Type 2 diabetes mellitus (T2DM) is a chronic inflammatory disease that is mostly accompanied by many complications especially those related to the cardiovascular system(Reference Einarson, Acs and Ludwig1,Reference Akash, Rehman and Chen2) . CHD accounts as one of the common problems and the major cause of morbidity and mortality in diabetic patients(Reference Beckman, Creager and Libby3). The contributing factors of T2DM, including insulin resistance, dyslipidaemia and increased inflammatory cytokines, play important roles in the occurrence of endothelial damages and atherosclerosis(Reference Yahagi, Kolodgie and Lutter4).
Today, there is an increasing interest in applying nutritional interventions to provide antioxidant and anti-inflammatory effects in diabetic patients(Reference Abdali, Samson and Grover5). Fish oil is not always palatable and often causes belching complaint. While it may provide health-related benefits, patients’ compliance would be low. Because of the concerns regarding the palatability of fish oil, there is a need for alternative sources of n-3 fatty acids. Flaxseed oil is comprised of 50 % PUFA and is one of the richest vegetarian sources of α-linolenic acid(Reference Raper, Cronin and Exler6). Current evidence supports flaxseed oil as an advantageous dietary supplement for maintaining healthy cholesterol levels(Reference Prasad and Jadhav7,Reference Prasad8) . By incorporating flaxseed oil in the diet, a modest reduction was observed in both total- and LDL-cholesterol concentrations among healthy volunteers(Reference Cunnane, Hamadeh and Liede9,Reference Cunnane, Ganguli and Menard10) . Several studies have investigated the effects of flaxseed oil supplementation on endothelial function(Reference Dupasquier, Dibrov and Kneesh11,Reference Ren, Chen and Chen12) . In addition, in a meta-analysis conducted by Ren et al. (Reference Ren, Chen and Chen12), supplementation with flaxseed oil or its derivatives led to a significant reduction in C-reactive protein (CRP) levels in obese individuals. Moreover, flaxseed oil consumption by patients with metabolic disease led to promising effects on glucose and lipid metabolism. Previously, we observed that flaxseed oil supplementation significantly reduced insulin resistance in patients with diabetic foot ulcer(Reference Soleimani, Hashemdokht and Bahmani13). However, in another study, it did not show any beneficial effect on glycaemic control and lipid profiles in diabetic patients(Reference Zheng, Lin and Fang14). Flaxseed oil contains essential fatty acid that may inhibit the production of inflammatory markers(Reference Zhao, Etherton and Martin15) and affect mRNA expression of transcription factors involved in the metabolic pathways(Reference Devarshi, Jangale and Ghule16). On the other hand, current evidence indicates that serum levels of 25-hydroxyvitamin D (25(OH)D) are negatively associated with carotid intima–media thickness (CIMT)(Reference Wang and Zhang17). In addition, vitamin D supplementation improved markers of systemic inflammation in patients with chronic heart failure(Reference Jiang, Gu and Zhang18). Moreover, the results of meta-analysis studies revealed that vitamin D supplementation decreased insulin resistance(Reference Li, Liu and Zheng19) and improved a few markers of lipid profile in patients with T2DM(Reference Jafari, Fallah and Barani20). Vitamin D is involved in insulin sensitivity and glucose metabolism(Reference Maestro, Campion and Davila21). It also exerts anti-inflammatory and antifibrotic effects on vessels(Reference de Boer, Kestenbaum and Shoben22). n-3 Fatty acids have been shown to increase the concentrations of active form of vitamin D. Hence, the cardioprotective roles of n-3 fatty acids can partly be explained by their impact on activating vitamin D(Reference An, Lee and Son23). Recently, it is suggested that the combination of n-3 fatty acids and vitamin D may improve the function of pancreatic β-cells(Reference Baidal, Ricordi and Garcia-Contreras24).
Considering the existing evidence that vitamin D and n-3 fatty acids’ intake may have glucose-lowering and anti-inflammatory effects, we hypothesised that vitamin D and n-3 fatty acids’ co-supplementation might benefit diabetic patients with CHD. The present study was, therefore, performed to evaluate the effects of long-term vitamin D and n-3 fatty acids’ co-supplementation on CIMT, glucose homeostasis parameters, lipid concentrations and inflammatory markers in diabetic patients with CHD.
Materials and methods
Participants
The present study was a randomised, double-blinded, placebo-controlled trial that was registered in the Iranian registry of clinical trials (http://www.irct.ir:IRCT2017090133941N15) and was performed at a cardiology clinic affiliated to Kashan University of Medical Sciences (KAUMS), Kashan, Iran, between November 2017 and June 2018. Inclusion criteria were as follows: vitamin D-deficient (serum 25(OH)D levels in the range of 6·3–19·9 ng/ml) type 2 diabetic patients with diagnosed CHD and aged 45–85 years. Diabetes and CHD were diagnosed according to the criteria of American Diabetes Association(25) and American Heart Association(Reference Luepker, Apple and Christenson26). Exclusion criteria included those consuming vitamin D supplements and n-3 fatty acids within the last 3 months, who experienced an acute myocardial infarction or who underwent cardiac surgery within the past 3 months or with significant renal or hepatic failure.
Ethics statements
This investigation was conducted according to the principals of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of KAUMS. All subjects were informed about the aims and protocol of the study. Written informed consent was obtained from all subjects prior to the intervention.
Study design
At first, all participants were stratified according to age, BMI, sex, dosage and type of medications. Then participants were randomly allocated into two treatment groups either taking 50 000 IU vitamin D supplements every 2 weeks plus 2× 1000 mg/d n-3 fatty acids from flaxseed oil containing 400 mg α-linolenic acid in each capsule (n 30) or placebo (n 31) for 6 months. Vitamin D, n-3 fatty acids supplements and placebos were produced by Zahravi Pharmaceutical Company, Barij Essence Pharmaceutical Company and Barij Essence Pharmaceutical Company, respectively. Vitamin D, n-3 fatty acids supplements and placebos had similar packaging. Patients and researchers were unaware of the content of the package until the end of study. Quality control of vitamin D and n-3 fatty acids’ supplements was conducted in the laboratory of Food and Drug Administration in Tehran, Iran, by HPLC and GC methods, respectively. Patients were instructed to preserve their regular diet and levels of physical activity throughout the intervention. All participants completed the 3-d dietary records (two weekdays and one weekend) at baseline and at months 1, 3 and 6 of the trial. To calculate participants’ nutrient intakes using the 3-d food records, we applied Nutritionist IV software (First Databank) adopted for the Iranian food pattern. In the current study, physical activity was described as metabolic equivalents (MET) in h/d. To determine the MET for each patient, we multiplied the times (in h/d) of physical activity reported each day by its related MET coefficient using standard tables(Reference Ainsworth, Haskell and Whitt27).
Randomisation
Randomisation and allocation were blinded from the researcher and subjects until the main analyses were completed. At the cardiology clinic, a trained staff followed the randomised enrolment and assignment of the patients to the groups.
Treatment adherence
The consumption of supplements and placebos was monitored by examining the returned capsule containers as well as by evaluating the serum 25(OH)D levels using the ELISA method, at the beginning and end of the intervention.
Assessment of anthropometric measures
Patients’ weight and height (Seca) were measured without shoes and in minimal clothing at baseline and after intervention, in the cardiology clinic by a trained nutritionist. BMI was calculated as weight in kilograms divided by height in metres squared.
Assessment of outcomes
CIMT was considered as the primary outcome; and glycaemic control, lipid profile and inflammatory markers were considered as the secondary outcomes. At baseline and after the 12-week intervention, thickness was measured at the 2-cm distance of the common carotid bifurcation, by the same sonographist, using a Doppler ultrasonography device (Samsung Medison V20) with linear multi-frequencies of 7·5- to 10-MHz probe. The physician was blinded to patient-related clinical information.
Fasting blood samples (10 ml) were collected at the beginning and 6 months after intervention at Kashan reference laboratory. Blood was collected in two separate tubes: (1) one without EDTA to separate the serum to quantify serum insulin, lipid profile and high-sensitivity CRP (hs-CRP) concentrations and (2) another containing EDTA to separate plasma for measuring total nitrite. Since fasting plasma glucose (FPG) is not a stable marker, we measured it on the day blood was collected. Then the samples were stored at –80°C until the final analysis at the KAUMS’s reference laboratory. HbA1c levels in the whole blood was measured using a Glycomat kit (BiocodeHycel) with the exchange chromatography method. Serum 25(OH)D levels were assessed by an ELISA kit (IDS) with inter- and intra-assay CV below 7 %. Serum insulin levels were quantified using an ELISA kit (DiaMetra) with inter- and intra-assay CV below 5 %. The homeostatic model assessment of insulin resistance (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI) were determined according to the standard formula(Reference Pisprasert, Ingram and Lopez-Davila28). Enzymatic kits (Pars Azmun) were used to quantify FPG and lipid profile with inter- and intra-assay CV below 5 %. Serum hs-CRP levels were determined by an ELISA kit (LDN) with inter- and intra-assay CV below 7 %. The plasma total nitrite concentrations were measured using the Griess method(Reference Tatsch, Bochi and da Silva Pereira29) with CV below 5 %.
