Introduction
Dengue is a mosquito-borne zoonotic disease that has become endemic in many countries in the tropical and sub-tropical regions in the world. It is among the most widespread diseases worldwide with an estimated 390 million cases annually [Reference Bhatt1]. It has been reported in over 125 countries globally including many with cases reported from travellers returning from known dengue-endemic regions [Reference Ravanini2–Reference Huang4]. There are four distinct dengue virus (DENV) serotypes known to cause human infections: DENV-1, DENV-2, DENV-3 and DENV-4. In general, there are no differences in the clinical manifestations of dengue caused by any of the virus serotypes. Immunity developed against one serotype usually confers long-lasting protection against the same serotype, but limited and transient protection against the subsequent infection by a different serotype [Reference Sabin5]. Repeated infections, however, are possible and common, especially in endemic/hyperendemic regions where multiple DENV serotypes co-circulated [6].
Malaysia is a dengue-hyperendemic country with all four DENV serotypes co-circulating since the 1960s [Reference AbuBakar and Shafee7–Reference Chee and AbuBakar10]. The homotypic transmission cycle with DENV-3 as the dominant serotype occurred in the years 1982, 1986, 1992–1995 and 2002 [Reference AbuBakar and Shafee7]. In 2010, we reported the emergence of DENV-3 genotype III (DENV-3/III) [Reference Tan11] that coincided temporally with the anticipated DENV-3 major outbreak cycle [Reference Tan12]. The DENV-3/III is one of the DENV genotypes that has been associated with high transmissibility and widespread global distribution [Reference Messer13]. The strains recovered in Asia, America and Europe appeared to have originated from the Indian subcontinent [Reference Messer13, Reference Gutierrez14]. The virus has been associated with several major outbreaks/epidemics in places where it emerged [Reference Gutierrez14–Reference Guzman16]. However, there was no upsurge of DENV cases following the emergence in Malaysia in 2008. Furthermore, the anticipated DENV-3-dominated cycle in 2006–2012 was also not observed [Reference Tan12]. The high genetic semblance between the DENV-3/III recovered from the outbreak and non-outbreak regions suggests the possible existence of factors, other than the virus intrinsic differences, which could have shaped the amplitude of transmissibility of DENV-3/III. Earlier studies based on epidemiological observations and mathematical predictions have suggested the potential importance of population immunity in mitigating the spread of dengue [Reference Ooi, Goh and Gubler17–Reference Salje19]. Its potential role in influencing the emergence and transmission of the virus at the genotype level, however, remained unclear. Hence, investigation of the factors that could influence virus emergence at the genotype level could provide a better understanding on the cyclical recurrence of dengue, especially in the dengue-endemic regions. The emergence of DENV-3/III in Malaysia in the midst of circulating homotypic DENV-3 genotype I (DENV-3/I) and other DENV serotypes provides an excellent opportunity for such study. Here, we detailed the recent introduction event of DENV-3/III into Malaysia and investigated the factors that potentially affected its spread. We performed genetic analysis to determine the genetic diversity of DENV-3 isolates recovered between 2005 and 2011. Spatiotemporal distribution of different DENV-3 isolates was mapped to examine the DENV-3/III movement and spread pattern after its emergence. Lastly, we used the immune sera obtained from patients infected with homotypic DENV (DENV-3) and heterotypic DENV (DENV-1 and DENV-2) to determine the neutralisation capacity of these sera against the newly emerged DENV-3/III strains and the pre-existing circulating DENV-3/I strains.
Methods
Ethics statement
The human serum samples used in this study were obtained from the archived collection of the WHO Collaborating Centre for Arbovirus Reference & Research (Dengue/Severe Dengue) Virus Repository at the University of Malaya (UM). This study was approved by the Medical Ethics Committee of the University Malaya Medical Centre (UMMC) (MEC Ref No: 806.23 and 806.24).
Data collection
Records of laboratory-confirmed DENV-3 cases between 2005 and 2011 were obtained from the UMMC Medical Record Department. Isolates with defined DENV-3 genotype were matched to the database to identify the patients’ places of residence. All datasets without location of residence were excluded. The hospital and patient's address of residence were geocoded and entered into ArcGIS V9.3 (ESRI, Redlands, CA) to generate the DENV distribution map [20]. No personal identifying information other than the place of residence was included in the analysis.
