data were collected from 10 community healthcare centres that belong to the Senegalese Syndromic Sentinel Surveillance referred to as 4S. These sentinel sites conduct population-based surveillance for ILI outpatients’ visits and are distributed geographically in diverse areas across the Country (Fig. 1).
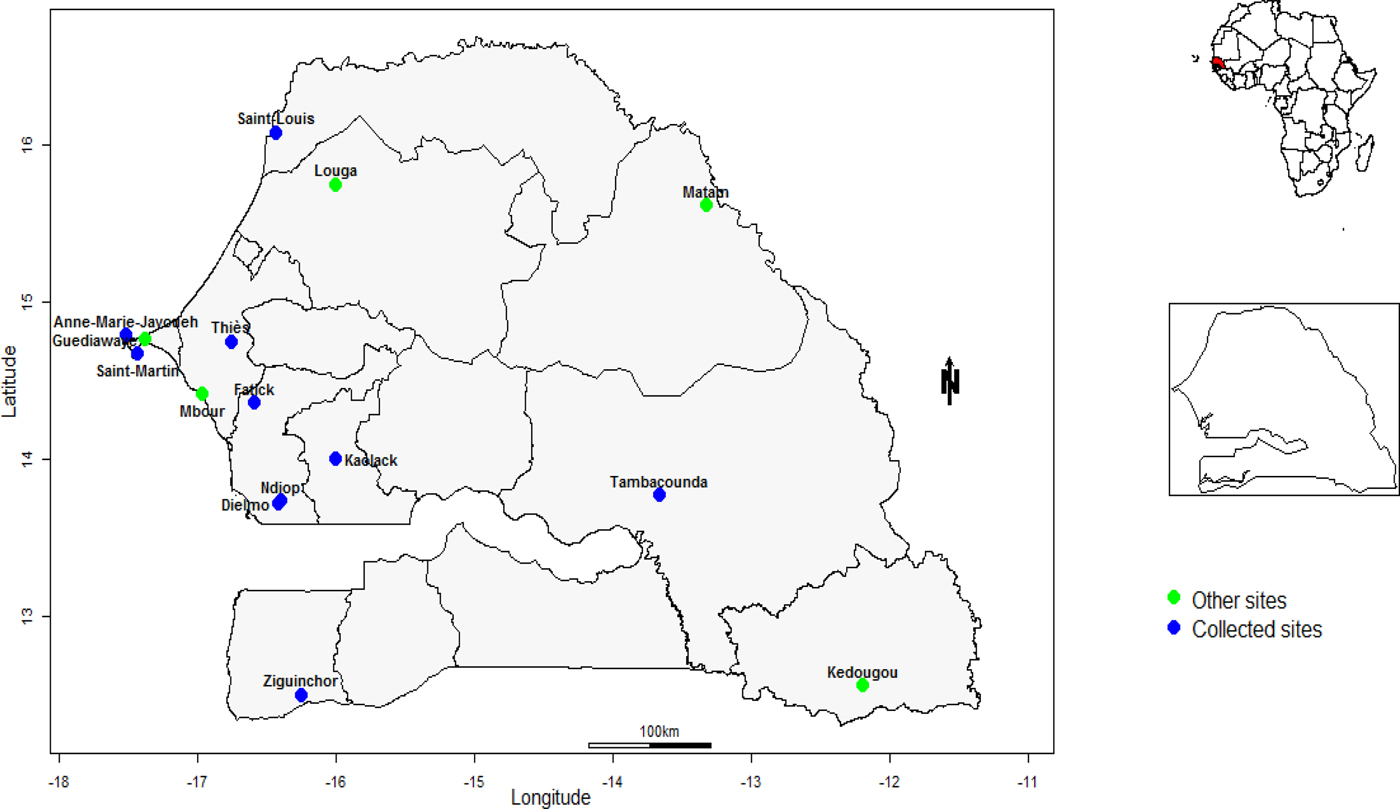
Fig. 1. Senegal map with sentinel sites implemented for the influenza surveillance. Only data from sentinel sites represented in blue dots are included in the present study.
Cases definition and data collection
In accordance with the sentinel surveillance system in Senegal based on clinical pre-diagnostic data using standard WHO case definitions to ensure comparability, case definitions for the study were: Fever: defined as an axillary temperature of more than 37.5 °C; ILI: defined as fever with cough or fever with sore throat with onset within the last 10 days [12].
Eligible subjects were patients who sought care at study sites and meet the aforementioned case definition. At each sentinel site, trained General Practitioners (GPs) use a structured standard questionnaire to record both clinical and demographic data of registered participants. Information such as clinical symptoms, date of enrollment, sex, age, previous treatments, travelling history, vaccination status for influenza was anonymously transferred at IPD on a weekly basis. Furthermore, GPs were expected to transmit on a daily basis to the IPD epidemiology unit, encrypted short messages (number of total visits, fever cases, Influenza Like illness) from android phone. For each sentinel site and per week, nasopharyngeal and/or oropharyngeal swabs were collected from the first five ILI cases with at most 3 days of onset of symptoms. Samples were collected in 2 mL of viral transport medium (Universal Transport Medium) and stored at 4 °C before shipment to the National Influenza Centre (NIC) located in IPD on a weekly basis. Next, total viral ribonucleic acid (RNA) was extracted using the QIAamp® Viral RNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer's recommendations. Influenza virus was detected using CDC standardised rRT-PCR protocols [13]. Briefly, rRT-PCR assays were performed with AgPath One-Step rRT-PCR kit (Thermo Fisher Scientific, Inc., Waltham, Massachusetts, USA) using the Applied Bios systems 7500 fast rRT-PCR device (Thermo Fisher Scientific, Inc., Waltham, Massachusetts, USA).
For the present study, data were collected from (i) both medical records of patients and consultation registers in selected sentinel sites, (ii) database of epidemiologic surveillance and (iii) virological surveillance database.
Data analysis
Statistical analyses were performed using R software (R Core team 2017). The Chi-square test was used to compare both observed proportion and P-value of <0.05 considered as significant. R 3.0.1 software tool was used for statistical analysis. The annual proportional contribution of influenza-associated ILI to outpatient load for the 3 consecutive years was calculated using the method described in WHO's Manual for Estimating Disease Burden Associated with Influenza [14]. Different age-groups were divided as defined by WHO: 0 to <2 years, 2 to <5 years, 5 to <15 years, 15 to <50 years, 50 to <65 years, ⩾ 65 years. The total number of influenza-associated ILI (I) visits for each age-group in selected sites was calculated as follows:
I = influenza positive proportion of enrolled ILI cases × Total ILI cases in selected sites
Thus, the burden of influenza-associated ILI (p) to annual outpatient load was calculated by estimating the proportion of the total number of influenza-associated ILI visits for each year among all outpatient visits in the same year
 $$P(\% ) = \displaystyle{ \matrix{\hbox{Estimated number of influenza-associated} \cr \hbox{ILI visits in a calendar year}} \over \matrix{\hbox{Total number of outpatient visits at the} \cr \hbox{sentinel site in the same year}} } \times 100$$
$$P(\% ) = \displaystyle{ \matrix{\hbox{Estimated number of influenza-associated} \cr \hbox{ILI visits in a calendar year}} \over \matrix{\hbox{Total number of outpatient visits at the} \cr \hbox{sentinel site in the same year}} } \times 100$$Binomial proportion confidence interval was used to estimate the 95% confidence intervals (CIs) of the proportion for influenza-associated ILI.
Ethical issues
As part of the national influenza surveillance network, this study was approved by the Senegalese National Ethical Committee of the Ministry of Health. Throughout the surveillance, the generated database was shared with the Epidemiology Department at the Senegalese Ministry of Health and Prevention for appropriate public health actions.