Sample size
In this study, we used randomised clinical trial sample size calculation formula where type one (α) and type two errors (β) were 0·05 and 0·20 (power = 80 %), respectively. According to the previous trial(Reference Karakas, Sahin and Urfali30), we used 0·16 mm as the SD and 0·116 mm as the change in mean (d) of the mean levels of left CIMT as the primary outcome. Based on the formula, we needed thirty subjects in each group; after allowing for seven dropouts in each group, the final sample size was thirty-seven persons in each group.
Statistical methods
The Kolmogorov–Smirnov test was applied to determine the normal distribution of the variables. The independent-samples t test was used to detect the differences in the general characteristics and daily dietary macro- and micronutrient intake between the two groups. The Pearson χ2 test was used for the comparison of categorical variables. Multiple linear regression models were used to assess treatment effects on the study outcomes after adjusting for confounding variables including the baseline values, age and BMI. The effect sizes were presented as the mean differences with 95 % CI. A P value below 0·05 was considered significant. All statistical analyses were performed by the Statistical Package for Social Science version 18 (SPSS Inc.).
Results
In the present study, seven participants in the supplemented group (moving to other city (n 4) or loss of interest for participation in the research (n 3)) and six in the placebo group (moving to other city (n 2) or loss of interest for participation in the research (n 4)) withdrew from the study and final analyses (Fig. 1). Finally, sixty-one patients (treatment (n 30) and placebo (n 31)) completed the trial. Overall, the compliance rate was high, such that more than 90 % of capsules were consumed throughout the study in both groups. Throughout the study, no side effects were reported while taking vitamin D, n-3 fatty acids and placebos in diabetic patients with CHD.
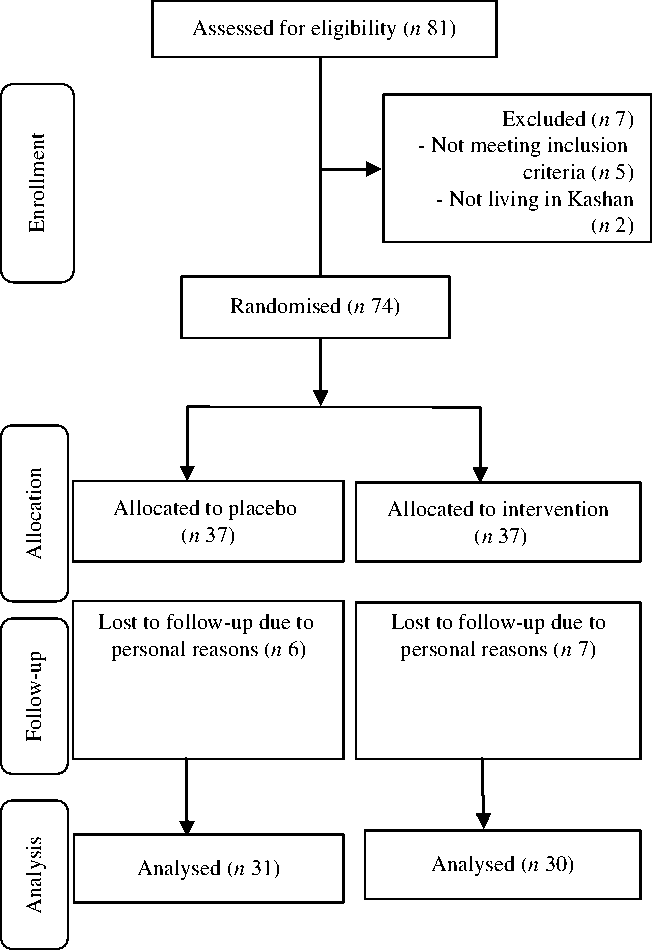
Fig. 1. Summary of patient flow diagram.
There were no significant differences between the two groups in terms of distribution of sex, mean age, height, baseline weight, baseline BMI and mean changes in weight and BMI during the trial (Table 1).
Table 1. General characteristics of study participants at baseline
(Mean values and standard deviations; numbers of participants and percentages)
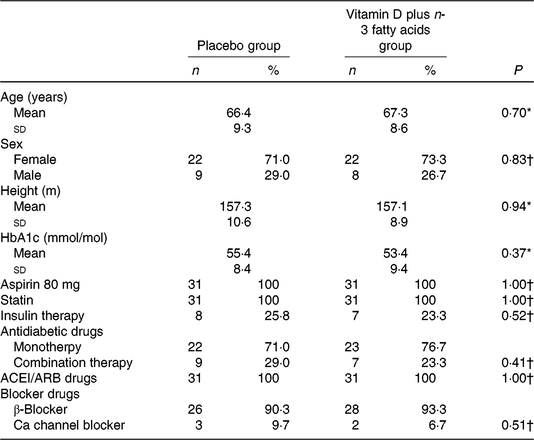
ACEI, angiotensin-converting enzyme inhibitors; ARB, aldosterone receptor blockers.
* Obtained from independent-samples t test.
† Obtained from Pearson’s χ2 test.
Based on the 3-d dietary records obtained at baseline and throughout the intervention, we observed no significant change in dietary macro- and micronutrient intake between the two groups (online Supplementary Table S1).
Vitamin D and n-3 fatty acids’ co-supplementation resulted in a significant reduction in mean (P = 0·01) and maximum levels of left CIMT (P = 0·004) and mean (P = 0·02) and maximum levels of right CIMT (P = 0·003) compared with the placebo (Table 2). In addition, vitamin D and n-3 fatty acids’ co-supplementation resulted in a significant increase in serum 25(OH)D levels (P < 0·001) and a significant reduction in FPG (P = 0·03), insulin (P < 0·001), HOMA-IR (P < 0·001), LDL-cholesterol (P = 0·04) and total-/HDL-cholesterol (P =0·01) and a significant increase in QUICKI (P = 0·001) and HDL-cholesterol (P = 0·02) compared with the placebo. Additionally, hs-CRP (P = 0·005) was significantly reduced in the supplemented group compared with the placebo group. We did not see any significant effect of co-supplementation on changes in other lipid concentrations and plasma total nitrite levels compared with the two intervention groups.
Table 2. Markers of cardiometabolic risk at baseline and 6 months after the intervention in type 2 diabetic patients with CHD
(Mean values and standard deviations; β coefficients and 95 % confidence intervals)
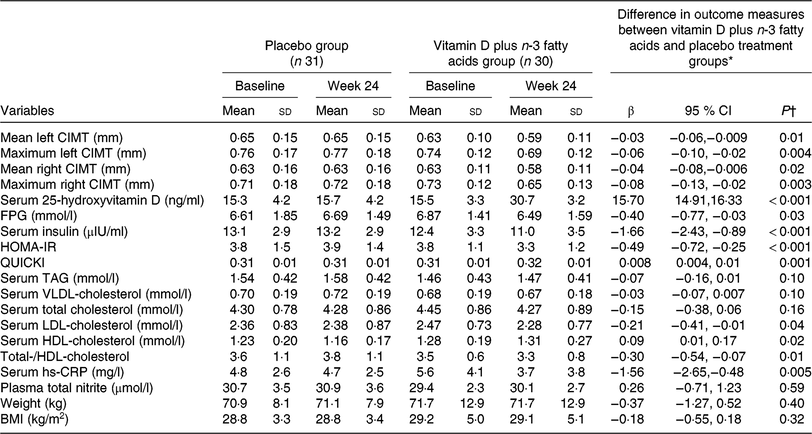
CIMT, carotid intima–media thickness; FPG, fasting plasma glucose; HOMA-IR, homeostatic model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; hs-CRP, high-sensitivity C-reactive protein.
* ‘Outcome measures’ refers to the change in values of measures of interest between baseline and week 24. β (Difference in the mean outcomes measures between treatment groups (vitamin D plus n-3 fatty acids group = 1 and placebo group = 0)).
† Obtained from multiple regression model (adjusted for baseline values of each biochemical variables, age and baseline BMI).
Discussion
In the present study, for the first time we evaluated the effects of 6-month vitamin D and n-3 fatty acids’ co-supplementation on CIMT, glucose homeostasis parameters, lipid concentrations and inflammatory markers among diabetic patients with CHD. We demonstrated that long-term vitamin D and n-3 fatty acids’ co-supplementation had beneficial effects on mean and maximum levels of left and right CIMT, glycaemic control, LDL-, HDL-, total-/HDL-cholesterol and hs-CRP levels among diabetic patients with CHD.
Effects on carotid intima–media thickness
Patients with diabetes and CHD are at higher risk of CIMT progression(Reference Katakami, Kaneto and Shimomura31), which is closely associated with dyslipidaemia and diabetic state(Reference Bots, Evans and Tegeler32). The present study demonstrated that 6-month vitamin D and n-3 fatty acids’ co-supplementation in diabetic patients with CHD significantly reduced mean and maximum levels of left and right CIMT compared with the placebo. Similarly, an 8-week trial of vitamin D supplementation to haemodialysis patients decreased CIMT(Reference Karakas, Sahin and Urfali30). Our previous study indicated that vitamins D and K and Ca co-supplementation reduced the maximum levels of left CIMT, yet did not change the mean levels of left and right CIMT in overweight T2DM patients(Reference Asemi, Raygan and Bahmani33). In another study, the consumption of flaxseed oil for 90 d reduced CIMT in obese and overweight non-diabetic elderly patients (Reference de Oliveira, Kovacs and Moreira34). However, vitamin D supplementation for 16 weeks did not change CIMT in patients with metabolic syndrome(Reference Salekzamani, Bavil and Mehralizadeh35). CIMT is a prognostic predictor of future cardiovascular events. Greater CIMT increases the risk of myocardial infarction(Reference Katakami, Kaneto and Shimomura31). Vitamin D may affect CIMT through the regulation of expression matrix Gla-poretin(Reference Fraser and Price36). It is indicated that flaxseed oil inhibited the atherosclerosis via antiproliferative and anti-inflammatory actions(Reference Dupasquier, Dibrov and Kneesh11). n-3 Fatty acids increase the active form of vitamin D levels, providing cardioprotective effects(Reference An, Lee and Son23).
Effects on glycaemic control and lipid profiles
We found that 6-month vitamin D supplementation in diabetic patients with CHD was associated with a significant reduction in FPG, insulin, HOMA-IR, LDL- and total-/HDL-cholesterol but a significant increase in QUICKI and HDL-cholesterol levels compared with the placebo but did not affect other lipid profiles. In a meta-analysis conducted by Li et al. (Reference Li, Liu and Zheng19), vitamin D supplementation reduced HOMA-IR in patients with T2DM. In another study, vitamin D supplementation to women with gestational diabetes mellitus decreased FPG, insulin levels, HOMA-IR and LDL-cholesterol but increased HDL-cholesterol levels(Reference Zhang, Gong and Xue37). Moreover, taking 10 g flaxseed pre-mixed in cookies twice per d by constipated patients with T2DM for 12 weeks was shown to improve FPG, LDL- and HDL-cholesterol levels(Reference Soltanian and Janghorbani38). Furthermore, a 12-week flaxseed oil supplementation was effective in improving insulin levels, HOMA-IR and QUICKI in patients with diabetic foot ulcer(Reference Soleimani, Hashemdokht and Bahmani13). However, flaxseed oil supplementation (2·5 g/d) for 6 months did not improve glycaemic control and lipid profiles in patients with T2DM(Reference Zheng, Lin and Fang14). In another study, 6-week vitamin D supplementation had no significant effect on glycaemic control and lipid profiles in overweight and obese women(Reference Khosravi, Kafeshani and Tavasoli39). Insulin resistance and dyslipidaemia play an important role in the exacerbation of atherosclerosis in diabetic patients(Reference Gärtner and Eigentler40). In addition, total-/HDL-cholesterol ratio is associated with the incidence of CHD events(Reference Ingelsson, Schaefer and Contois41). Flaxseed oil may decrease sterol regulatory element binding protein-1, which in turn reduces lipogenesis(Reference Devarshi, Jangale and Ghule16). Ingestion of n-3 fatty acids may improve lipid profiles through inhibiting the signalling pathway of phosphatidylinositol 3-kinase and protein kinase B(Reference Chen, Li and Chen42) and modulating the activities of oxidative stress-induced NF-κB pathway(Reference Huang, Liang and Han43). Taking n-3 fatty acids may also improve glycaemic control by modulating the secretion of adipocytokines and inflammatory markers and enhancing fatty acid β-oxidation(Reference Liu, Xue and Liu44). Improved insulin sensitivity and parathyroid hormone reduction following vitamin D intake might cause decreased lipid levels(Reference Wang, Xia and Yang45). Insulin decreases the biosynthesis of cholesterol via increased β-hydroxy β-methylglutaryl-CoA reductase activity(Reference Kaplan, Kerry and Aviram46). In addition, the beneficial effects of vitamin D on glycaemic control might be explained by its effect on Ca and P metabolism and by up-regulation of the insulin receptor genes(Reference Maestro, Molero and Bajo47) and increased transcription of insulin receptor genes(Reference Maestro, Molero and Bajo47). Moreover, the combination of n-3 fatty acids and vitamin D may improve the activity of pancreatic β-cells(Reference Baidal, Ricordi and Garcia-Contreras24).
Effects on inflammatory markers
This study showed that compared with the placebo, taking vitamin D plus n-3 supplements for 6 months by diabetic patients with CHD decreased hs-CRP but did not influence the plasma total nitrite levels. Similar to our findings, vitamin D supplementation lowered inflammatory markers in patients with chronic heart failure(Reference Jiang, Gu and Zhang18). In addition, vitamin D supplementation significantly reduced CRP concentrations in women with gestational diabetes mellitus(Reference Zhang, Gong and Xue37) and patients with T2DM(Reference Mousa, Naderpoor and Teede48). Moreover, the 8-week flaxseed oil supplementation led to a significant decrease in CRP levels in haemodialysis patients(Reference Mirfatahi, Tabibi and Nasrollahi49). The consumption of flaxseed reduced inflammatory markers in patients with coronary artery disease(Reference Khandouzi, Zahedmehr and Mohammadzadeh50). Furthermore, taking flaxseed oil with isolated soya protein for 3 weeks lowered blood CRP values in burn patients(Reference Babajafari, Akhlaghi and Mazloomi51). Although in another study, 6-week supplementation with flaxseed oil (containing 3·5 g α-linolenic acid) did not affect hs-CRP levels in polycystic ovary syndrome patients(Reference Vargas, Almario and Buchan52). In addition, vitamin D supplementation for 6 weeks did not change CRP values in overweight and obese women(Reference Khosravi, Kafeshani and Tavasoli39). It is suggested that elevated CRP levels may be one of the factors causing vascular endothelial dysfunction in diabetic patients with CHD(Reference Zhang, Zhang and Zhao53). The mechanism by which flaxseed oil lowers inflammatory markers is attributed to the activation of PPAR-γ(Reference Zhao, Etherton and Martin54) and down-regulation of NF-κB(Reference Jangale, Devarshi and Dubal55). Intake of n-3 fatty acid may provide substrates for the synthesis of the proinflammatory lipid mediator’s protectins and reduce adipokines through the inhibition of NF-κB signalling(Reference Trayhurn and Wood56,Reference Ajuwon and Spurlock57) . Up-regulation of PPAR-γ expression by n-3 fatty acids may inhibit TNF-α and IL-1β-induced NF-κB transcriptional activity in skeletal muscle cells(Reference Remels, Langen and Gosker58). Moreover, vitamin D modulates inflammatory response through the regulation of proinflammatory gene and interference with NF-κB and mitogen-activated protein kinase cascade(Reference Wobke, Sorg and Steinhilber59).
The present study had a few limitations. Due to the limited funding, we could not assess the effects of vitamin D and n-3 fatty acids’ co-supplementation on circulating fatty acids. In addition, we did not evaluate gene expression related to insulin, lipids and inflammation. We did not control for sun exposure in a day-to-day basis among study participants; therefore, this point has been considered in the interpretation of our findings. Furthermore, we could not evaluate HbA1c levels at the end of trial. Most study participants were female of the older age, so current findings cannot be generalised and may be more reflective of the older women population.
Conclusions
In summary, vitamin D and n-3 fatty acids’ co-supplementation for 6 months had beneficial effects on the markers of cardiometabolic risk among diabetic patients with CHD.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519001132
Acknowledgements
The present study was funded by a grant from the Vice-Chancellor for Research, KAUMS, Iran.
Z. A. contributed to conception, design, statistical analysis and drafting of the manuscript. H.-R. T., V. N., F. R., V. O., N. M., E. A., M. T., M. H. and R. Sh. contributed to data collection and manuscript drafting. All authors approved the final version for submission. Z. A. supervised the study. All authors confirmed the final version for submission.
No conflicts of interest are declared.