Virus isolates
Dengue-positive sera were obtained from the WHO Collaborating Centre for Arbovirus Reference and Research (Dengue/Severe Dengue) Virus Repository at UM. For virus isolation, sera were inoculated into C6/36 mosquito cells (Aedes albopictus cell line). The supernatant was harvested at day 7 post-infection as previously described [Reference AbuBakar, Wong and Chan8]. The infecting DENV serotype was confirmed using an in-house multiplex reverse-transcription polymerase chain reaction assay and virus genome sequencing, performed as previously described [Reference Teoh9]. The genotype of each DENV-3 was assigned according to the phylogenetic tree constructed from the complete envelope (E) gene. Virus titres were determined using a focus-forming assay as previously described [Reference Wong, Abd-Jamil and Abubakar21, Reference Okuno22].
Serological characterisation of human serum
Acute DENV infection was confirmed in the laboratory using the dengue IgM enzyme-linked immunosorbent assay (ELISA; Standard Diagnostics, Gyeonggi-do, South Korea) and virus isolation. The presence of DENV-specific IgG was determined using dengue-specific IgG ELISA (Standard Diagnostics, Gyeonggi-do, South Korea). The IgM/IgG ratio was used to distinguish between dengue primary and secondary DENV infection, with a discriminatory factor that ranges from 1.2 to 1.4 [23]. In order to gain better sample selection criteria, patients’ sera were classified as primary infection if the IgM/IgG ratio was more than 1.4. Patients’ sera were classified as from secondary infection when (1) the sample had IgM/IgG ratio <1.2 and (2) the detection of anti-dengue IgG concurrent with the isolation of DENV in the first serum sample. The serotype-specific neutralising capacity of serum obtained from patients with primary and secondary DENV-3 infections was determined against the circulating DENV-3 isolates. Cross-neutralizing capacity was determined using serum obtained from patients infected with DENV-1 and DENV-2. These sera were used to represent the dominant circulating DENV serotypes (DENV-1 and DENV-2) during the study period [Reference Teoh9].
Foci Reduction Neutralisation Test
Neutralisation assay was performed as previously described using Vero cells with only minor modifications [Reference Teoh9]. Briefly, pooled sera were first heat-inactivated at 56 °C for 30 min. The heat-inactivated sera were then serially diluted in twofold (1/20 to 1/2560) in Dulbecco's phosphate-buffered saline (dPBS; Gibco, Invitrogen, Thermo Fisher Scientific, Grand Island, NY). Then, the diluted DENV-3 (120 FFU; 100 µl) was mixed with foetal bovine serum (FBS)-free Dulbecco's Modified Eagle's Medium (DMEM; Gibco, Invitrogen, Thermo Fisher Scientific, Grand Island, NY) (100 µl) or serial diluted heat-inactivated serum (100 µl). The virus–medium/serum mixture was incubated at 37 °C for 1 h. The virus–serum mixtures were transferred onto a monolayer of Vero cells in a 24-well plate and incubated at room temperature for 2 h. The inoculum was then discarded, and the infected cell monolayers were rinsed with dPBS and overlaid with DMEM (1 ml) containing 2% FBS (Bovogen Biologicals, Victoria, Australia) and 1.5% of carboxymethylcellulose (Sigma-Aldrich, Steinheim, Germany). The plate was incubated at 37 °C for 4 days. Foci formation was visualised as previously described [Reference Wong, Abd-Jamil and Abubakar21, Reference Okuno22]. The foci reduction neutralisation titres (FRNT) of the respective serum was expressed as the reciprocal of the serum dilution in which 50% foci reduction (FRNT50; semi-neutralisation) was observed.
Results
DENV-3 genetic diversity during the period between 2005 and 2011
We investigated the genetic diversity of DENV-3 isolates obtained during the period between 2005 and 2011. A total of 166 isolates were identified as DENV-3 with the number of isolates obtained annually ranging from 10 to 38. A subset of the isolates (n = 99) was recovered, sequenced and genotyped. Three major DENV-3 genotypes; i.e., genotypes I, II and III were identified. During the period between 2005 and 2006, all genotyped DENV-3 belonged to DENV-3/I (Fig. 1). In 2007, DENV-3/III was first identified from the DENV-3 population (Fig. 1). DENV-3/III accounted for 45% of the total genotyped DENV-3 cases in 2008. A steady increase in the isolation of DENV-3/III was observed in the subsequent years; it accounted for 50% (10/20) and 57% (4/7) of the genotyped DENV-3 in years 2010 and 2011, respectively.
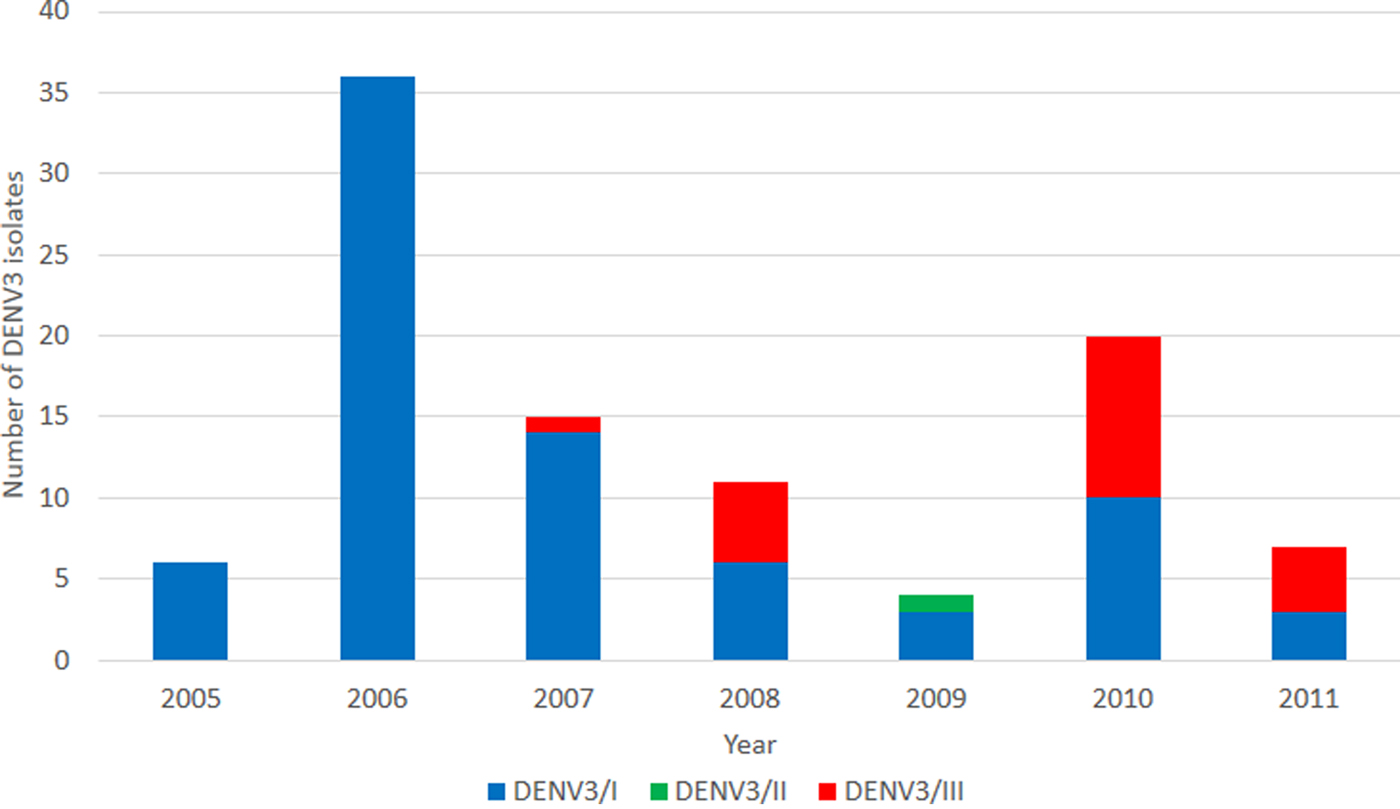
Fig. 1. Overview of DENV-3 isolated from the UMMC between 2005 and 2011. A total of 99 DENV-3 isolates were identified and sequenced. DENV-3/I (blue) was the main DENV-3 recovered during the period 2005–2009. DENV-3/III (red) was first recovered in 2007.
