Impact statement
Ocean acidification (OA) is recognised as a significant aspect of global change that will have widespread impacts on marine ecosystems. There has been a recent increase in published research that acknowledges the potential for marine vegetation, such as macroalgae, to modulate local pH conditions through biotic processes and thereby serve as OA refugia for marine organisms. However, the specific role that macroalgae play in the carbonate chemistry dynamics of shallow coastal marine environments has not yet been reviewed in detail. This review assesses the available literature documenting the distribution patterns and structural complexities of macroalgae and how this informs their role in pH modulation over various temporal and spatial extents. A wholistic understanding on the role of macroalgal marine vegetation as OA refugia can facilitate improved local OA management and protected area management to benefit impacted coastal marine species.
Introduction
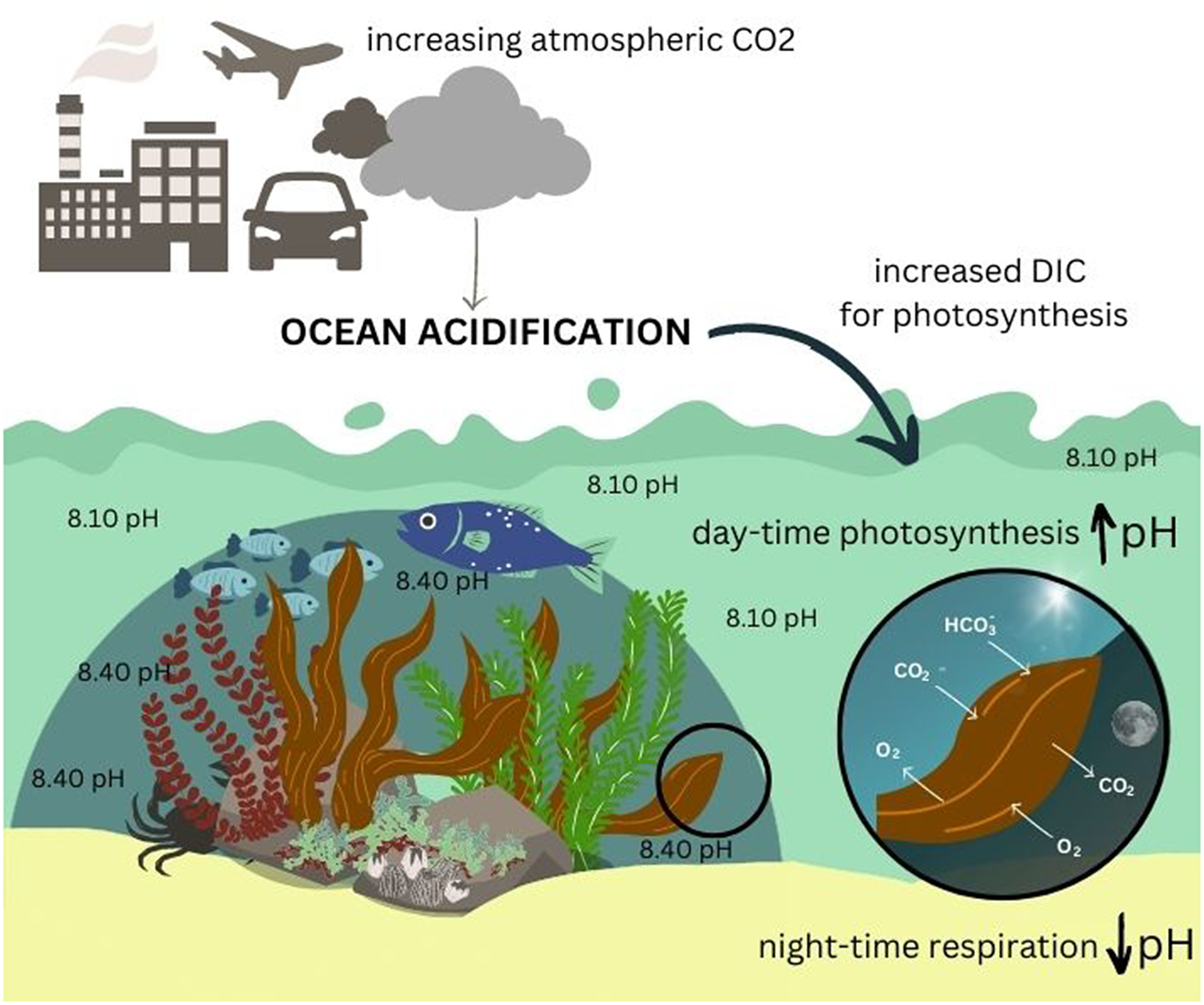
In the marine environment, ocean acidification (OA) refugia refers to locations where naturally higher pH levels are observed, through biotic or physical drivers, providing periodic or sustained relief from global OA for marine organisms (Kapsenberg and Cyronak, Reference Kapsenberg and Cyronak2019). OA is a consequence of increasing anthropogenic emissions of carbon dioxide (CO2) into the earth’s atmosphere (Caldeira and Wickett, Reference Caldeira and Wickett2003; Sabine et al., Reference Sabine, Feely, Gruber, Key, Lee, Bullister, Wanninkhof, Wong, Wallace and Tilbrook2004; Doney et al., Reference Doney, Fabry, Feely and Kleypas2009). This global process results from increased absorption of atmospheric CO2 by the oceans, which