Results
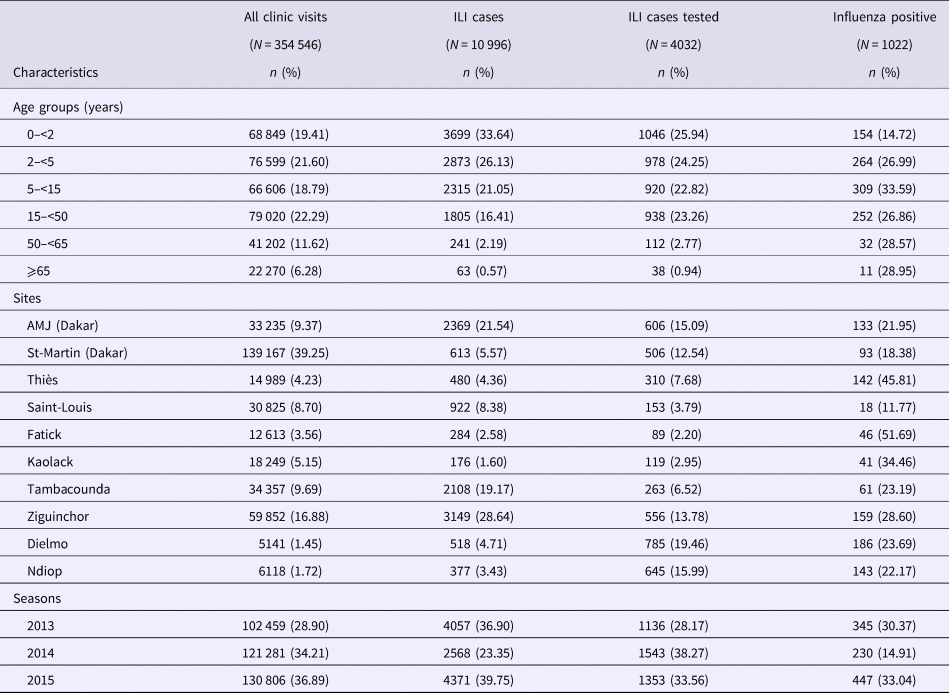
ILI surveillance and influenza detectionOver the 3 consecutive years (January 2013 to December 2015), we identified 10,996 (3.1%) ILI cases within 354 546 outpatient visits. Among the 10 996 identified ILI cases, 4032 (36.7%; 4032/10 996) were tested for influenza and 1022 (25.3%; 1022/4032) were found positive for influenza. ILI cases were more common in children under 2-years-old with 3699 (33.6%) cases recorded. As shown in Table 1, more than half of patients with ILI (59.8%; 6572/10 996) were children under 5-years-old. Trends of ILI cases varied across years of study with 4057 (36.9%), 2568 (23.3%) and 4371 (39.7%) cases in 2013, 2014 and 2015, respectively. ILI cases reporting were greater in sites located in Senegalese's capital city Dakar (AMJ and St-Martin) and southern regions of Senegal (Tambacounda and Ziguinchor) with respectively, 2982 (27.1%) and 5257 (47.8%) of ILI enrolled patients (Table1).
Table 1. All clinic visits, ILI cases and Influenza-positive included in the study, by age groups, sites and season, 4S Network, 2013–2015. Positive and negative rates for influenza are calculated based on the number of ILI cases tested for each age group, site and year
Of the 1022 ILI cases confirmed for influenza, 484 (47.3%; 484/1022) were tested positive for influenza A and 538 (52.6%; 538/1022) for influenza virus B. Influenza A positives subtyping revealed that out of 484 cases, 246 (24.1%; 246/1022) were A/H3N2 and 238 (23.3%; 238/1022) were A/H1N1pmd09. In general, over the study period, Influenza B was the most common type among ILI patients particularly in 2013 (77.1%) and 2015 (50.5%). Notably, A/H1N1pmd09 subtype was predominant in 2014 with 70.9% of influenza detected viruses. Moreover, influenza A and B co-infections were observed in 32 cases (3.1%; 32/1022).
The highest detection rate for seasonal influenza viruses (33.6%; 309/920) occurred in the 5 to <15 age group (Table 1). The proportions of positive cases among age groups 0 < 2, 2 < 5, 15 < 50, 50 < 65, ⩾65 throughout the 3-years-period were 14.7%, 27%, 26.9%, 28.6% and 28.9%, respectively.
We also noted significant disparities in influenza detection proportions between sites, with lowest positive cases (11.8%; 18/153) registered in Saint-Louis (Northern Senegal) while the highest rate was found in a central region, Fatick (51.7%; 46/89).
In the present study, Influenza viruses were continuously detected over the 3 years, with varying rates of positivity: 30.4% in 2013, 14.9% in 2014 and 33% in 2015 (χ 2 = 146.8, df = 2, P < 0.001).
Next, we sought to determine the temporal distribution change of influenza positives samples during the 3 years of surveillance. The Figure 2 shows different circulation profiles. For the year 2013 (Fig. 2a) influenza B was detected almost all year long. Indeed, the flu B type circulated exclusively from week 2 to week 37, with a significant activity increasing from week 9. From week 38 the A/H1N1pdm09 subtype arises. Then the 2 subtypes co-circulate until week 46 which coincides with the end of the influenza annual peak circulation. Regarding the A/H3N2 subtype, only a few cases are detected at week 41.