Spatiotemporal analysis of DENV-3 isolation (2005–2011)
Spatiotemporal analysis suggested that the majority of DENV-3 cases were from patients residing within a 15 km radius of the UMMC (study site). We divided the study period into two distinct phases representing pre-emergence of DENV-3/III (2005–2007, Fig. 2) and post-emergence (2008–2011, Fig. 3). During the pre-emergence period, DENV-3 cases concentrated mainly in the city of Petaling Jaya. A DENV-3/I cluster was located in Petaling Jaya, approximately 5 km south of UMMC (Fig. 2a). DENV-3/I was the sole genotype isolated in years 2005 and 2006 (Figs 2b and c). Only one DENV-3/III was isolated from the midst of the DENV-3/I cluster located in Petaling Jaya, south-western area (5 km) of UMMC in 2007 (Fig. 2d).
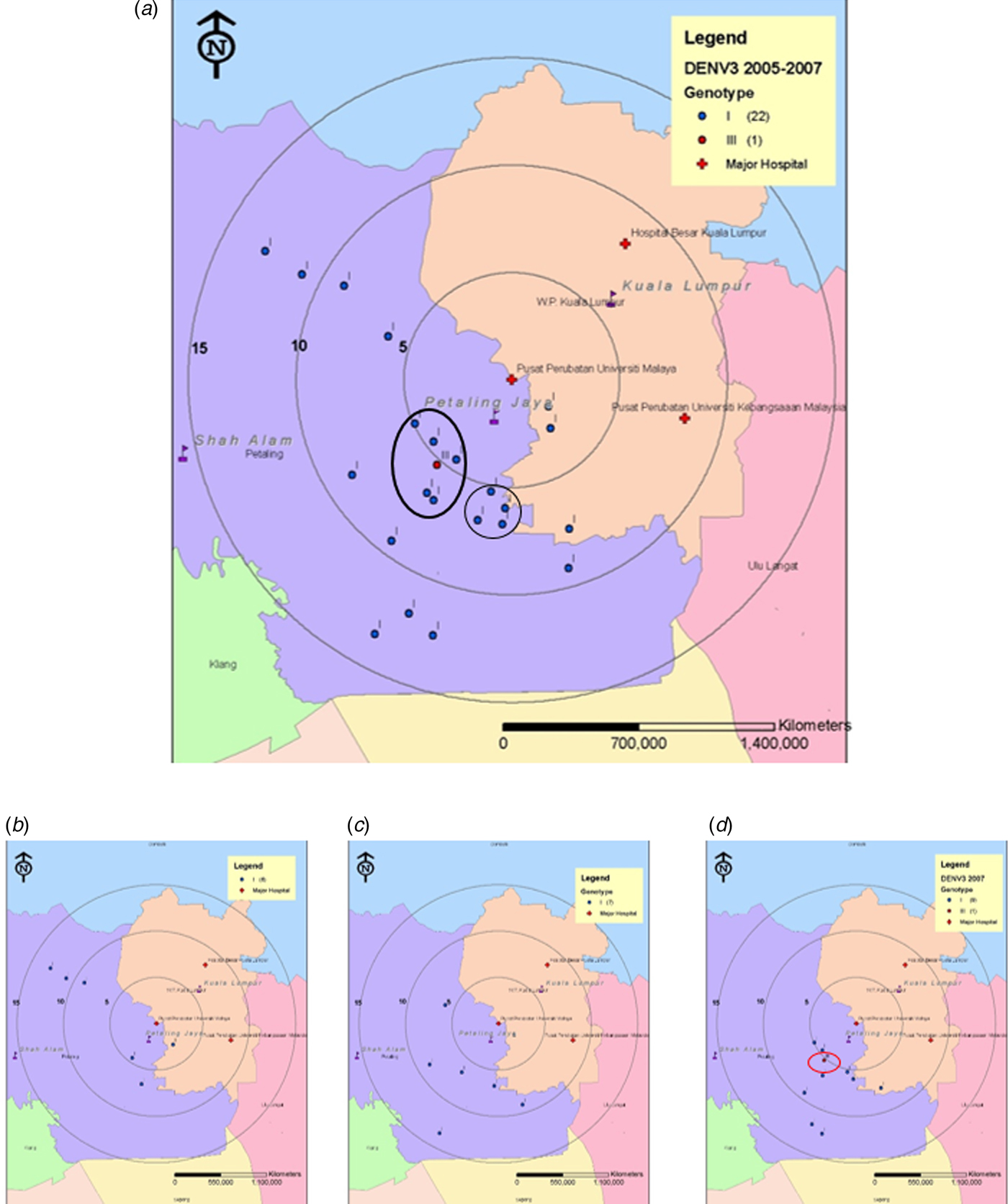
Fig. 2. Map of the Klang Valley, showing the geographical distribution of DENV-3 isolates recovered from UMMC between 2005 and 2007. (a) 2005–2007, (b) 2005, (c) 2006 and (d) 2007. Phylogenetic analysis was performed on the DENV-3 isolates and linked to their corresponding genotype. Blue and red markers indicate the Malaysian DENV-3/I and DENV-3/III, respectively.