ultimately shifts the carbonate chemistry equilibrium of seawater resulting in a decline in average seawater pH (Doney et al., Reference Doney, Fabry, Feely and Kleypas2009; Dickson, Reference Dickson, Riebesell, Fabry, Hansson and Gattuso2010). Concurrent changes in carbonate chemistry associated with a decline in pH include changes to the concentrations of inorganic carbon, such as an increase in dissolved CO2 and bicarbonate (HCO3−) and a decrease in carbonate ions (CO32−) (Feely et al., Reference Feely, Sabine, Lee, Berelson, Kleypas, Fabry and Millero2004; Dickson, Reference Dickson, Riebesell, Fabry, Hansson and Gattuso2010).
In addition to OA, other natural and anthropogenic processes (e.g., pollution, mariculture, aquaculture, upwelling and freshwater inputs) can result in intensified acidification, resulting in even more complex carbon system dynamics, especially in coastal areas (termed coastal acidification; e.g., Wallace et al., Reference Wallace, Baumann, Grear, Aller and Gobler2014; Doney et al., Reference Doney, Busch, Cooley and Kroeker2020; Isah et al., Reference Isah, Enochs and San Diego-McGlone2022; Savoie et al., Reference Savoie, Moody, Gilbert, Dillon, Howden, Shiller and Hayes2022). These changes in ocean chemistry have implications for marine organisms and ecosystems by reducing carbonate ion availability for calcification (Hofmann et al., Reference Hofmann, Barry, Edmunds, Gates, Hutchins, Klinger and Sewell2010) and disrupting key biological processes through changes in pH (e.g., organism physiology and behaviour; Pörtner, Reference Pörtner2008; Heuer and Grosell, Reference Heuer and Grosell2014; Clements and Hunt, Reference Clements and Hunt2015; Nagelkerken and Munday, Reference Nagelkerken and Munday2016).