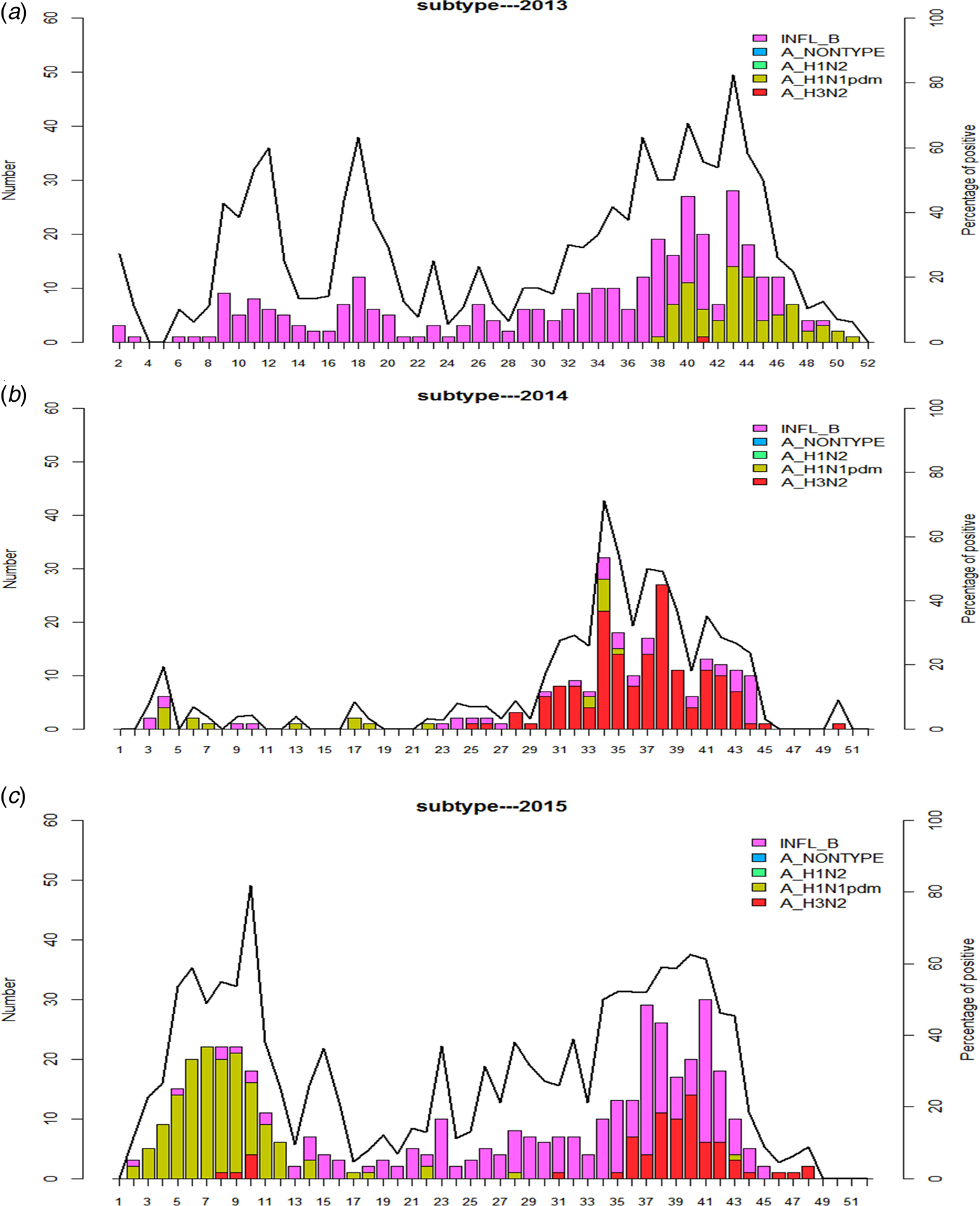
Fig. 2. Weekly number of positive cases for each targeted virus, with the percentage of positive influenza cases in 2013 (2a), 2014 (2b), 2015 (2c). Bars, in different colours (one colour for each type or subtype), represent the number of ILI cases samples positive. Line, for each year, represents the percentage of positive samples tested for influenza.
A sporadic circulation of both influenza B and A/H1N1pdm09 subtype was reported from the early 2014 to week 29. From that period an influenza circulation peak has started, mainly due to the A/H3N2 subtype arising and lasted until week 44.
Two peaks of detection were observed in 2015 (Fig. 2c). A first one between weeks 5 and 11 characterised by a high activity of A/H1N1pdm09 subtype and a second peak which has occurred between weeks 35 and 45 with influenza B and A/H3N2 subtype co-circulation.
Estimating the burden of influenza-associated ILI outpatient visits
Estimations of the proportional contribution of influenza-associated ILI to annual outpatient load in the selected sentinel sites were, per 100 population, 1.2 (95% CI 1.1–1.3), 0.3 (95% CI 0.28–0.35), 1.1 (95% CI 1.05–1.16) from January to December in 2013, 2014, 2015, respectively (Table 2).
Table 2. Proportion of influenza-associated ILI in the selected sentinel sites, 4S Network Senegal, January 2013–December 2015 (95% CI)

(c): Proportion of influenza-associated ILI cases (%) was estimated from total number of cases of ILI positive for human influenza viruses reported to the total number of ILI cases from whom clinical specimens were collected for laboratory confirmation of influenza virus (%) (c) = [(a)/(b)) × 100]; (d): Include ILI outpatient visits from all selected sentinel sites (cases tested or not tested for influenza). (e): Estimated by multiplying the number of outpatient visits for ILI at selected sites by the proportion testing positive for influenza virus; (e) = (c) × (d); (g): was estimated by the proportion of the total number of influenza-associated ILI visits for each study year among all outpatient visits in the same year (%) (g) = [(e)/(f)) × 100].
The distribution by group of individual children, adults and elderly showed that the highest proportion of influenza-associated ILI occurred among children with 0.6%; 0.7%, 0.8% of positivity in age groups 0–<2; 2–<5; 5–<15 years, respectively. In adults between 15 and <65-years-old the proportion was 0.56% while the lowest proportion was noted in older adults aged ⩾65 years with 0.05%.
Discussion
The present study is the first attempt for Senegal to estimate the proportional contribution of influenza-associated ILI outpatient visits using surveillance data for ILI in 10 selected sentinel sites through 4S Network [Reference Dia11].
Regarding age groups, overall, the rate of outpatient visits due to ILI was at least 4 times higher among pediatric patients (0–<2; 2–<5; 5–<15) than among adults, which is concordant with findings in USA [Reference Fowlkes15].
The proportional contribution of influenza-associated ILI to annual outpatient visits was highest among children under 15 years of age which are in line with observations reported in others studies. In a previous burden assessment study in a rural area sentinel site in Senegal, authors had also concluded that influenza was the most frequent infection among the youngest children [Reference Diene Sarr16]. In addition, in an influenza incidence study conducted in Ghana, Ntiri et al. [Reference Ntiri17] also noted the highest incidence of influenza-associated ILI among children. Children vulnerability to influenza has also been reported in a school prospective survey study conducted in USA [Reference Kathleen M18]. Significant influenza morbidity in this age group has been also described through the high rates of hospitalisation and mortality by Peebles et al. [Reference Peebles19]. In the elderly (50–< 65 and ⩾65 years age groups), the low proportions noted could be partly explained by the reduced number of elderly presenting at healthcare centres for ILI consultation compared with other age groups (children and young adults). Indeed, as reported in a previous study conducted in elderly in Senegal, self-medication, bed rest or traditional medicine are generally adopted against ILI episodes in this group [Reference Dia20].
The overall influenza virus detection rate in this study (25.3%) is significantly higher than rates reported in similar studies conducted in many other countries, including Ghana (18%) [Reference Ntiri17], Cambodia (16.9%) [Reference Horm21], Bangladesh (16.6%) [Reference Azziz-Baumgartner22] or Kenya (14%) [Reference Emukule23]. However, our results are very close to other data obtained in a similar study conducted from 2012 to 2015 in Tunisia with a detection rate of 25.8% [Reference Chlif24] and in Philippines as reported as reported by Tallo et al. [Reference Tallo25] in Philippines and in China by Fu et al. [Reference Fu26] with 21.7% and 21.3%, respectively. However, significantly higher rates have been reported in the USA with 35.3% [Reference Fowlkes15] or in Peru with 34.5% [Reference Forshey27]. These discrepancies noted in influenza detection rates among patients with ILI, beyond technical approaches sensitivities, can be attributed to geographical differences in virus burden, the number of patients tested, periods of samples collection and even the duration of the study.
The rate of outpatient visits for ILI in all clinical visits (3.1%) in our study is similar to that observed in the USA [Reference Fowlkes15] with 3.2%, in China [Reference Guo28] with 3.8% or in Peru [Reference Forshey27] with 3.9%. However, significantly highest rate was reported in a study conducted in Philippines [Reference Tallo25] with 18.2% or Salvador with 6% [Reference Oliveira29]. Regarding the estimated cumulative incidence of influenza-associated ILI outpatient visits, our rate in 2013–2105 period (0.9/100 population) is within the range of those from studies in the USA [Reference Fowlkes15] or Thailand [Reference Simmerman30] with respectively, 8.7 and 14.2/1000 population. In studies focused on children younger than 5 years, estimated at 14.6 and 14-100 population were reported in China [Reference Zhang31] and India [Reference Broor32]. For these comparisons, it must be kept in mind that, overall, it is difficult to directly compare ILI and influenza-associated ILI incidence rates from location to location due to potential differences in case capture, in case definitions, year-to-year variability and rarity of unbiased population-based data, especially in tropical zones.
Influenza circulated year round in Senegal during the study period with two distinct patterns: 1 year peak in both 2013 and 2014 while 2 peaks were recorded in 2015. In Senegal, a sub-Saharan country, influenza epidemics seasonality has been previously defined after 16 years of regular influenza monitoring [Reference Niang10] with influenza annual peaks during rainy seasons (June–October) and a very low viral detection level during dry seasons. This profile was confirmed in the years 2013 and 2014. In 2015, the two peaks pattern seen was atypical. Indeed, Senegal had already experienced such unusual flu pattern circulation with the A/H1N1pdm09 outbreak in early (January) in 2010 [Reference Dia33]. For the second time, peak out of rainy season was exclusively due to a high activity of the pandemic subtype. In a previous study, Dia et al. had already reported that the A/H1N1pdm09 subtype had a seasonal flow profile significantly different from that of other seasonal viruses (A/H3N2 and Influenza B) [Reference Dia, Diop and Niang34].
Our study has several limitations. Our results might underestimate the burden of influenza-associated ILI in Senegal as only medically attended ILI were assessed. Indeed, in Senegal, the majority of patients with ILI do not refer to any health facility for treatment and either self-medicate. In addition, since not all influenza infections require medical attention, the absolute incidence of influenza could not be determined accurately using our approach. A second limitation is that the weekly number of samples taken for virological surveillance is somewhat capped because physicians are requested to take only five specimens per week. It is expected that this might create a bias in our estimations. Moreover, the unavailability of data over the whole study period for all sites (sites excluded because they have less than 3 years activity), creates another bias regarding areas representativeness in this study. Another limitation in our estimation is the use of data which lack a population denominator. Nevertheless, we have launched a pilot phase in a few 4S sentinel sites to assess the feasibility of estimating the annual incidence of ILI combined to SARI cases before any generalisation.
In conclusion, this study reports the result of a first influenza burden assessment attempt in Senegal, based on flu-association ILI visits. Our preliminary results indicate that Influenza contributed significantly to outpatient clinic visits in Senegal, especially in children under 5 of age group and highlight the importance of considering public health action. The next step will be incidence studies that will include severe acute respiratory infections and population covered by each sentinel site in order to better understand influenza disease burden. This will be a crucial step before the set up of influenza control strategies and more importantly prevention using available flu vaccines in targeted groups.
Acknowledgements
We sincerely appreciate the contribution of ANSD, the focal points and staff of sentinel sites. We also thank Debora Goudiaby, Joseph Faye, Marie Louise Senghor, Davy Kiori, Sara Sy and all those who have contributed, either directly or indirectly, to the realisation of this work. This work was supported by WHO PIP.
Conflict of interest
None of the authors have any conflicting interests to declare. All authors have contributed materially to the research, have approved the submitted manuscript and concur with its submission to Epidemiology and Infection.







