The prevalence of abdominal obesity has increased substantially worldwide, in parallel with the increase of overall body adiposity( Reference Li, Ford and McGuire 1 ). The deleterious effects of abdominal fat accumulation in the general population have been well recognised, and there is increasing evidence of its harmful effect on cardiometabolic abnormalities among patients with chronic kidney disease (CKD) as well( Reference Odamaki, Furuya and Ohkawa 2 – Reference Kato, Ishida and Endo 5 ). In the past, studies have reported a relationship between abdominal obesity and mortality among non-dialysis and haemodialysis patients( Reference Postorino, Marino and Tripepi 6 – Reference Kamimura, Carrero and Canziani 10 ). Body-fat gain is a common finding among peritoneal dialysis (PD) patients, particularly after commencing dialysis therapy( Reference Stenvinkel, Lindholm and Lönnqvist 11 – Reference Jolly, Chatatalsingh and Bargman 16 ). Further, there is evidence that the accumulation of body fat occurs predominantly in the abdominal area( Reference Fernstrom, Hylander and Moritz 17 , Reference Choi, Kim and Hong 18 ). However, the impact of abdominal obesity on clinical outcomes has been scarcely investigated among patients undergoing PD( Reference Jin, Shin and Seung 19 , Reference Lu, Cheng and Wang 20 ).
Waist circumference (WC) is a simple and reliable marker of abdominal fat. In PD patients, some aspects related to the therapy, such as abdominal distension, presence of a catheter and frequent hernia, raised questions regarding the usefulness of WC as a surrogate marker of abdominal adiposity( Reference Iglesias and Diez 21 ). However, when the reproducibility of WC was tested in a recent analysis, WC proved to be a reliable marker of abdominal adiposity in patients undergoing PD( Reference Bazanelli, Kamimura and Manfredi 22 ). In addition, available data have demonstrated the use of WC as predictor of adipokines and cardiovascular complications( Reference Bazanelli, Kamimura and Canziani 23 , Reference Asicioglu, Kahveci and Arikan 24 ). Here, we aimed to investigate whether WC measurements at baseline and, particularly, whether 6-month changes in WC were able to predict mortality in PD patients during the 48 months of follow-up.
Methods
Patients
A total of 109 patients undergoing PD (seventy-two on automated PD and thirty-seven on continuous ambulatory PD) were recruited from the Dialysis Unit of the Nephrology Division of the Federal University of São Paulo, Brazil. Only patients older than 18 years, undergoing PD for >3 months, free of peritonitis for at least 3 months, and with no catabolic condition were included in the study. Exclusion criteria were treatment with corticosteroid or immunosuppressive drugs, presence of ascites, severe hernia or malignant disease.
Written informed consent was obtained from each patient. The study was approved by the Ethics and Research Committee of the Federal University of São Paulo.
Study design and protocol
In this prospective study, WC was measured at baseline and at 6 months. Nutritional status and laboratory parameters were also evaluated. Mortality was recorded over 48 months.
Anthropometry and waist circumference
BMI was calculated as body weight divided by squared height( Reference Keys, Fidanza and Karvonen 25 ). Measurements of WC were taken when the abdominal cavity was empty. WC was measured at the umbilicus level using a flexible plastic tape measure, while patients were standing with their weight equally distributed on both feet and had their heads facing forward. The mean of three measures was considered for analyses. All WC measurements were performed by the same trained observer.
Values >88 cm for women and >102 cm for men were considered to be indicative of high risk, in accordance with the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adults Treatment Panel III) (NCEP-ATPIII)( 26 ).
Laboratory parameters
Blood samples were drawn after an overnight fast of 12 h for assessment of serum levels of glucose, albumin (bromocresol green method) and high-sensitive C-reactive protein (immunochemiluminescence). Patients were instructed to collect the 24-h dialysate and urine samples for weekly measurements of total K t /V urea and weekly peritoneal creatinine clearance. Presence of residual renal function was considered when urinary volume was >200 ml/24 h.
Statistical analyses
Data are expressed as means and standard deviations for normally distributed variables and as medians and interquartile ranges for skewed variables. Paired or independent Student’s t test was applied, as appropriate. Pearson’s correlation test was used to evaluate the association between WC and the studied variables. Cox’s regression analysis was applied to investigate whether high WC at baseline and the 6-month changes in WC were predictors of mortality. The variables that significantly affected survival in the univariate analyses, or those known to affect survival according to literature, such as diabetes, inflammation, albumin and BMI, were considered in the multivariate models. In order to respect the proportion of covariates per outcome, we demonstrated models by including different covariates step-by-step. Hazard ratios and 95 % CI were calculated. Differences with P<0·05 were considered statistically significant. The analyses were performed using SPSS software, version 18.0 (SPSS Inc.).
Results
The mean age of the studied patients was 52 (sd 16) years and the median duration on PD was 12 (range 3–104) months. A majority of the patients were male (57 %), 32 % were diabetics, 64 % had residual renal function and 48 % had BMI≥25 kg/m2. At baseline, high WC was observed in 55 % of women and in 23 % of men (P<0·001). Patients with higher WC did not differ from those with lower WC in regards to demographic and clinical characteristics. Overall, WC increased from 92·6 (sd 11·9) to 94·1 (sd 12·4) cm (P=0·006) after 6 months. No other changes were observed during this period.
All through the 48 months of the study period, twenty-seven deaths were recorded. The causes of the death were CVD (n 12), sepsis (n 11), infection (n 2), multiple organ failure (n 1) and respiratory failure (n 1). The baseline characteristics of the survivor and non-survivor groups are demonstrated in Table 1. As can be seen, the non-survivor group was older and no other differences were found between the groups. Fig. 1 depicts the WC measurements at baseline and after 6 months in the survivor and non-survivor groups. Of note, a significant increase in WC was observed only in the non-survivor group. In the Cox regression analysis adjusting for sex, age, time on dialysis, presence of diabetes, BMI, serum albumin and C-reactive protein, high WC at baseline (Table 2) and the 6-month increase in WC (Table 3) were independently associated with mortality in PD patients. The incidence of peritonitis, changes in residual renal function, volume status, and PD modality were not associated with mortality in our study.

Fig. 1 Waist circumference at baseline and after 6 months in surviving and non-surviving peritoneal dialysis patients. * P<0·05 (independent Student’s t test).
Table 1 Characteristics of the patients at baselineFootnote * (Mean values and standard deviations; numbers and percentages; medians and interquartile ranges (IQR))

APD, automated peritoneal dialysis.
* Student’s t test.
Table 2 Cox’s regression analysis for high waist circumference at baseline (Hazard ratios and 95 % confidence intervals)

DM, diabetes mellitus; PD, peritoneal dialysis; CRP, C-reactive protein.
Table 3 Cox regression analysis for 6-month increase in waist circumference (Hazard ratios and 95 % confidence intervals)
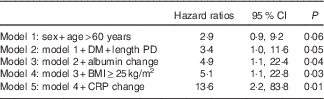
DM, diabetes mellitus; PD, peritoneal dialysis; CRP, C-reactive protein.
Discussion
In the present study, high WC was a predictor of mortality in PD patients. And, more importantly, we showed that changes over time in WC were associated with higher mortality in these patients. The current finding is relevant as available data show that PD patients accumulate body fat over time, particularly in the abdominal area( Reference Fernstrom, Hylander and Moritz 17 , Reference Choi, Kim and Hong 18 ). In fact, Fernstrom et al. ( Reference Fernstrom, Hylander and Moritz 17 ) showed that visceral fat, measured by computed tomography, increased substantially in a sample of incident PD patients followed up for 15 months. In addition, a subsequent investigation including sixty patients demonstrated that both visceral and subcutaneous fat increased during the first 6 months on PD therapy( Reference Choi, Kim and Hong 18 ).
WC is the most simple and inexpensive surrogate of abdominal adiposity, and its reliability against ‘gold standard’ methods has been well recognised in the general population( Reference Despres, Prud’homme and Pouliot 27 – Reference Vega, Adams-Huet and Peshock 30 ). In the CKD population, Sanches et al. ( Reference Sanches, Avesani and Kamimura 31 ) showed in a cross-sectional study including 122 non-dialysed patients that WC was strongly correlated with visceral as well as with subcutaneous fat as assessed by computed tomography. Moreover, the authors found that WC was associated with cardiovascular risk factors in the same manner as was visceral fat. In a subsequent study including the same cohort, Velludo et al.( Reference Velludo, Kamimura and Sanches 32 ) found an agreement between changes over time in WC and visceral fat. The use of WC in PD patients has been avoided by some researchers( Reference Iglesias and Diez 21 , Reference Zhe, Zeng and Tian 33 ) owing to some aspects related to the PD therapy, such as abdominal distension due to fluid infusion into the peritoneal cavity, presence of catheter and hernia, which could compromise the accuracy of WC measurements( Reference Nurmi, Korkeila and Honkanen 34 ). However, taking into consideration some features such as emptied abdominal cavity, absence of severe hernia and, finally, standardisation and training of the WC measurement technique, WC was shown to be a reliable marker of abdominal adiposity among PD patients( Reference Bazanelli, Kamimura and Manfredi 22 ).
The association of WC with mortality, however, has been scarcely reported in a PD population. A Korean retrospective study, which explored this relationship, followed eighty-four patients on PD for 53·2 (sd 34·4) months found that both all-cause and cardiovascular-cause mortality were not associated with the presence of abdominal obesity assessed by WC. However, the cut-off points used for classification were different from ours (90 cm for males and 80 cm for females)( Reference Jin, Shin and Seung 19 ). In haemodialysis patients, Postorino et al.( Reference Postorino, Marino and Tripepi 6 ) have demonstrated a direct association between WC and all-cause and cardiovascular death. Kramer et al.( Reference Kramer, Shoham and McClure 7 ) reported that WC was able to determine the mortality risk associated with obesity in a study including a large sample of non-dialysis patients. By using waist:hip ratio (W/H) as a central-obesity marker, Su et al.( Reference Su, Clase and Brimble 35 ) found that a high W/H was associated with increased cardiovascular events and all-cause mortality in a small number of PD patients followed up for 3·1 years.
In line with the previous studies in CKD, we demonstrated here that the simple anthropometric measure of WC was a predictor of mortality in PD patients. This is the first prospective study to demonstrate the association of high WC with mortality in PD patients, and the first one to establish the relationship between changes over time in abdominal obesity with mortality in a CKD population.
The exact mechanisms associated with high abdominal adiposity and mortality in these particular patients are not well established. A potential reason could be related to the fact that increased abdominal adiposity is strongly associated with insulin resistance, dyslipidaemia and systemic inflammation, which play essential roles in the pathogenesis of CVD in patients with CKD( Reference Odamaki, Furuya and Ohkawa 2 – Reference Kato, Ishida and Endo 5 ). In fact, there is evidence of increased WC with reduced adiponectin levels among PD patients( Reference Bazanelli, Kamimura and Canziani 23 ). Moreover, a recent study demonstrated that WC was independently associated with carotid intima media thickness in PD patients( Reference Asicioglu, Kahveci and Arikan 24 ). Accordingly, cardiovascular complications were the main cause of mortality in the present cohort (44 %).
It has been suggested that subjects with higher WC have a greater death risk than those with normal WC, regardless of BMI values( Reference Postorino, Marino and Tripepi 6 ). Accordingly, the mortality predictability of WC was observed in the present study even after adjustment for BMI. Thus, in agreement with other investigators, we highlight that caution is needed when interpreting the relationship between BMI and mortality in PD patients without taking into account the WC measurement.
The limitation of our study is the relatively small cohort of patients; hence, studies with larger samples are required. Besides, an additional prevalent characteristic of the cohort could be added in order to better explore the impact of other variables on mortality. The lack of ‘gold standard’ methodologies for abdominal adiposity such as computed tomography or MRI could be another limitation in the current study. However, the validity of WC has been previously demonstrated in cross-sectional as well as prospective studies. As WC cut-off values employed in the current study were based on a non-CKD-specific population (NCEP-ATPIII), studies addressing the optimal WC values linked to mortality in PD patients might provide further insight.
In conclusion, this study demonstrated that high WC as well as the 6-month increase in WC were both predictors of mortality in PD patients. We suggest WC to be incorporated as an important tool in the routine care of patients undergoing PD therapy.
Acknowledgements
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and the Oswaldo Ramos Foundation.
A. C. M. C. received a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. This manuscript was supported by FAPESP (Process no. 06/56124-6) and the Oswaldo Ramos Foundation.
A. C. M. C., M. A. K. performed statistical analysis and the interpretation of data. A. C. M. C, M. A. K. and F. B. N. drafted the manuscript. A. P. B. was responsible for data collection and review of the content. F. B. N. and L. C. critically reviewed the paper. L. C. and M. A. K. were responsible for the conception and design and final approval of the study. L. C. obtained funding. M. A. K. supervised the study.
The authors declare that there are no conflicts of interest.