While lowered seawater pH caused by OA can have substantial impacts on coastal vegetation (Koch et al., Reference Koch, Bowes, Ross and Zhang2013; Narvarte et al., Reference Narvarte, Nelson and Roleda2020), these species can in turn also affect seawater pH. For example, seagrass and macroalgae can raise pH on a local scale by taking up carbon through photosynthesis (Krause-Jensen et al., Reference Krause-Jensen, Duarte, Hendriks, Meire, Blicher, Marbà and Sejr2015). The role of coastal vegetated habitats, such as seagrass, mangroves, salt marshes and macroalgae as carbon sinks is widely acknowledged (e.g., Bouillon et al., Reference Bouillon, Borges, Castañeda-Moya, Diele, Dittmar, Duke, Kristensen, Lee, Marchand and Middelburg2008; Duarte et al., Reference Duarte, Marbà, Gacia, Fourqurean, Beggins, Barrón and Apostolaki2010; Alongi, Reference Alongi2012; Fourqurean et al., Reference Fourqurean, Duarte, Kennedy, Marbà, Holmer, Mateo, Apostolaki, Kendrick, Krause-Jensen and McGlathery2012; Chmura, Reference Chmura2013; Krause-Jensen and Duarte, Reference Krause-Jensen and Duarte2016). However, the role that coastal vegetated habitats play in influencing localised carbonate chemistry and pH of surrounding seawater, particularly in the context of OA, has only come to the forefront more recently, consequent to the increased attention given to coastal acidification.
Macroalgal vegetation can play a significant role in influencing the carbonate chemistry of seawater over various spatial and temporal scales (Middelboe and Hansen, Reference Middelboe and Hansen2007; Wahl et al., Reference Wahl, Schneider Covachã, Saderne, Hiebenthal, Müller, Pansch and Sawall2018; McNicholl et al., Reference McNicholl, Koch and Hofmann2019) through autotrophic, calcification and respiration processes (Middelboe and Hansen, Reference Middelboe and Hansen2007; Krause-Jensen and Duarte, Reference Krause-Jensen and Duarte2016). These localised zones of elevated pH associated with macroalgal beds could potentially serve as OA refugia (Noisette and Hurd, Reference Noisette and Hurd2018). For example, Wahl et al. (Reference Wahl, Schneider Covachã, Saderne, Hiebenthal, Müller, Pansch and Sawall2018) found that dense beds of brown algae and seagrass in the Western Baltic increased the overall mean pH of the surrounding water by as much as 0.3 units relative to other similar habitats with no macrophytes and also imposed strong diurnal pH fluctuations (due to photosynthetic activity). This allowed mussels (Mytilus edulis) to maintain calcification even under acidified conditions and suggests that seagrass and macroalgae may mitigate the impact of OA on organisms living in these habitats.
Macroalgal physiology and carbon use strategies
Photosynthesis and respiration
The process of photosynthesis (which occurs only during the day) in macroalgae requires the uptake of dissolved inorganic carbon (DIC), which is ultimately required in the form of CO2, together with water and light, to produce glucose and oxygen (Hanelt et al., Reference Hanelt, Wiencke, Bischof, Larkum, Douglas and Raven2003). In aquatic and marine environments, DIC exists in different forms: CO2, HCO3− and CO32− (Cornwall et al., Reference Cornwall, Revill and Hurd2015; Stepien et al., Reference Stepien, Pfister and Wootton2016). In seawater, DIC predominantly occurs in the form of HCO3− (~90%) and not as dissolved CO2 gas (<1%) due to the rapid dissociation of CO2 in seawater (Park, Reference Park1969). As such, most macroalgae have evolved carbon concentrating mechanisms (CCMs), which facilitate the active uptake of DIC in the form of HCO3− using various energy-driven mechanisms, such as external conversion of HCO3− to CO2 catalysed by the enzyme carbonic anhydrase (common in red, green and brown seaweeds; e.g., Flores-Moya and Fernández, Reference Flores-Moya and Fernández1998; Axelsson et al., Reference Axelsson, Larsson and Ryberg1999; Mercado et al., Reference Mercado, Viñgla, Figueroa and Niell1999) or through the uptake of HCO3− directly in this form via anion exchange proteins or proton pumps (Fernández et al., Reference Fernández, Hurd and Roleda2014). Once taken up into the cells, the enzyme carbonic anhydrase facilitates the interconversion of DIC to make it available for photosynthesis (Raven, Reference Raven1995). In many macroalgal species, CCMs are used in addition to passive CO2 diffusion (Maberly, Reference Maberly1990; Raven, Reference Raven2003; Giordano et al., Reference Giordano, Beardall and Raven2005; Cornwall et al., Reference Cornwall, Revill and Hurd2015; Stepien et al., Reference Stepien, Pfister and Wootton2016) depending on the availability and forms of DIC in surrounding seawater. Very few macroalgal species rely on passive CO2 diffusion alone for DIC uptake (Raven, Reference Raven2003; Giordano et al., Reference Giordano, Beardall and Raven2005; Raven and Hurd, Reference Raven and Hurd2012; Stepien et al., Reference Stepien, Pfister and Wootton2016).
The DIC acquisition strategies employed vary among macroalgal species and taxonomic groups (Maberly, Reference Maberly1990; Raven, Reference Raven1997). Green algae, like Ulva, for example, can efficiently use both CO2 and HCO3− as inorganic carbon sources (Beer and Eshel, Reference Beer and Eshel1983; Rautenberger et al., Reference Rautenberger, Fernández, Strittmatter, Heesch, Cornwall, Hurd and Roleda2015). Brown algae species rely almost exclusively on the uptake of DIC in the form of HCO3− (Surif and Raven, Reference Surif and Raven1989; Zou and Gao, Reference Zou and Gao2010; Fernández et al., Reference Fernández, Hurd and Roleda2014). The red algae are also primarily HCO3− users, with some exceptions, like Lomentaria articulata and Delesseria sanguinea, which lack this ability and rely on CO2 as a DIC source (Johnston et al., Reference Johnston, Maberly and Raven1992; Kubler and Raven, Reference Kubler and Raven1994). The ability and efficiency with which different DIC sources are used by macroalgal species appears to be related to habitat rather than specific to particular taxonomic groups (Maberly, Reference Maberly1990; Kubler and Raven, Reference Kubler and Raven1994; Murru and Sandgren, Reference Murru and Sandgren2004).
During periods when photosynthesis is not occurring or limited (e.g., at night or under reduced light availability), there is a net release of CO2 gas into surrounding seawater through respiration (Duarte et al., Reference Duarte, Middelburg and Caraco2005; Middelboe and Hansen, Reference Middelboe and Hansen2007; Semesi et al., Reference Semesi, Kangwe and Björk2009). This process also influences the carbonate chemistry and forces the equilibrium to a state that decreases pH (Middelboe and Hansen, Reference Middelboe and Hansen2007; Saderne et al., Reference Saderne, Fietzek and Herman2013; Wahl et al., Reference Wahl, Schneider Covachã, Saderne, Hiebenthal, Müller, Pansch and Sawall2018). The influence of photosynthesis and respiration on seawater carbonate chemistry is most significant over larger spatial scales when macroalgal growth and density is high. Since the carbonate chemistry equilibrium of seawater is dynamic, the periodic uptake of DIC for photosynthesis by macroalgae acts to increase pH, and CO2 released by respiration, conversely, decreases pH often resulting in diurnal pH cycles. Despite these complex and ongoing changes to this equilibrium, which result in highly variable conditions in these habitats, there is evidence to suggest that macroalgae raise the overall average pH in surrounding seawater, which can have temporary or long-term benefits for the organisms that live in these habitats (Krause-Jensen and Duarte, Reference Krause-Jensen and Duarte2016; Koweek et al., Reference Koweek, Zimmerman, Hewett, Gaylord, Giddings, Nickols, Ruesink, Stachowicz, Takeshita and Caldeira2018; Wahl et al., Reference Wahl, Schneider Covachã, Saderne, Hiebenthal, Müller, Pansch and Sawall2018).
Calcification
In addition to photosynthetic needs, some macroalgal species rely on DIC in the form of CO32−, HCO3− or CO2 to build and maintain calcium carbonate structures through the precipitation of CaCO3 (Roleda et al., Reference Roleda, Boyd and Hurd2012a; Hofmann and Bischof, Reference Hofmann and Bischof2014). This process may also modulate the carbonate chemistry equilibrium of surrounding seawater through both the absorption of DIC and the release of CO2 (Kalokora et al., Reference Kalokora, Buriyo, Asplund, Gullström, Mtolera and Björk2020). As such, the role that calcifying species have on carbon dynamics of surrounding seawater is complex, as processes of photosynthesis, calcification and respiration simultaneously influence the carbon system, and ultimately pH, in counteracting ways (Kalokora et al., Reference Kalokora, Buriyo, Asplund, Gullström, Mtolera and Björk2020).
The best-known calcifying group of algae are the order of red algae, the coralline algae, that form crustose or articulated structures by depositing CaCO3 extracellularly (Hofmann and Bischof, Reference Hofmann and Bischof2014; McCoy and Kamenos, Reference McCoy and Kamenos2015). These algae can form large aggregations spanning several square kilometres or also occur as smaller crusts or as epiphytes on other living organisms (McCoy and Kamenos, Reference McCoy and Kamenos2015). Although calcification in this group is considered to be sensitive to OA, as CO32− is less available in seawater under acidic conditions (Feely et al., Reference Feely, Sabine, Lee, Berelson, Kleypas, Fabry and Millero2004; Fabry et al., Reference Fabry, Seibel, Feely and Orr2008; Raven, Reference Raven2011; Stepien et al., Reference Stepien, Pfister and Wootton2016), the fact that many calcifying macroalgae utilise HCO3− or CO2 as substrate for calcification, and not carbonate may limit this sensitivity (Roleda et al., Reference Roleda, Boyd and Hurd2012a). Furthermore, carbonate concentrations can be increased by these algae through alteration of pH of water in close association with the thallus (e.g., intercellular spaces) during photosynthetic use of CO2 and/or HCO3− (Digby, Reference Digby1977; Borowitzka and Larkum, Reference Borowitzka and Larkum1987), reducing reliance on elevated ambient carbonate ion concentrations. In fact, dissolution of calcified structures because of OA might be a greater problem than reduced calcification rates (Doney et al., Reference Doney, Fabry, Feely and Kleypas2009; Hofmann and Todgham, Reference Hofmann, Barry, Edmunds, Gates, Hutchins, Klinger and Sewell2010).
Through these interactions with the carbonate system of seawater, macroalgae influence the carbon equilibrium of surrounding seawater over various scales. However, for macroalgal vegetation to play a meaningful role as OA refugia for other marine organisms, the density of these primary producers needs to be high enough. Macroalgal vegetation is not evenly distributed with respect to species and biomass and certain regions would be of greater significance in their effect on local seawater carbon fluxes and pH. The greatest potential for OA mitigation is likely to be in coastal areas with large, dense and complex macroalgal communities or seaweed farms (Chung et al., Reference Chung, Oak, Lee, Shin, Kim and Park2013; Zacharia et al., Reference Zacharia, Kaladharan and Rohith2015; Fernández et al., Reference Fernández, Leal and Henríquez2019; Xiao et al., Reference Xiao, Agustí, Yu, Huang, Chen, Hu, Li, Li, Wei, Lu, Xu, Chen, Liu, Zeng, Wu and Duarte2021).
Macroalgal distribution patterns
Globally, macroalgal distribution is determined by seawater surface temperature, while local scale distribution is established in response to substrate properties and depth. Lüning (Reference Lüning1991) described macroalgal floras of the world in detail and the regions identified based on macroalgal vegetation closely resemble the seven temperature zones adopted by Briggs (Reference Briggs1995) (Figure 1).

Figure 1. Distribution of coastal temperature zones based on Briggs, Reference Briggs1995 (reproduced from Bartsch et al., Reference Bartsch, Wiencke, Laepple and Wiencke2012). Blue, polar regions; green, cool temperate; yellow, warm temperate; red, tropical.
Marine forests
Canopy-forming macroalgae occur along various coastlines and include large brown species, such as large Laminarian kelps and smaller Fucalean genera, which dominate marine benthic communities known as marine forests. Large robust canopy-forming brown algae are recognised habitat engineers known for their ability to alter physical conditions in the surrounding benthos (Steneck et al., Reference Steneck, Graham, Bourque, Corbett, Erlandson, Estes and Tegner2002; Schiel and Foster, Reference Schiel and Foster2015; Teagle et al., Reference Teagle, Hawkins, Moore and Smale2017; Wernberg et al., Reference Wernberg, Krumhansl, Filbee-Dexter, Pedersen and Sheppard2019). These subtidal brown algae are the most important macroalgal primary producers based on area cover and net primary production (Duarte et al., Reference Duarte, Bruhn and Krause-Jensen2022). Kelp forest communities include a variety of canopy-forming species that differ widely in stature. Steneck et al. (Reference Steneck, Graham, Bourque, Corbett, Erlandson, Estes and Tegner2002) recognised at least three different groups of large forms that represent the kelp-component in kelp forests. The floating canopy kelps (e.g., Macrocystis) that grow up to 45 m in length, smaller canopy kelps like Ecklonia and Nereocystis (<10 m); and kelps that are held upright by rigid stipes (Laminaria and Ecklonia radiata) (<5 m). These ecosystem engineers create forest-like marine vegetation types that have complex three-dimensional structures. Like terrestrial forests, kelp communities have associated understory algal communities (Leliaert et al., Reference Leliaert, Anderson, Bolton and Coppejans2000; Bennett and Wernberg, Reference Bennett and Wernberg2014; Leclerc et al., Reference Leclerc, Riera, Lévêque and Davoult2016; Smale et al., Reference Smale, Pessarrodona, King, Burrows, Yunnie, Vance and Moore2020).
The smaller canopy-forming algae are from the Fucales, of which Sargassum and Cystoseira species are the most widespread (Nizamuddin, Reference Nizamuddin1970). Sargassum is more common in the warm-temperate, subtropical and tropical coasts (Yip et al., Reference Yip, Quek and Huang2020), while Cystoseira is most diverse in the Mediterranean (Nizamuddin, Reference Nizamuddin1970). This pattern of canopy-forming algal distribution is reflected in the mapped brown algal marine forests, which show strong representation in cool- to cold-temperate regions, but also in some warmer tropical coasts such as the Caribbean and Indo-China (Figure 2) (Assis et al., Reference Assis, Fragkopoulou, Frade, Neiva, Oliveira, Abecasis, Faugeron and Serrão2020).

Figure 2. Modelled distribution of brown forest forming macroalgal species (after Assis et al., Reference Assis, Fragkopoulou, Frade, Neiva, Oliveira, Abecasis, Faugeron and Serrão2020).
Lower canopy-forming algae like Gelidium corneum are also considered foundational, or habitat forming species (Quintano et al., Reference Quintano, Díez, Muguerza, Figueroa and Gorostiaga2017; Borja et al., Reference Borja, Chust, Fontan, Garmendia and Uyarra2018; Muguerza et al., Reference Muguerza, Díez, Quintano and Gorostiaga2022), particularly on some European coasts, with Gelidium canariense representing an important canopy-forming species on Macronesian islands (Alfonso et al., Reference Alfonso, Sangil and Sansón2017; Hernández, Reference Hernández2021). While these algae are not as tall as the kelps, the canopies formed are similar in height (20–30 cm) to some of the fucalean-dominated communities (Robertson, Reference Robertson1987; Quintano et al., Reference Quintano, Díez, Muguerza, Figueroa and Gorostiaga2018; Alfonso et al., Reference Alfonso, Hernández, Sangil, Martín, Expósito, Díaz and Sansón2021; Hernández, Reference Hernández2021). Smaller brown and red foliose species are usually important components of the under-story vegetation and occur both under canopies of larger brown algae as well as in the gaps between stands of taller algae. However, temperate G. corneum (Quintano et al., Reference Quintano, Díez, Muguerza, Figueroa and Gorostiaga2017, Reference Quintano, Díez, Muguerza, Figueroa and Gorostiaga2018; Casado-Amezúa et al., Reference Casado-Amezúa, Araújo, Bárbara, Bermejo, Borja, Díez, Fernández, Gorostiaga, Guinda and Hernández2019) and Gelidium canariense (Alfonso et al., Reference Alfonso, Hernández, Sangil, Martín, Expósito, Díaz and Sansón2021; Hernández, Reference Hernández2021) are both recognised as short canopy-forming species in their own right.
Polar macroalgae
The polar macroalgae flora is confined predominantly to the subtidal zone due to seasonal ice scour, which abrades rock surfaces on the intertidal reefs (Zacher et al., Reference Zacher, Rautenberger, Hanelt, Wulff and Wiencke2009). Light limitation during winter months limits the poleward distribution of kelps that form such extensive forests in temperate regions (Steneck et al., Reference Steneck, Graham, Bourque, Corbett, Erlandson, Estes and Tegner2002). However, a few Laminarian species do occur in these cold polar regions (Filbee-Dexter et al., Reference Filbee-Dexter, Wernberg, Fredriksen, Norderhaug and Pedersen2019), with macroalgal communities in cold polar waters generally comprised of a canopy of large brown algae, with an understory community of foliose and coralline macroalgae (Brouwer et al., Reference Brouwer, Geilen, Gremmen and Lent1995; Wiencke and Amsler, Reference Wiencke, Amsler, Wiencke and Bischof2012). Although the Arctic has limited potential for substantial macroalgae communities, since only 35% of the benthos in this region has hard substrate that can support large attached macroalgae like kelp forests (Filbee-Dexter et al., Reference Filbee-Dexter, Wernberg, Fredriksen, Norderhaug and Pedersen2019), subtidal macroalgae communities can reach biomass values comparable to temperate kelp communities (Wiencke and Amsler, Reference Wiencke, Amsler, Wiencke and Bischof2012).
Temperate algal vegetation
In cold- and warm-temperate regions, the large brown algae also dominate the subtidal zone (Flores-Moya, Reference Flores-Moya, Wiencke and Bischof2012; Huovinen and Gómez, Reference Huovinen, Gómez, Wiencke and Bischof2012). The most prominent group in this regard are the kelps (Laminariales), which attain the largest size of these brown canopy-forming algae (Steneck et al., Reference Steneck, Graham, Bourque, Corbett, Erlandson, Estes and Tegner2002). These are common in cooler regions (warm and cold temperate) but relatively rare along warmer coastlines (Bolton, Reference Bolton2010). Dense beds of smaller macroalgal groups are also common in temperate areas (Lüning, Reference Lüning1991; Shepherd and Edgar, Reference Shepherd and Edgar2013). Estimates based on habitat availability models show that, while these smaller algae potentially cover a substantial area, they make a limited contribution to primary production (Duarte et al., Reference Duarte, Bruhn and Krause-Jensen2022).
Tropical algal vegetation
Subtidal algal vegetation in tropical regions usually forms a mosaic with coral reefs. These metazoan colonies share space with both turf and foliose macroalgae communities, the relative abundance of which is dependent on the interplay between herbivory, nutrients and light availability (Hurd et al., Reference Hurd, Harrison, Bischof and Lobban2014). Foliose algae are usually rare in coral reef systems, unless there is reduction in herbivory or an increase in nutrients (Lobban and Harrison, Reference Lobban and Harrison1994). These algae are often fucoids like Cystoseira, Turbinaria and Sargassum (Mejia et al., Reference Mejia, Puncher, Engelen, Wiencke and Bischof2012). Foliose algae, along with some coralline algae, usually form communities on the shallow shoreward reef flats of coral atolls, while the seaward slopes are usually occupied by corals, coralline algae and calcified macroalgae such as Halimeda (Littler and Littler, Reference Littler, Littler, Lobban and Harrison1994). One of the reasons for this is the availability of hard substrate on a relatively flat surface to which they can attach (Fong and Paul, Reference Fong, Paul, Dubinsky and Stambler2011). Extensive macroalgal communities are not common in tropical regions, however, Wilson et al. (Reference Wilson, Depczynski, Fisher, Holmes, O’Leary and Tinkler2010) and Evans et al. (Reference Evans, Wilson, Field and Moore2014) report substantial algal meadows off the coast of Australia.
Based on macroalgal distribution models, there appears to be great potential for OA mitigation in algal beds, both in temperate and tropical regions. However, the greatest opportunities for larger scale OA refuge would lie within the high biomass areas offered by temperate marine forests. High biomass can also be created through coastal seaweed aquaculture initiatives, which may alleviate OA stresses in shallow coastal areas.
Trends in ocean acidification refuge provision by macroalgae
Temporal trends
Refuge from OA provided by macroalgae can be temporally consistent or cyclical, depending on several factors such as seasonal productivity (Middelboe and Hansen, Reference Middelboe and Hansen2007), photosynthetic state (diurnal cycles), water retention time and mixing (Middelboe and Hansen, Reference Middelboe and Hansen2007; Buapet et al., Reference Buapet, Gullström and Björk2013; Koch et al., Reference Koch, Bowes, Ross and Zhang2013) and nutrient availability (Gao and McKinley, Reference Gao and McKinley1994; Celis-Pla et al., Reference Celis-Pla, Hall-Spencer, Horta, Milazzo, Korbee, Cornwall and Figueroa2015).
Macroalgae are subject to seasonal differences in productivity and photosynthetic rates, like many other plant species, related to temperature preferences, irradiance and nutrient availability (Takahashi et al., Reference Takahashi, Sutherland, Sweeney, Poisson, Metzl, Tilbrook, Bates, Wanninkhof, Feely and Sabine2002; Saderne et al., Reference Saderne, Fietzek and Herman2013; Attard et al., Reference Attard, Rodil, Berg, Norkko, Norkko and Glud2019; Kapsenberg and Cyronak, Reference Kapsenberg and Cyronak2019). This means that their provision as OA refuges may vary with seasonal differences in productivity (Li et al., Reference Li, Moon, Kang, Kim, Kim, Lee, Kwak, Kim, Kim and Park2022). Studies have found that in most regions macroalgal productivity is typically higher in summer and spring months. For example, Middelboe and Hansen (Reference Middelboe and Hansen2007) identified seasonal pH variability associated with macroalgal productivity (Fucus vesiculosus, F. serratus, Ceramium rubrum and Ulva spp.) on the northeast coast of Zealand (Denmark), evidenced by higher average pH in summer and lower pH in winter, which they attributed mostly to seasonal differences in irradiance. These findings are similar to other studies that also found higher average pH and higher variability in carbonate parameters (pCO2 and DIC) in productive summer months, for example, Krause-Jensen et al. (Reference Krause-Jensen, Duarte, Hendriks, Meire, Blicher, Marbà and Sejr2015) in a subarctic fjord in Greenland and Delille et al. (Reference Delille, Borges and Delille2009) in a Southern Ocean Archipelago. As such, macroalgal-dominated habitats may provide seasonal relief for marine organisms from OA during periods of higher productivity.
Many studies have identified diurnal cycles in seawater pH adjacent to macroalgal vegetation, with the common pattern being higher pH during the day (driven by photosynthesis) and lower pH at night (driven by respiration; e.g., Middelboe and Hansen, Reference Middelboe and Hansen2007; Semesi et al., Reference Semesi, Kangwe and Björk2009; Frieder et al., Reference Frieder, Nam, Martz and Levin2012; Cornwall et al., Reference Cornwall, Hepburn, McGraw, Currie, Pilditch, Hunter, Boyd and Hurd2013; Krause-Jensen et al., Reference Krause-Jensen, Duarte, