Gestational diabetes mellitus (GDM) is a form of hyperglycaemia that is first recognised during pregnancy. GDM is widely debated in view of steadily rising trend in case and constituting a major economic burden as it increased the risk for the offspring to develop obesity, as well as the risk for both mothers and children to develop type 2 diabetes mellitus later in life( Reference Reece, Leguizamon and Wiznitzer 1 – Reference Bellamy, Casas and Hingorani 4 ). Although effective strategies to prevent GDM are mandatory, they are inadequate. Many factors have been shown to increase GDM risk, such as pre-pregnancy overweight or obesity, advanced maternal age, inadequate physical activity, multiparous status and first-degree family history of diabetes( Reference Reece, Leguizamon and Wiznitzer 1 , Reference Catalano, McIntyre and Cruickshank 5 – Reference Boudet-Berquier, Salanave and Desenclos 9 ). Furthermore, epidemiological studies have found a link between oxidative stress and GDM( Reference Coughlan, Vervaart and Permezel 10 ), which indicated that dietary antioxidant nutrition may play a critical protective role against GDM development( Reference Zhang, Williams and Sorensen 11 – Reference Bo, Lezo and Menato 13 ).
Recently, carotenoids have received considerable interest due to their biological antioxidant activity in maintaining positive health( Reference Zielinska, Wesolowska and Pawlus 14 , Reference Fiedor and Burda 15 ). Carotenoids, a group of natural fat-soluble pigments, are abundant in dark, green leafy and yellow-orange vegetables and fruits( Reference Couillard, Lemieux and Vohl 16 ). More than 600 carotenoids have so far been identified in nature, but six carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lycopene and lutein/zeaxanthin) account for more than 95 % of total blood carotenoids( Reference Fiedor and Burda 15 ).
Observational studies have suggested that carotenoids play a protective role in preventing chronic diseases, such as cancer, cardiovascular diseases and metabolic syndrome( Reference Zhou, Wang and Meng 17 – Reference Liu, Shi and Cao 19 ). Meanwhile, large numbers of studies have shown the protective effect of carotenoids on diabetes risk( Reference Montonen, Knekt and Jarvinen 20 – Reference Akbaraly, Fontbonne and Favier 22 ), but the findings remained inconclusive. Randomised controlled trials with β-carotene supplementation consistently showed no effect on diabetes( Reference Song, Cook and Albert 23 – Reference Kataja-Tuomola, Sundell and Mannisto 25 ). A cross-sectional study showed an inverse relationship between dietary lycopene and fasting plasma glucose concentration( Reference Ylonen, Alfthan and Groop 26 ), and three cohort studies found no association with diabetes among the general population or male smokers( Reference Montonen, Knekt and Jarvinen 20 , Reference Wang, Liu and Manson 27 , Reference Kataja-Tuomola, Kontto and Mannisto 28 ). The latest meta-analysis confirmed no significant association between lutein intake and diabetes risk( Reference Leermakers, Darweesh and Baena 29 ).
Considerable evidence indicates that GDM and type 2 diabetes share a common pathogenesis as insulin resistance or β-cell progressive dysfunction( Reference Law and Zhang 30 ). However, research on the effect of carotenoids on GDM is limited. We found a single case–control study that suggested no impact of β-carotene on GDM( Reference Parast and Paknahad 31 ). Thus, we aimed to investigate whether higher dietary carotenoid intake during mid-trimester pregnancy could reduce the risk of GDM.
Methods
Study design and population
The present study population was drawn from the Tongji Maternal and Child Health Cohort (TMCHC) study, a prospective cohort study conducted from September 2013 to May 2016 at three public hospitals in Wuhan, Hubei Province, China. The study aimed to determine the impact of maternal diet and lifestyle on pregnancy outcomes. The inclusion criteria were: (1) gestational age from 8 to 16 weeks, (2) maternal age >18 years, (3) no communication problem and (4) resident of Wuhan. Face-to-face interviews on the socioeconomic characteristics, dietary intake and lifestyles of the participants during each trimester were conducted by trained investigators. An oral glucose tolerance test (OGTT) was conducted at 24–28 weeks to diagnose GDM. All procedures performed in the present study were in accordance with the ethical standards of the Ethics Review Committee of Tongji Medical College of Huazhong University of Science and Technology (no. 201302). All participants provided informed written consent upon recruitment. This study was registered at clinicaltrials.gov as NCT03099837.
We collected the dietary data of 2750 participants by a researcher-administered semi-quantitative FFQ survey during 20–28 weeks, but subjects with unavailable dietary data (blank items > 10 on FFQ: n 8, missing values for any vegetables or fruits: n 170, extreme energy (<700 kcal (<2930 kJ) or >5000 kcal (>20920 kJ)): n 2), unavailable OGTT data (missing OGTT data: n 198, OGTT performed before FFQ: n 321), multiple pregnancies (n 59) and pre-gestational diabetes (n 14) were excluded. Finally, the data of 1978 participants were obtained and analysed (online Supplementary Fig. S1).
Dietary assessment
Dietary data were collected using an FFQ before OGTT was conducted. The developed FFQ consisted of sixty-one food items, which were assembled into ten food groups: cereals, meat, fish, eggs, beans, vegetables, fruits, milk and milk products, nuts and beverages. The vegetable and fruit food groups were used to assess the dietary carotenoid intake. The vegetable group mainly included dark green leafy vegetables, light leafy vegetables, carrots and pumpkins, fresh beans, melons and solanaceous vegetables, including tomatoes and peppers. The fruit group mainly included citrus fruits, pomes, bananas, berries, watermelons and mangoes( Reference Zhang, Qiu and Zhong 32 ). Participants were asked to report their usual food consumption during the past 4 weeks, including the type of food, frequency and amount. Daily energy and nutrient consumption (except for carotenoids) were calculated using a dietary software based on the China Food Composition Database( Reference Yang 33 ); carotenoids intake was calculated based on the United States Department of Agriculture Nutrient Database( 34 ). The FFQ was a reasonably reliable and valid tool to investigate the diet of urban participants, as the reproducibility and validity coefficients of food and nutrition were all statistically significant( Reference Zhang, Qiu and Zhong 32 ). The correlation coefficients between two FFQ were 0·34 for α-carotene, 0·37 for β-carotene, 0·38 for β-cryptoxanthin, 0·36 for lycopene and 0·31 for lutein/zeaxanthin. The de-attenuated correlation coefficients between the average of two FFQ and three 24 h recalls were 0·33 for α-carotene, 0·37 for β-carotene, 0·41 for β-cryptoxanthin, 0·45 for lycopene and 0·40 for lutein/zeaxanthin (online Supplementary Tables S1 and S2).
Assessment of other covariates
We collected information on socioeconomic characteristics (age, ethnicity, education and income level, gravidity, parity and family history of diabetes), and anthropometric data including height, pre-pregnancy weight, weight at enrolment and weight at OGTT screening, systolic blood pressure and diastolic blood pressure at enrolment, and lifestyle habits such as smoking status and alcohol consumption before pregnancy, physical activity, sleep status and dietary supplements use during mid-trimester. Pre-pregnancy BMI (pre-BMI) was calculated as pre-pregnancy weight divided by height squared (kg/m2). Pre-pregnancy weight was self-reported, and height was measured at the first antenatal visit. If pre-pregnancy weight was missing, it was substituted with the enrolment weight. We examined two weight gain rate patterns before OGTT screening: the first trimester weight gain rate was measured as the enrolment weight minus pre-pregnancy weight and divided by the gestational age at enrolment; the second trimester weight gain rate was measured as the OGTT screening weight minus the enrolment weight and divided by the gestational age at OGTT screening minus gestational age at enrolment. If the OGTT screening weight was missing, it was replaced with the weight closest to the screening and no more than 3 weeks before or 1 week after the screening. Sleep time referred to the duration of night time sleep during pregnancy. Maternal sleep quality during pregnancy was classified as ‘never’, ‘occasionally’, ‘sometimes’ and ‘often insomniac’; poor sleep quality referred to those participants who selected the answer ‘often insomniac’. No participants used pure carotenoids supplementation in our study, so we recorded the use of compound supplements containing vitamin A, vitamin C or vitamin E during mid-pregnancy.
Oral glucose tolerance test assessment
All participants received routine screening by means of 75 g 2-h OGTT after at least 8 h overnight fast at 24–28 gestational weeks. Fasting blood glucose (FBG), 1-h postload blood glucose (PBG) and 2-h PBG concentration were collected from the laboratory test report. The International Association of Diabetes and Pregnancy Study Groups (IADPSG) recommends GDM to be diagnosed if one or more of the following criteria are met: FBG concentration ≥ 5·1 mmol/l, 1-h PBG concentration ≥ 10·0 mmol/l and 2-h PBG concentration ≥ 8·5 mmol/l( Reference Metzger 35 ).
Statistical analysis
Demographic characteristics were described as means for parametrically distributed data, medians for non-parametrically distributed data and percentages for categorical data. Characteristics among quartile groups of carotenoids were compared using ANOVA for normally distributed variables, or the Kruskal–Wallis rank test for non-normally distributed variables, and the χ2 test for categorical variables. All dietary nutrients and carotenoids intake were energy adjusted using the residual method( Reference Willett and Stampfer 36 ).
We demonstrated the correlation between dietary carotenoids intake and GDM prevalence by scatterplot smoothing. Binary logistic regression analysis was applied to evaluate the OR of GDM among different dietary carotenoids intake quartiles and per unit (1 mg) increment of dietary carotenoids intake. Linear trends were evaluated by creating a continuous variable for carotenoids using the median value of each quartile. Furthermore, multivariate linear regression analysis was adopted to demonstrate the relationship between lycopene consumption and FBG, 1-h and 2-h PBG concentration, and the results were presented as β with 95 % CI. We then demonstrated the association of carotenoids and GDM risk in subgroups including the age group (≤28 years or >28 years), pre-BMI group (<24·0 kg/m2 or ≥24 kg/m2) and gravidity group (1 or ≥ 2). The interactions between these stratification variables and carotenoids intake were tested using the multivariate logistic regression model, and the likelihood ratio test was used to assess the statistical significance of the interaction terms. All analyses used the same covariates, with some of them reported as common confounders or GDM factors( Reference Reece, Leguizamon and Wiznitzer 1 , Reference Catalano, McIntyre and Cruickshank 5 – Reference Boudet-Berquier, Salanave and Desenclos 9 , Reference Zhang, Williams and Sorensen 11 , Reference Ley, Hanley and Sermer 12 , Reference MacDonald, Bodnar and Himes 37 , Reference Zhang and Rawal 38 ), and were those changed the effect estimate over 10 % for corresponding models. In addition, the season of FFQ was adjusted to account for the variation in diet for different seasons. The first model was adjusted for maternal age, pre-BMI, first trimester weight gain rate, second trimester weight gain rate, systolic blood pressure, diastolic blood pressure, gravidity, parity, family history of diabetes, smoking and alcohol use status, physical activity and poor sleep quality. The second model was further adjusted for the gestational age of FFQ, season of FFQ and dietary intake of vitamin C, vitamin E, fibre, cholesterol, retinol, Se, Zn, Mg, Fe and Cu intake, which were all adjusted for energy.
All data were analysed using statistical packages R (The R Foundation; http://www.r-project.org; v. 3.1.2) and Empower(R) (http://www.empowerstats.com; X&Y Solutions Inc.). All tests were conducted assuming a two-sided alternative hypothesis and P < 0·05 was considered statistically significant.
Results
In the present study, 1978 participants were included for analysis (age: 28·1 ± 3·5 years and pre-BMI: 20·7 ± 2·6 kg/m2) and 152 (7·7 %) were diagnosed GDM. Median intakes were as follows: α-carotene, 0·277 (interquartile range (IQR) 0·135, 0·650) mg/d, β-carotene, 3·091 (IQR 2·089, 4·566) mg/d, β-cryptoxanthin, 0·293 (IQR 0·156, 0·477) mg/d, lycopene 2·082 (IQR 0·145, 5·266) mg/d and lutein/zeaxanthin, 2·499 (IQR 1·572, 3·803) mg/d. Table 1 shows the distribution of socio-economic, anthropometric and lifestyle characteristics and dietary intake of the sample according to quartiles of lycopene intake. The characteristics of other carotenoids are shown in online Supplementary Table S3. Subjects with higher lycopene intake had higher vitamin C, Mg, Fe and Cu consumption, as well as lower second trimester weight gain rate, GDM prevalence and FBG.
Table 1. Basic characteristics of study subjects by quartiles (Q) of dietary lycopene intake from the Tongji Maternal and Children Health Cohort (TMCHC) study
(Mean values, median values; numbers of participants; percentages)
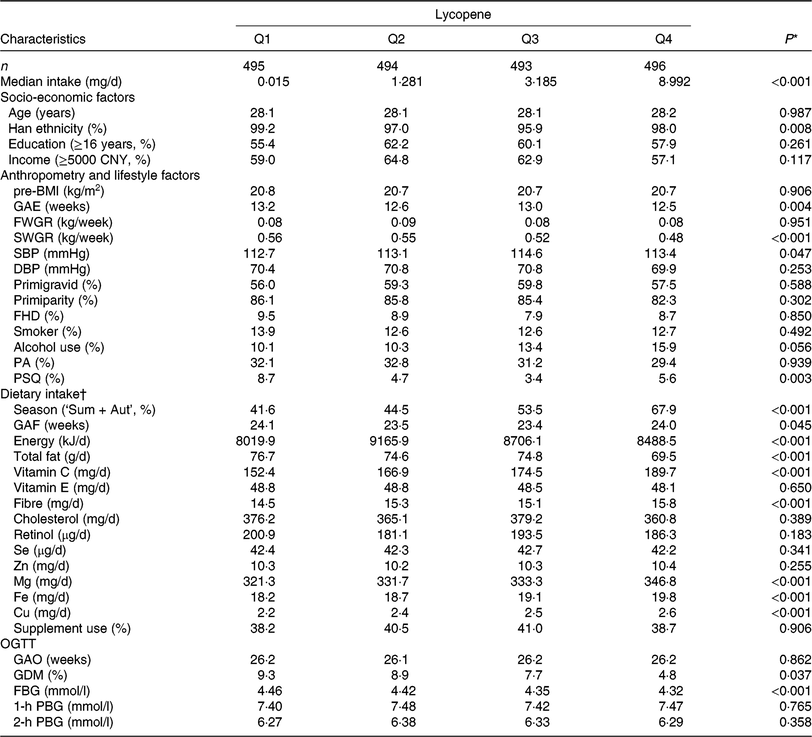
pre-BMI, pre-pregnancy BMI; GAE, gestational age at enrolment; FWGR, first trimester weight gain rate; SWGR, second trimester weight gain rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; FHD, family history of diabetes; PA, physical activity; PSQ, poor sleep quality; Sum + Aut, summer and autumn; GAF, gestational age at FFQ survey; OGTT, oral glucose tolerance test; GAO, gestational age at OGTT screening; GDM, gestational diabetes mellitus; FBG, fasting blood glucose; PBG, postload blood glucose.
* ANOVA or χ2 test or the Kruskal–Wallis rank test was used as appropriate.
† Values of dietary intake were medians and were energy-adjusted by residual methods (except for energy).
The associations between carotenoids intake and GDM prevalence by scatterplot smoothing were displayed in online Supplementary Fig. S2. An inverse correlation was observed between lycopene intake and GDM risk (P = 0·032) after adjusting for covariates. OR for GDM risk according to quartiles of carotenoids consumption are shown in Table 2. In model 1 logistic regression analyses, we found that the OR for the highest v. the lowest quartiles of β-cryptoxanthin and lycopene were 0·61 (95 % CI 0·38, 0·98; P for trend = 0·084) and 0·50 (95 % CI 0·30, 0·84; P for trend = 0·005), respectively. After further adjusting for potential confounders, the protective effect of β-cryptoxanthin was attenuated (OR 0·65; 95 % CI 0·35, 1·21; P for trend = 0·278), but the effect of lycopene was still significant (OR 0·50; 95 % CI 0·29, 0·86; P for trend = 0·007); each 1 mg increase in lycopene consumption was associated with 5 % (OR 0·95; 95 % CI 0·91, 0·99; P for trend = 0·020) decrease in GDM risk. However, no significant effect of α-carotene, β-carotene and lutein/zeaxanthin on GDM risk was found in our research.
Table 2. Association of dietary carotenoids intake with gestational diabetes mellitus (GDM) risk
(Odds ratios and 95 % confidence intervals)
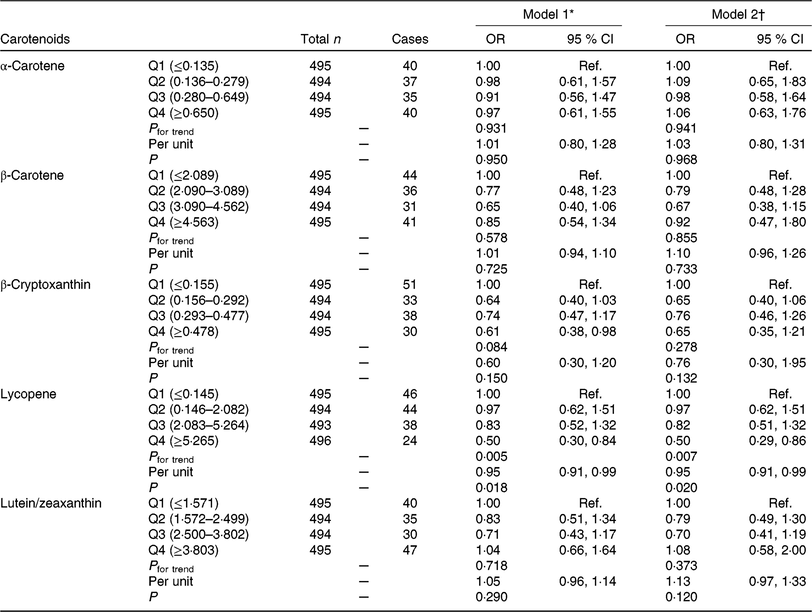
Q, quartile; Ref., reference; pre-BMI, pre-pregnancy BMI; FWGR, first trimester weight gain rate; SWGR, second trimester weight gain rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; FHD, family history of diabetes; PA, physical activity; PSQ, poor sleep quality; GAF, gestational age at FFQ survey; Sum + Aut, summer and autumn.
* Model 1 was adjusted for maternal age, pre-BMI, FWGR, SWGR, SBP, DBP, gravidity (1 as reference), parity (0 as reference), FHD (‘no’ as reference), smoking status (‘no’ as reference), alcohol use status (‘no’ as reference), PA (‘yes’ as reference), PSQ (‘no’ as reference).
† Model 2 was further adjusted for GAF, season of FFQ (‘Sum + Aut’ as reference), supplement use (‘no’ as reference), as well as for vitamin C, vitamin E, fibre, cholesterol, retinol, Se, Zn, Mg, Fe and Cu intake, which were all adjusted for energy.
Table 3 presents further multivariate linear regression analysis to examine the impact of lycopene consumption on blood glucose concentration. Compared with the lowest lycopene intake quartile, the β-coefficients across quartiles of lycopene intake with FBG were −0·032 (95 % CI −0·074, 0·011), −0·105 (95 % CI −0·148, −0·062) and −0·119 (95 % CI −0·163, −0·075) (P for trend < 0·001) after adjusting for potential confounders; each 1 mg increase in lycopene intake was associated with 0·005 (95 % CI 0·002, 0·007) mmol/l decrease in FBG concentration (P < 0·001). No significant associations were observed between lycopene intake and 1-h or 2-h PBG concentration.
Table 3. Association between dietary lycopene intake and blood glucose
(β-Coefficients and 95 % confidence intervals)

Q, quartile; FBG, fasting blood glucose; PBG, postload blood glucose; pre-BMI, pre-pregnancy BMI; FWGR, first trimester weight gain rate; SWGR, second trimester weight gain rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; FHD, family history of diabetes; PA, physical activity; PSQ, poor sleep quality; GAF, gestational age at FFQ survey; Sum + Aut, summer and autumn.
* Model 1 was adjusted for maternal age, pre-BMI, FWGR, SWGR, SBP, DBP, gravidity (1 as reference), parity (0 as reference), FHD (‘no’ as reference), smoking status (‘no’ as reference), alcohol use status (‘no’ as reference), PA (‘yes’ as reference), PSQ (‘no’ as reference).
† Model 2 was further adjusted for GAF, season of FFQ (‘Sum + Aut’ as reference), supplement use (‘no’ as reference), as well as for vitamin C, vitamin E, fibre, cholesterol, retinol, Se, Zn, Mg, Fe and Cu intake, which were all adjusted for energy.
Stratified analysis was used to examine potential modification, according to age, pre-BMI and gravidity strata (Fig. 1). The protective effect of lycopene against GDM was consistent for each age and pre-BMI subgroups. However, the primigravid women was found to exhibit enhanced protection (OR 0·20; 95 % CI 0·07, 0·55 in the highest v. the lowest quartile of intake; P for interaction = 0·036).
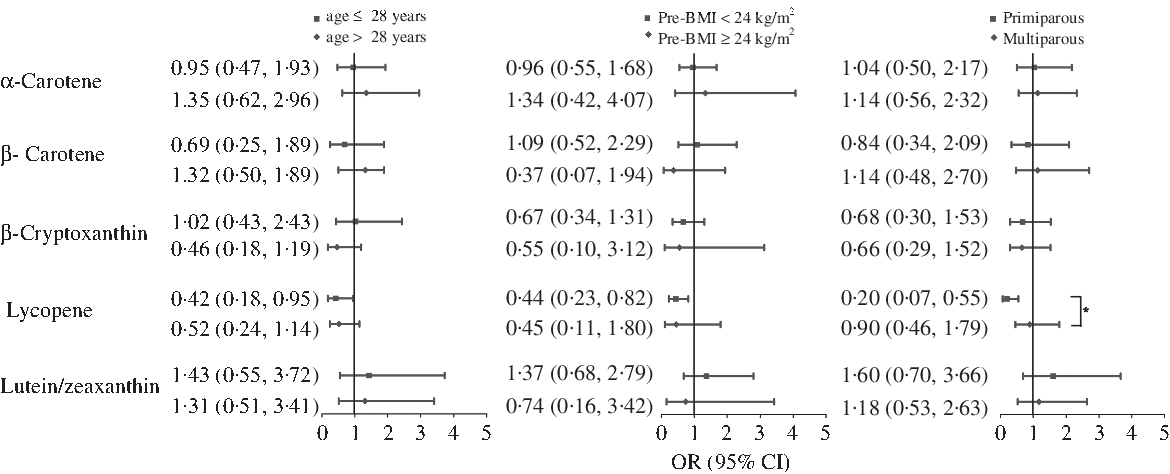
Fig. 1. Multivariate-adjusted OR (95 % CI) of gestational diabetes mellitus by age, pre-pregnancy BMI (pre-BMI) and gravidity, comparing quartile 4 with quartile 1 of dietary intake of carotenoids. Model adjusted for maternal age, pre-BMI, first trimester weight gain rate, second trimester weight gain rate, systolic blood pressure, diastolic blood pressure, gravidity (1 as reference), parity (0 as reference), family history of diabetes (‘no’ as reference), smoking status (‘no’ as reference), alcohol use status (‘no’ as reference), physical activity (‘yes’ as reference), poor sleep quality (‘no’ as reference) and further adjusted for gestational age at FFQ survey, season of FFQ (‘summer + autumn’ as reference), supplement use (‘no’ as reference), as well as for vitamin C, vitamin E, fibre, cholesterol, retinol, selenium, zinc, magnesium, iron and copper intake, which were all adjusted for energy. *P for interaction< 0·05.
Discussion
In this large cross-sectional study, we established that dietary lycopene intake, rather than the intake of other carotenoids, was inversely associated with GDM risk after adjusting for a wide range of potentially confounding factors. Furthermore, the protection was mainly assumed due to reducing FBG, instead of 1-h and 2-h PBG. Notably, the observed protective effect was stronger in primigravid than in multiple pregnancies women.
To the best of our knowledge, no large-scale study has demonstrated the association between carotenoids consumption and GDM risk. In fact, carotenoids, the marker of fruits and vegetables intake, has less been focused on maternal health. As aforementioned, only one case–control study (n 80) revealed no intergroup difference of β-carotene intake between GDM and non-GDM groups( Reference Parast and Paknahad 31 ), which was consistent with our results. Another nested case–control study confirmed the protective effect of serum lutein and zeaxanthin on maternal health, but the outcome was preeclampsia( Reference Cohen, Kramer and Platt 39 ). We found no significant association between dietary α-carotene, β-carotene, β-cryptoxanthin and lutein/zeaxanthin intake and GDM risk, which was consistent with most previously mentioned studies on type 2 diabetes( Reference Song, Cook and Albert 23 – Reference Ylonen, Alfthan and Groop 26 , Reference Leermakers, Darweesh and Baena 29 ).
Our finding that lycopene intake was significantly associated with a reduced risk of GDM was supported by previous studies. Based on reported studies, a daily lycopene intake of 5–7 mg in normal healthy human may be sufficient to maintain circulating levels, combat oxidative stress and prevent chronic diseases( Reference Rao and Shen 40 ). In this study, the median of lycopene intake in the highest quartile was 8·992 mg/d, which indicated the intake may be enough for pregnant women to prevent GDM. Intriguingly, the protection was mainly manifested via reducing FBG, instead of 1-h or 2-h PBG, which was in line with the previous study. The cross-sectional study of Ylonen et al. suggested that a higher lycopene intake was related to lower FBG concentration( Reference Ylonen, Alfthan and Groop 26 ). By contrast, a multi-centre study proposed that serum lycopene was inversely related to PBG concentration( Reference Coyne, Ibiebele and Baade 41 ). However, many studies have found no relationship between lycopene and diabetes, which maybe due to differences in study design, population, range of carotenoids and sample size. For instance, one nested case–control study confirmed a crude inverse relationship between plasma lycopene and type 2 diabetes, but the effect was attenuated after adjusting for potential confounding factors( Reference Wang, Liu and Pradhan 42 ).
Although the mechanism of the association between dietary lycopene intake and GDM risk has yet not to be clarified, several biological properties of lycopene may account for its protective effect. Lycopene, mainly found in tomato, tomato products and watermelon, possesses more potent antioxidative activity than α- or β-carotene( Reference Di Mascio, Kaiser and Sies 43 , Reference Wang 44 ). Given the association between oxidative stress and prevalence and development of GDM, the benefit of lycopene against oxidative damage is indispensable( Reference Wang 44 , Reference Cocate, Natali and Alfenas 45 ). Indeed, insulin resistance due to progressive β-cell dysfunction has also been recognised as a fundamental predictor for GDM development( Reference Buchanan and Xiang 46 , Reference Buchanan, Xiang and Kjos 47 ). High hepatic insulin resistance is generally manifested by overproduction of glucose in the basal state despite fasting hyperinsulinaemia( Reference Vangipurapu, Stancakova and Kuulasmaa 48 ). Intriguingly, animal experiments showed that lycopene ameliorated insulin resistance by inhibiting the pathogenesis of hepatic steatosis and improving the lipid profiles( Reference Ahn, Lee and Jung 49 , Reference Zeng, He and Jia 50 ). Beyond that, lycopene may induce β-cell intercellular communication through gap junctions( Reference Stahl and Sies 51 ), which contributed to the control of insulin secretion and glucose tolerance( Reference Charollais, Gjinovci and Huarte 52 ). All these properties potentially explained the regulation of FBG by higher dietary lycopene consumption.
However, the lack of association between other carotenoids and GDM risk cannot be explained. Of all carotenoids addressed in this study, β-carotene is known to be a strong antioxidant. In our analysis the increase in β-carotene intake has been associated with the decrease in GDM risk; however, the association was not statistically significant. One possible reason is that lycopene possesses more potent antioxidative activity( Reference Di Mascio, Kaiser and Sies 43 , Reference Wang 44 ). In addition, the intake of β-carotene was narrower than the intake of lycopene in this study. The narrow intake of β-carotene may be difficult to find the significant effect on GDM risk. All the above may partly explain why β-carotene exerted no effect on GDM risk. We also found no association between α-carotene and GDM risk, which could possibly be explained by the high correlation between β-carotene and α-carotene. In our analysis, the correlation coefficient between α-carotene and β-carotene was 0·535 (data were not shown). Meanwhile, no significant effect of lutein/zeaxanthin on GDM risk was found in this study, which was consistent with the result of lutein/zeaxanthin on type 2 diabetes in a meta-analysis( Reference Leermakers, Darweesh and Baena 29 ).
Notably, this study provided new evidence that the protection of lycopene against GDM was significantly stronger in primigravid women, and several possible mechanisms might account for this result. Pregnancy itself could be regarded as a state of increased insulin resistance, and recurrent pregnancies may result in an additive effect( Reference Zhang, Shu and Gao 53 ). Moreover, previous research demonstrated the increased pre-BMI and complicated metabolic changes among multiparous women, which may increase GDM risk( Reference Boudet-Berquier, Salanave and Desenclos 9 , Reference Koski-Rahikkala, Pouta and Pietilainen 54 ). The protection of lycopene against GDM may be overwhelmed by complicated pro-oxidant activity generated by multiple pregnancies. However, the potential mechanism remains poorly understood and should be elucidated by further animal and epidemiological research.
The main strength of our study is that the dietary survey was conducted via face-to-face interview using a validated FFQ. The researcher-administered FFQ survey was considered as the proper approach, while a self-administered FFQ survey was not advisable in China. Furthermore, the large size of the study sample, different categories of carotenoids, high quality of data and adjustment for a large numbers of potential confounding variables are also the major strength.
Nevertheless, there are some limitations to our study. First, based on a cross-sectional study design, we cannot draw a conclusion about causality. However, the main strength of this study might reduce the risk of report bias and recall bias as the participants had not known their GDM status when FFQ survey was conducted. Second, although numerous confounding factors were adjusted, we could not exclude uncontrolled factors, such as dietary lipid content, which may influence the bio-accessibility and bioavailability of carotenoids( Reference Kim, Gordon and Ferruzzi 55 ). However, we pronounced carotenoids-independent effect by adjusting for dietary vitamin C and vitamin E intake, which maybe the main confounding factors and negatively related with GDM risk( Reference Zhang, Williams and Sorensen 11 , Reference Ley, Hanley and Sermer 12 ). Finally, despite the large sample size of the study, the number of GDM cases was relatively small for stratified analyses. However, all GDM cases in our study were strictly diagnosed based on FBG, as well as 1-h and 2-h postload glucose and according to new diagnostic criteria, rather than directly copying from hospitalised medical records. In fact, the prevalence of GDM in the present study (7·7 %) was lower than the incidence from medical records (8·2 %) (data not shown). We admitted that we might have missed some cases of GDM rather than erroneously considered a non-GDM participant as a GDM case. Of course, the potential bias would most likely attenuate our results, and we would have a higher statistical power if we had more cases of GDM.
In conclusion, the findings from this large cross-sectional study investigating the associations between carotenoids consumption and GDM risk suggest that dietary lycopene intake is associated with a lower risk of GDM in a Chinese population, particularly among primigravid women. This work has important implications for public health with respect to the prevention of GDM. Further and larger studies should be conducted to confirm our findings and to elucidate the underlying mechanisms in the future.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519000606
Acknowledgements
The authors are indebted to all the pregnant women included in the TMCHC study for their continued cooperation and participation. The authors also express their thanks to the staff of The Central Hospital of Wuhan, Hubei Maternal and Child Health Hospital and Jiang’an Maternal and Child Health Hospital for their considerable assistance with many aspects of this study. The authors gratefully acknowledge everyone in the TMCHC study group.
The TMCHC study was supported by the National Program on Basic Research Project of China (N. Y., no. 2013FY114200) and the National Natural Science Foundation of China (L. H., no. 81573149).
The authors’ responsibilities were as follows: Q. G. collected data, analysed the data and drafted the manuscript; C. Z., X. Z., R. C., T. X., M. H. and Q. L. collected data and contributed to the discussion and reviewed/edited the manuscript; M. K., W. H. and G. S. coordinated data collection in the field; L. H., X. Y. and N. Y. participated study design and reviewed and edited the manuscript; N. Y. is the guarantor of this study, who had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis; all the authors read and approved the final manuscript.
All the authors declare that they have no conflict of interest relevant to the content of this article.