Fig. 3. Map of the Klang Valley, showing the geographical distribution of DENV-3 isolates recovered from UMMC between 2008 and 2011. (a) 2008–2011, (b) 2008, (c) 2009, (d) 2010 and (e) 2011. Phylogenetic analysis was performed on the DENV-3 isolates and linked to their corresponding genotype. Blue, green and red markers indicate the location of patients infected with Malaysian DENV-3/I, DENV-3/II and DENV-3/III, respectively.
During the post-emergence period, DENV-3 cases were demonstrated to have wider distribution, scattered across the cities with no noticeable clustering (Fig. 3a). It was noted, however, that the majority of DENV-3/I from the post-emergence period were isolated from areas within 5–15 km radius from UMMC in a southward direction (Fig. 3). In 2008, DENV-3/III cases increased; mainly focused in Kuala Lumpur, north-eastern area (<5 km from UMMC; Fig. 3b). There was no previous DENV-3 isolation from these areas, suggesting a possible introduction of DENV-3/III into a sufficiently susceptible population. During 2010 and 2011, DENV-3/III showed wider geographical distribution, mostly among patients from the north of UMMC (Fig. 3), encompassing areas in Kuala Lumpur, Petaling Jaya and Ulu Langat with a small cluster detected within 5 km north-eastern of UMMC (Figs 3d and e). The dispersal pattern of the DENV-3/III virus on the map suggested the autochthonous spread of DENV-3/III. The virus, however, did not spread into areas in which DENV-3/I genotype foci was already present (south-western from UMMC).
Neutralisation of DENV-3 strains by DENV immune sera
The possibility that pre-existing immunity in the population affects the transmission of the newly introduced DENV-3/III was investigated using sera from patients with the respective virus infection. Overall, the control sera from donors with no previous DENV exposure exhibited weak non-specific neutralisation responses against DENV-3/I and DENV-3/III at FRNT50 of only 34.3 and 27.2, respectively (Table 1). Sera of patients with secondary DENV-3 infection showed similar neutralisation titres against DENV-3/I (S3/I-S: FRNT50 ⩾ 5120; S3/III-S: FRNT50 = 4740.5) and DENV-3/III (S3/I-S: FRNT50 ⩾ 5120; S3/III-S: FRNT50 = 3910.2). The possible existence of previous infecting serotypes antibodies in the sera of patients with secondary dengue infection, however, was not determined due to the limited volume of serum and unavailability of previous clinical samples and records. Hence, in this study, the potential influence of the previous infecting serotypes on the neutralisation capacity was not evaluated. The primary homotypic immune serum was obtained from patients infected with DENV-3/I (S3/I). The sera neutralised DENV-3/I and DENV-3/III strains at titres of 775.9 and 1382.5, respectively (Table 1). There was no primary immune serum obtained from any patient infected with DENV-3/III that fulfilled the selection criteria. Therefore, neutralisation capacity of the S3/III against DENV-3/I and DENV-3/III was not evaluated.
Table 1. Neutralisation capacity of DENV immune serum against DENV-3/I and DENV-3/III strains

a Serum sample from S3/III not available.
b Control sera obtained from healthy donor with no previous dengue exposure; ‘–’ indicates that the test was not run.
