Whether the brain interprets somatosensory inputs that are encoded by particular afferent activity patterns or that transmit via modality specific, potentially anatomically segregated, labelled lines has been long debated.Reference Ma 1 According to the labelled line theory of somatosensation specialised receptors in the periphery capture specific attributes of a stimulus which is then conveyed to cortical regions, such as the somatosensory cortex. According to this theory, ascending spinal pathways also carry this sensory information in specific labelled lines.
The spinothalamic tract (STT), which ascends the anterolateral funiculus of the spinal cord, is long-established as the second-order pathway conveying information about innocuous cutaneous temperature and noxious mechanical and thermal stimulation from the dorsal horn of the spinal cord. Indeed STT lesioning with anterolateral cordotomy is used as a palliative procedure to ameliorate contralateral, intractable cancer-related pain.Reference Tasker 2
We present a case of a 62-year-old man who, following an anterolateral cordotomy, developed dissociated spinothalamic sensory loss implying segregation of ascending thermal and mechanical nociceptive pathways. The case is discussed in relation to both its clinical relevance and the labelled line theory of somatosensation.
Nine years following the initial mesothelioma diagnosis our patient developed chest wall bony destruction and pain affecting the left hemithorax and axilla. The pain was initially successfully controlled with chest wall radiotherapy and opioid analgesia. However, with disease progression, it became refractory to opioids. He was referred to our unit for consideration of palliative percutaneous anterolateral cordotomy. The pain was described as a persistent dull ache with severe paroxysms of sharp, shooting pain typically precipitated by movement. On examination there was a small area of dynamic tactile allodynia around the left nipple (Figure 1A). Otherwise the neurological examination was normal. His analgesic regime at presentation consisted of morphine slow release 500 mg twice daily, morphine immediate release liquid 100 mg up to five times day, amitryptiline 20 mg at night, paracetamol 4 g/day, and ibuprofen 1.2 g/day.
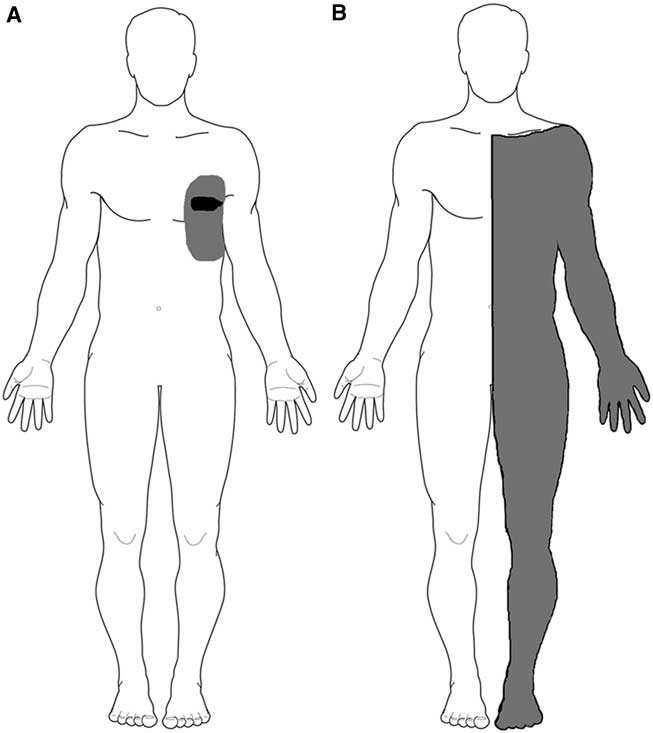
Figure 1 (A) Pre-lesion map of the region affected by the cancer-related pain (grey shading) and tactile allodynia (black shading); (B) post-lesion deficit to pinprick (grey shading).
A right-sided percutaneous cervical cordotomy between first and second cervical vertebrae was performed. The cordotomy electrode was inserted through a 20-gauge spinal needle into anterolateral spinal cord. Sensory stimulation through the cordotomy electrode at 50 Hz, rather than inducing the typical intense and unpleasant heat or cold sensation,Reference Lahuerta, Bowsher and Lipton 3 reproducibly evoked an unusual poorly described, although not unpleasant, cutaneous sensation over the left chest wall and upper limb, centred on the area of allodynia. No evoked motor phenomena were observed at 2 Hz stimulation indicating that the corticospinal tract was remote to the electrode tip. Three incremental heat lesions, each lasting 25 seconds, were performed at 75°C, 80°C, and 85°C. The patient described immediate and complete pain relief in his left chest wall. No demonstrable clinical deficits to temperature sensation were elicited in the operating room. It was decided not to progress to any further lesioning. On post-cordotomy review, the patient remained pain-free with no objective evidence of altered thermal perception. Pinprick sensation was lost in the left C4-S1 dermatomes (Figure 1B). Thermal detection and pain thresholds, performed with a Medoc II TSA (Medoc Ltd., Ramat Yishai, Israel) using the method of limits, demonstrated normal cold and warm detection thresholds in the left forearm (C6), chest wall (T2), and foot dorsum (L5) on both sides. Heat pain thresholds also showed no significant side-to-side asymmetry. Cold pain thresholds were at floor level bilaterally (Table 1).
Table 1 Pre- and post-lesion results for thermal detection and thermal pain thresholds
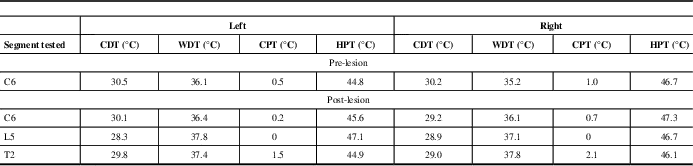
CDT=cold detection threshold; WDT=warm detection threshold; CPT=cold pain threshold; HPT=heat pain threshold.
The baseline thermode temperature was 32°C.
Pre-lesion studies were only performed in the arm (C6).
The patient had complete pain relief sustained at 4 weeks follow-up and indeed had discontinued all opioid and non-opioid analgesia.
Although the anterolateral cordotomy procedure typically produces contralateral deficits in pain and temperature sensation in the same segmental distribution,Reference Tasker 2 dissociations in STT modalities have been described previously in a small number of patients.Reference Friehs, Schröttner and Pendl 4 , Reference Bowsher 5 BowsherReference Bowsher 5 investigated STT sensory modalities using quantitative methods in a series of patients following anterolateral cordotomy. In some patients, pain relief and loss of mechanical pain to pinprick stimuli was associated with preserved cold and/or warm sensation. A single patient exhibited preservation of heat pain as well as innocuous heat and cold sensation. Evidence of segregation within ascending thermal and mechanical nociceptive pathways is also provided by microstimulation studies of thalamic nuclei during deep brain stimulation surgery in humansReference Lenz, Seike and Richardson 6 as well as in patients with lesions of the brainstem and thalamus.Reference Bowsher 5 Our patient also demonstrated a dissociation in nociceptive mechanical and heat sensation. Although a deal of interaction and processing of diverse modality-specific primary afferent inputs probably occurs in the spinal cord,Reference Ma 1 this case further demonstrates that there remains anatomical-functional segregation in broadly labelled lines within the STT.
It has been argued that more ventrally placed STT lesions will preferentially disrupt ascending nociceptive fibres, whilst sparing thermal pathways.Reference Friehs, Schröttner and Pendl 4 Indeed more ventral and medial lesions are associated with greater pain relief and loss of mechanical pain sensation.Reference Lahuerta, Bowsher and Lipton 3 In the current case neither neuroimaging nor pathological information is available to support a ventromedial lesion. However, a more ventrally placed lesion may be suggested by the unusual non-painful, non-temperature-related cutaneous sensation elicited during 50 Hz high-frequency stimulation, the nature of which suggests activation of ascending non-nociceptive pathways. This potentially reflects activation of the ventral STT. Although poorly described in humans fibres destined for the ventral STT in cats arise in lamina VII and VIII of the dorsal horn and have been shown to respond to both innocuous as well as noxious range mechanical stimuli to deep and superficial tissues from the contralateral body side.Reference Meyers, Kelly and Snow 7 It is also possible that the evoked sensations could reflect stimulation of ascending second-order pathways of primary mechanosensitive low threshold A-δ or C-tactile afferent fibres.Reference McGlone, Wessberg and Olausson 8
The findings have potential clinical implications. An appropriately placed lesion may produce a beneficial outcome without sacrifice of temperature, including thermo-noxious pathways. Furthermore the absence of clear deficits in cold sensation in the operating room does not necessarily imply that additional lesions, with the associated potential risk of motor tract damage, are required. Careful questioning about ongoing pain and assessment of noxious mechanical stimulation are of importance in this respect.
Acknowledgements
We would like to thank the operating theatre medical and nursing staff for their assistance with the procedure.
Disclosure
MLS, KM, FPM, MG, and AGM have nothing to disclose.




