Phytate is known to have an inhibitory effect on mineral absorption by forming insoluble complexes with essential minerals, such as Zn, Ca, Cu and Fe(Reference Cheryan1, Reference Lonneral2). Experimental data have shown the negative effects of phytate on mineral absorption in human subjects. Zn absorption averaged about 35 % when young and elderly subjects were fed dephytinised diets (phytate:Zn molar ratio = 0), but the absorption declined to about 20 % when the phytate:Zn molar ratio was increased to 20(Reference Couzy, Kastenmayer and Mansourian3). In human subjects, Fe absorption from oat porridge with a phytate content of 107 μmol/l was shown to be 60 %, compared with only 44 % absorption from oat porridge containing phytate at 432 μmol/l(Reference Larsson, Rossander-Hulthen and Sandstrom4).
It has been found that phytate is not hydrolysed by humans due to our lack of phytase activity(Reference Sandberg and Andersson5). Recently it was observed that a considerable amount of dietary phytate was degraded in the human gut(Reference Joung, Jeun and Li6). This degradation is of importance because the mineral-binding capacity decreases when the phosphate groups are removed and thus may reduce the adverse effects of phytate on mineral bioavailability. However, the effect of undegraded dietary phytate on the absorption of minerals such as Ca, Fe, Cu and Zn has not been elucidated yet. Therefore, it is necessary to identify the impact of undegraded dietary phytate on mineral excretion when a substantial amount of phytate degradation has occurred in the gastrointestinal (GI) tract of humans.
Concerns about the impact of dietary phytate on mineral status have been raised among certain vulnerable subpopulations, including children, pregnant women, the elderly and vegetarians(Reference Couzy, Kastenmayer and Mansourian3, Reference Gibson, Donovan and Heath7–Reference Mendoza, Viteri and Lonnerdal11). The elderly are thought to be at higher risk of mineral deficiency than younger adults due to a lower intake and/or a reduced absorption with ageing(Reference August, Janghorbani and Young12–Reference Turnlund, Durkin and Costa14). Some studies reported that adjustment of Zn absorption or homeostasis with the change of dietary phytate did not differ between young and elderly women(Reference Couzy, Kastenmayer and Mansourian3, Reference Kim, Paik and Joung15). On the contrary, the inhibitory effect of dietary phytate on apparent P absorption was not observed in elderly women and was found to be unlikely in young women(Reference Joung, Jeun and Li6). Hence, further studies of the effect of age on mineral absorption or metabolism are needed.
The objectives of the present study were to determine the effect of phytate on mineral excretion, and the effect of dietary phytate and age on the relationships between faecal phytate and mineral excretion in Korean women.
Experimental methods
Subjects
Fourteen healthy younger women (aged 19–24 years) and fourteen healthy elderly women (aged 64–75 years) were recruited for the study through word of mouth and flyers on the campus of Seoul National University (Seoul, South Korea), and in neighbouring areas. Exclusion criteria included BMI of < 17 or >26 kg/m2, smoking, chronic use of alcohol, prescription drugs, oral contraceptives, vitamin or mineral supplements, Hb level of less than 105 g/l, the presence of acute disease or chronic disease such as diabetes, GI disorder, hyperlipidaemia, and a usual dietary Zn intake of less than 5 mg/d or greater than 15 mg/d.
All subjects gave their informed consent to participate in the present study. The study protocol was reviewed and approved by the Committee on Human Research of the College of Human Ecology at Seoul National University and the Davis Office of Human Research Protection at the University of California.
Study design
The metabolic balance study was divided into two 10 d metabolic periods (MP) and the subjects lived in a metabolic unit during the two MP and consumed diets provided from the metabolic kitchen. Upon completion of the first MP of the high-phytate (HP) diet, subjects returned to their homes to commence a 10 d washout period before starting the second period of the low-phytate (LP) diet. While subjects were on their usual diet during the washout period, they were instructed to avoid HP foods so that they could adapt more easily to the LP diet of the second period. Complete faecal samples were collected for 5–6 consecutive days starting from day 5 of each MP. A 3 d diet record for young women and 24 h dietary recall for elderly women were completed before MP1 for estimating usual intakes of energy, protein, phytate and minerals as described in Kim et al. (Reference Kim, Paik and Joung15). The usual intakes of nutrients were calculated by the estimated values from a nutrient database developed by the Korean Nutrition Society(16).
Diets
A 2 d cycle menu, composed of common Korean foods, was used for the two controlled metabolic diets (Table 1). The HP diet was fed in MP1 (phytate:Zn molar ratios were 24 for young women and 27 for elderly women); the lower-phytate diet was fed in MP2 (phytate:Zn molar ratios were 10 for young women and 12 for elderly women). On the day 1 menu, a phytase enzyme from Aspergillus niger (5000 U/g; BASF, Mount Olive, NJ, USA) was added to the brown rice gruel served at breakfast and the soyabean curd served for lunch to lower the phytate content of the diet during MP2(Reference Manary, Hotz and Krebs17). A quantity of 2 mg phytase was added to 100 g brown rice and incubated at 4°C for 6 h and 4 mg phytase was added to 100 g soyabean curd residue and incubated at 4°C for 3 h. On day 2, white rice replaced brown rice at lunch and dinner to reduce the phytate content of the total day's diet.
Table 1 Menus for the controlled diets for young women*

* A few adjustments were made in food items for the elderly; see the Diets section of the Experimental methods.
† These foods were treated with phytase to reduce the phytate content.
Several items on the younger women's menus were substituted to accommodate the food preferences of the elderly women without changing the energy, protein, Ca, Fe and Cu content of the menus or the Zn or phytate content. The ham and cheese sandwich was substituted with beef and vegetable gruel and grilled seaweed for elderly women at breakfast on day 2. Soya milk replaced milk for lunch on days 1 and 2 and tangerine replaced watermelon at dinner on days 1 and 2.
All food and water provided were prepared in the metabolic kitchen during the two MP. The women either ate their meals in the metabolic kitchen or living room in the metabolic unit, or in their workplace with members of our staff. Diet samples of each meal were prepared and analysed for minerals and phytate. The energy and protein contents of the study diets were determined by the estimated values from a nutrient database developed by the Korean Nutrition Society (Table 2) (16, Reference Kim, Paik and Joung18).
Table 2 Baseline characteristics of the study participants
(Mean values, standard deviations and ranges)

* Mean value was significantly different from that of the young women (P < 0·05; t test).
† Values were estimated by 1 d dietary recall at screening interviews using a database from the Korean Nutrition Society(16) and a previous report(Reference Kim, Paik and Joung18).
‡ Phytate:Zn molar ratio = (phytate intake/phytate molecular weight (660))/(Zn intake/Zn molecular weight (65·4)).
Sample collection and analyses
Each meal and every faecal sample were stored in polyethylene bags at − 20°C. Stored diets and faecal samples were freeze-dried, homogenised using a blender, and stored in desiccators until analysis. Samples of the freeze-dried diets (0·2–0·4 g) and faecal samples (0·1–0·2 g) were microwave digested (MARS 5; CEM Corp., Matthews, NC, USA) with 4 ml concentrated HNO3 (trace-metal grade; Fisher Scientific, Pittsburgh, PA, USA). Diet and faecal samples were diluted with 1 % HNO3 (trace-metal grade; Fisher Scientific) before mineral analysis. Mineral content in the diet and faecal samples was determined by inductively coupled plasma–atomic emission spectrometry (Vista; Varian Inc., Walnut Creek, CA, USA). Myo-inositol 1, 3, 4, 5, 6-pentakis-phosphate (IP5) and myo-inositol 1, 2, 3, 4, 5, 6-hexakis-phosphate (IP6) forms of phytate contents were determined by the Dionex Liquid Chromatograph System (Dionex Corp., Sunnyvale, CA, USA) after phytate extraction. Phytate (IP6), IP5 and phosphate ion (![]() ) were extracted using a modification of the procedure from Lehrfeld(Reference Lehrfeld19). All measurements were done in triplicate. Further details can be found in Kim et al. (Reference Kim, Paik and Joung15).
) were extracted using a modification of the procedure from Lehrfeld(Reference Lehrfeld19). All measurements were done in triplicate. Further details can be found in Kim et al. (Reference Kim, Paik and Joung15).
The degradation rate of dietary phytate was calculated as follows:
Statistical analysis
Results are expressed as mean values and standard deviations. Statistical analyses were conducted with SAS 9.1 (SAS Institute Inc., Cary, NC, USA). Differences of means between age groups were tested by t tests. Main effects and interactions for diet groups and age on faecal excretion of mineral and phytate were determined using ANOVA. Associations between faecal phytate and mineral excretion were examined using linear regression analyses. The statistical tests were executed using a two-sided significance level of 5 %. All significant differences were defined as P < 0·05.
Results
The characteristics of the young and elderly women in the study are described in Table 2. Their usual intakes of energy and minerals, such as Ca, Cu, Fe and Zn, did not differ significantly between the young and elderly women. Usual phytate intakes of the elderly women were about 67 % higher than those of the younger women.
Phytate excretion did change significantly with dietary phytate and age (Tables 3 and 4). Faecal phytate excretion was significantly higher during the HP period than the LP period in young women (P < 0·05). No significant differences were observed in the elderly women. Degradation rates of dietary phytate in the elderly subjects were higher compared with the young women on both the HP and LP diets (P = 0·023), and the degradation rates were significantly higher during the HP diet period compared with the LP diet period (P = 0·002).
Table 3 Nutrient intakes of phytate and minerals during the metabolic periods*
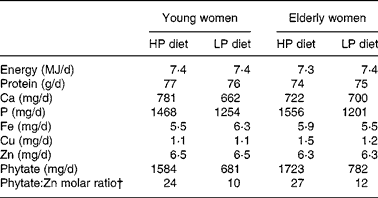
HP, high-phytate; LP, low-phytate.
* Nutrient contents were calculated using a database from the Korean Nutrition Society(16).
† Phytate:Zn molar ratio = (phytate intake/phytate molecular weight (660))/(Zn intake/Zn molecular weight (65·4)).
Table 4 Excretion of phytate and minerals during the metabolic periods
(Mean values and standard deviations)
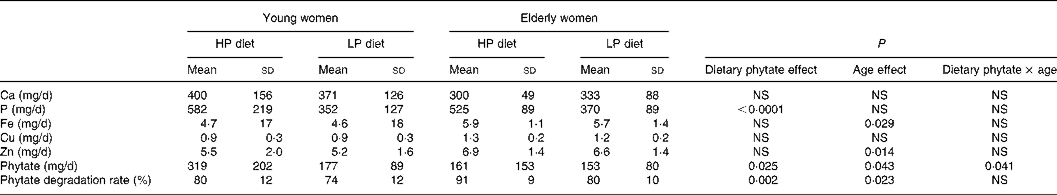
HP, high-phytate; LP, low-phytate.
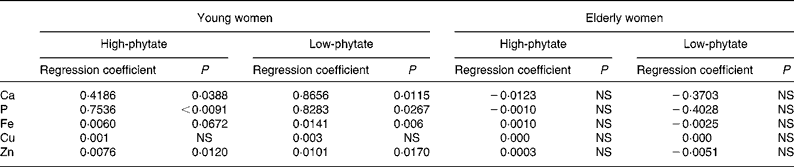
There were significant positive relationships between faecal phytate and mineral excretion in young women in both the HP diet and LP diet periods (P < 0·05; Table 5). Faecal minerals such as Ca, P, Fe and Zn were excreted more according to the increase of faecal phytate excretion in young women. The relationship between faecal phytate and mineral excretion tended to be greater during the LP diet period in young women. However, no association was found between phytate excretion and mineral excretion in elderly women.
Table 5 Relationship between faecal phytate and mineral excretion in young and elderly women (Regression coefficients)
Discussion
We found a positive relationship between faecal phytate and mineral excretion (Ca, P, Fe, Zn) in young women. The degradation rate of dietary phytate in young women was about 80 % in the present study. These results indicate that undegraded dietary phytate (IP5+IP6), about 20 %, inhibited absorption of minerals such as Ca, Fe and Zn, although considerable amounts of dietary phytate were degraded in the GI tract of young subjects.
Several studies have reported a negative effect of phytate on mineral bioavailability in animals and human subjects. A study of rats showed that apparent absorption and retention of Ca, P, Fe and Zn in rats fed the phytate-free diet were significantly higher than those in rats fed either a soyabean protein diet or a casein diet for 5 weeks, but not for 2 weeks(Reference Kamao, Tsugawa and Nakagawa20). In a study of piglets, mineral absorption (Ca, P) and urinary excretion (P, Cu) were increased by the addition of the phytase enzyme(Reference Kies, Gerrits and Schrama21). By reducing IP5 and IP6 in the diet with phytase treatment, Fe absorption in healthy adults increased by 78 %(Reference Zhang, Onning and Oste22). However, Davidsson et al. (Reference Davidsson, Ziegler and Kastenmayer23) reported no significant differences in apparent absorption of Ca, Fe, Cu and Zn between regular and dephytinised soya formula, as based on the faecal excretion of non-absorbed isotope during 72 h balances in healthy infants. A direct comparison is not possible because of many differences in the conditions of the studies (for example, species, age, the method of measuring mineral absorption).
Faecal mineral excretion was influenced by both dietary phytate and age of the women. The regression coefficients between faecal phytate and mineral excretion tended to be higher during the LP diet period than the HP diet period in young women (Table 5). However, it is not clear why the relationship between faecal phytate and mineral excretion was greater during the LP diet period in young women.
On the other hand, the positive relationship between faecal phytate and mineral excretion was not observed in elderly women. The lack of an inhibitory effect of phytate on mineral absorption with ageing may be due to an adaptation to HP diets occurring in the GI tract of elderly women, who are considered to have consumed high levels of phytate through their traditional Korean diet for a long time. The adaptation might lead to the higher phytate degradation rate in elderly subjects compared with younger subjects consuming HP diets. This increased rate of degradation during HP diets in elderly women would result in similar amounts of undegraded phytate between the two MP and it might change the inhibitory effect of phytate on mineral absorption. A previous study(Reference Joung, Jeun and Li6) reported the possibility of adaptation to an HP diet in the human gut by showing that the increased dietary phytate intake could stimulate phytate degradation in the gut over time.
Phytate degradation may occur from three sources of phytase found in the GI tract, i.e. dietary phytase, intestinal mucosal phytase, or phytase produced by the small-intestinal microflora(Reference Sandberg24). Some investigators suggested the induction of mucosal phytase by dietary phytate in rats(Reference Lopez, Leenhardt and Coudray25, Reference Yang, Matsuda and Sano26), but intestinal mucosal phytase seems to play a minor role compared with dietary phytase for phytate hydrolysis(Reference Sandberg and Andersson5). Lopez et al. (Reference Lopez, Leenhardt and Coudray25) showed that the enhancement of mucosal phytase induced by dietary phytate improves intestinal Ca absorption in rodents, demonstrating the adaptation of the small intestine to diets rich in phytate and poor in Ca. However, a direct application of the rat results to humans may be not possible because the ability of various species of single-stomached animals to hydrolyse phytate varies. Moore & Veum(Reference Moore and Veum27) showed that rats fed a marginal-P diet can compensate for the lack of available P by a greater phytate degradation, suggesting an adaptation in the intestinal microflora. This adaptation may result from enhanced phytase or alkaline phosphatase synthesis by the GI microflora stimulated by a lower level of phytate in the digesta(Reference Moore and Veum27). However, the role of small-intestinal microflora in phytate degradation is still disputed. On the other hand, Brune et al. (Reference Brune, Rossander and Hallberg28) observed that the inhibitory effect of phytate on Fe absorption did not differ between a group of vegetarians with a regular HP intake and a control group, suggesting no intestinal adaptation to an HP diet. The possible degree of the subjects' adaptation to HP diets is not yet clear. Thus, it is necessary to elucidate if adaptation to the long-term HP diet can occur in the human GI tract. Also, studies on the adaptation mechanism and influencing factors, such as the genetic difference of the phytase enzyme, are required.
Since the metabolic diet provided a much higher level of phytate compared with the habitual diet, the adaptation to the HP diet might cause greater inter-individual variation among subjects. A greater variation in baseline dietary intake for Zn and phytate among elderly women compared with younger women could affect the adaptation of elderly women to an HP diet and it might cause less impact on mineral excretion. Weaver(Reference Weaver29) showed that the inter-individual difference of Ca retention/absorption in the meals was a result of many factors, and individual absorptive efficiencies could account for a great part of the variations in absorption.
The extent of faecal mineral excretion, except for P, was similar between the HP and LP diet periods in the present study. The impact of dietary phytate on mineral excretion was not as great as we previously thought. Our findings were in contrast to the results of other human studies(Reference Brune, Rossander and Hallberg28, Reference Hallberg, Brune and Rossander30–Reference Fredlund, Isaksson and Rossander-Hulthen32). The addition of 100 mg or more phytate-P significantly decreased Ca retention compared with the test meals with no added phytate at day 7, suggesting the dose-dependent inhibitory effect of sodium phytate on retention of Ca in men(Reference Fredlund, Isaksson and Rossander-Hulthen32). The inhibitory effect of phytate on Fe absorption has been found to be dose dependent(Reference Hallberg, Brune and Rossander30, Reference Brune, Rossander-Hulten and Hallberg33). A recent study reported that the inhibitory effect of dietary phytate explained 82 % of the variance in Zn absorption as measured by stable isotope techniques in the mathematical model of quantity of Zn absorbed per d as a function of dietary Zn and phytate(Reference Miller, Krebs and Hambidge31). The results of the present study may indicate that a considerable amount of dietary phytate might have been degraded in the stomach and small intestine since the absorption of minerals mostly occurs in the small intestine. It has been known that phytate is not hydrolysed by humans due to the lack of phytase activity(Reference Sandberg and Andersson5). However, an ileostomy human study found that dietary phytate was partly digested in the stomach and small intestine (39–76 %), but did not impair the apparent absorption of Ca and Mg from the small intestine(Reference Sandberg, Hasselblad and Hasselblad34). On the other hand, Schlemmer et al. (Reference Schlemmer, Jany and Berk35) reported that the degradation of dietary phytate occurs in the colon as well as in the stomach and small intestine of pigs, irrespective of their diet. Therefore, further studies are needed on the extent of phytate degradation in different parts of the GI tract as well as on the influencing factors of phytate degradation, such as the age of subjects and the long-term phytate intake.
The present study has some limitations. First, no information was provided on the faecal excretion of phytate and Zn at baseline. Second, no correction was made for re-excreted and absorbed minerals when measuring faecal excretion. This correction may be more critical for some minerals (for example, Zn) than for others (for example, Fe). Thus, endogenous faecal excretion of some minerals should be considered when exploring the effect of phytate on mineral absorption in future studies.
Conclusion
The present study shows that faecal phytate excretion is positively related to mineral excretion, and undegraded phytate (about 10–20 %) has a negative impact on mineral absorption in young women. The inhibitory effect of phytate on mineral absorption was affected by both dietary phytate and age. The impact of phytate on mineral utilisation should be examined further considering the long-term phytate intake and age of subjects in future studies. Also, both beneficial and adverse health effects of phytate need to be studied since a considerable amount of dietary phytate was degraded in the human gut.
Acknowledgements
The present study was funded by the Korea Research Foundation (KRF 2001-003-D00119). The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Committee on Human Research of the College of Human Ecology at Seoul National University and the Davis Office of Human Research Protection at the University of California. Written informed consent was obtained from all subjects.
J. K. contributed to the analysis and interpretation of the data, and manuscript preparation. L. R. W. and J. C. K. were involved in coordinating the study and contributed to the study design. R. M. W. was involved in data collection and analysis. S. J. L. was the data manager and was also involved in data collection. H. Y. P. was the principal investigator at the early phase of the study and was involved in the study design. H. J. was responsible for all the process of the study. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.