The heterotypic DENV-1 immune sera showed varied degree of neutralisation against DENV-3/I, DENV-3/III and DENV-1 (Table 1). As expected, the DENV-1 serum (S1/I) neutralised DENV-1 at highest neutralisation titre (FRNT50 = 2031.9). Whereas, the same sera cross-neutralised DENV-3/I and DENV-3/III at FRNT50 of 284.0 and 190.3, respectively. Similar to DENV-1 serum, the heterotypic DENV-2 sera showed a varied degree of neutralisation against DENV-3/I, DENV-3/III and DENV-2 (Table 1). The DENV-2 sera neutralised its homotypic strain (DENV-2) at the highest neutralisation titre (FRNT50 = 905.1), compared with the neutralisation titres against the DENV-3/I (FRNT50 = 448.8) and DENV-3/III (FRNT50 = 50.4). The effects of heterotypic DENV-2 neutralisation response against the DENV-3/III strains was marginal, at a similar titre of the control sera (less than twofold differences). Whereas, the high neutralisation capacity of the DENV-2 serum against DENV-3/I strains was due to one of the DENV-3/I strains was efficiently neutralised by DENV-2 serum in high dilution (FRNT50 = 4063.8). The remaining two DENV-3/I strains were neutralised by the DENV-2 serum but at lower neutralisation titres (FRNT50 = 113.1 and 254.0).
Discussion
The ability of a newly introduced virus to spread and cause an outbreak in a population could be influenced by a number of intrinsic and extrinsic factors. For many of the viral diseases, population herd immunity is one of the most important factors. Herd immunity is also important in ensuring the success of a vaccination programme. Similarly, in dengue, it is expected that the previous DENV activities and the ongoing DENV circulation in the population would play a role in determining the impact of the introduction of new virus serotypes and genotypes into the population. Here, we examined the DENV-3 genotypes that caused outbreaks in Klang Valley, Malaysia, the spread pattern of the virus and investigated the potential effects of the homotypic and heterotypic neutralizing antibodies on the newly introduced DENV-3/III.
During the period between 2005 and 2011, DENV-1, followed by DENV-2 were the dominant DENV serotypes causing outbreaks in the Klang Valley, Malaysia [Reference Tan12]. The overall isolation of DENV-3, however, was low even after the introduction of the newly emerged DENV-3/III, in which our genetic diversity study suggested to have taken place as early as in 2007. This is in contrast to that observed in other countries where the introduction and the subsequent establishment of the new DENV genotype were associated with large epidemic [Reference Santiago15, Reference Guzman16, Reference Lee24–Reference Wang26]. Furthermore, looking at the distribution of dengue cases involving the DENV-3/III suggests that the virus was recovered mainly in the north-eastern areas of the UMMC, which had recorded no previous circulation of DENV-3/I viruses. Whereas, the recovery of DENV-3/III in the south-western part of the UMMC, where DENV-3/I was actively circulating, was much less. This implies that the population living in the south-western part of the UMMC had already been exposed to DENV-3/I. Hence, the homotypic immunity against it cross-protected them against infection by the newly introduced DENV-3/III. In contrast, those living in the north-eastern areas of the UMMC were likely immune naïve to DENV-3 allowing outbreaks involving DENV-3/III to occur. In support of this suggestion is our finding that sera of patients with DENV-3/I infection effectively neutralised DENV-3/III viruses (at nearly twofold), even when compared with neutralisation titre against its own genotype (DENV-3/I). This finding is consistent with many other earlier studies suggesting that immunity accorded to one DENV serotype would protect against the same serotype (homotypic) [Reference Carrington27].
The uneven distribution of the DENV-3 within the population of Klang Valley reflects the typical focal nature of DENV transmission [Reference Salje19]. This is probably due to the limited distance that DENV-infected mosquitoes could travel with an average of 400 m, to spread the infection. The most common vector for DENV, the Aedes aegypti, is also anthropophilic and prefers to stay indoor closer to humans. The low incidence of DENV outbreaks involving DENV-3 in the past decades could also contribute to increasing number of DENV-3-susceptible individuals, especially in the newer residences and townships. This would explain why in a certain locality, such as the north-eastern areas of the UMMC, transmission of the newly introduced DENV-3/III occurred. Hence, in general, geo-restricted homotypic population immunity in some parts of Klang Valley was probably one of the factors that contributed to the low amplitude of DENV-3/III-associated incidence in Malaysia, in comparison to its transmission into other countries that were previously DENV-3-free [Reference Santiago15, Reference Uzcategui28]. Our observation here is not dissimilar to that reported earlier in which the importance of pre-existing homotypic immunity in restricting the genetic diversity of the newly emerged DENV-2 genotype was described [Reference Carrington27].
Another factor that could have limited the spread of the newly introduced DENV-3/III is the presence of cross-protecting immunity accorded by earlier exposure to a different DENV serotype, heterotypic infection. Broad-ranged cross-protection derived from heterotypic DENV infection has been earlier suggested to provide short-term protection to the exposed population [Reference Sabin5, Reference Reich29, Reference OhAinle30] and limit the potential expansion of virus cluster [Reference Santiago15]. In the Klang Valley, the introduction of DENV-3/III coincided with the period of the prolonged DENV-1-dominant cycle (>10 years) within the same locality [Reference Teoh9, Reference Tan12]. The prolonged DENV-1 circulation in Klang Valley probably contributed to heightened population immunity that acted as transmission barrier against large-scale dispersal of DENV strains that are sensitive to DENV-1 immunity. Epidemiological observations in other countries [Reference Guzman16, Reference Khampapongpane31] suggested that the emergence of DENV-3/III with major epidemics takes place only in countries or regions with limited or no DENV-1 activities during the time of DENV-3/III introduction. Additionally, the introduction of DENV-3/III into areas with dominant DENV-1 circulation did not change the overall dengue incidence [Reference Rigau-Perez32, Reference Koh33], suggesting the susceptibility of DENV-3/III to DENV-1 immune sera probably lessen its tendency for its widespread transmission in the DENV-1-dominated area. Our findings that the heterotypic DENV-1 immune sera effectively neutralised both DENV-3/I and DENV-3/III is in support of these earlier epidemiological findings. Even though in our study, we only looked at the neutralising antibody responses, we would expect even more significant restriction of DENV-3 replication if the effects of the entire immune responses against DENV-1 are examined.
The recent reports of increasing the number of DENV cases caused by DENV-2, contributing to the switching of the dominant DENV serotype from DENV-1 in the Klang Valley is alarming. Our findings that the antibody responses against DENV-2 were only effective against DENV-3/I but not neutralising against DENV-3/III signal a possibility that DENV-3/III would eventually cause a major outbreak subsequent to the current DENV-2 outbreak cycle. In Malaysia, surveillance of dengue routinely includes identification and typing of the viruses [34]. It was usually performed by individual institute or research group as part of their research activities [Reference AbuBakar and Shafee7, Reference Tan12]. Findings from the study suggest that there is a need to undertake continuous molecular surveillance of DENV, identifying the virus serotypes and genotypes in addition to the reporting of clinical dengue cases. This would help the relevant authorities design and strategise better control and prevention activities relevant to the specific potential outbreak areas.
It is cautioned here nonetheless, that the current study is limited to using samples obtained from patients with symptomatic DENV infections and among those who sought medical care at the UMMC. The asymptomatic or inapparent DENV cases were not captured. Hence, we cannot rule out the possibility that the DENV-3/III transmission magnitude in the population is actually much higher. Furthermore, the DENV neutralisation studies presented here are limited to the use of pooled serum samples as only limited volume of archived dengue serum samples were available, and it was not possible to assess the potential importance of the T-cell responses using these archived materials.
In summary, we described herein the recent introduction event of DENV-3/III into Malaysia and investigated factors that potentially affected its spread. The inability of DENV-3/III to cause major outbreaks in areas where DENV-3/I and DENV-1 were already present and the findings that the respective homotypic and heterotypic antibodies were effective in neutralising the virus highlights the potential importance of herd immunity in shaping DENV outbreaks. These findings could be useful especially in the development of models for vaccine deployment strategies when one becomes available in the future.
Financial support
This study was supported in parts by Ministry of Science, Technology and Innovation, Malaysia (www.mosti.gov.my; Malaysia Genome Institute Initiative grant: 07-05-MGI-GMB015), Ministry of Higher Education, Malaysia (www.mohe.gov.my; Long Term Research Grant Scheme grant: LRGS/TD/2011/UM/Penyakit Berjangkit) and University Malaya (www.um.edu.my; University Malaya Postgraduate Research Fund: PS152/2008C). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Declaration of interest
None.







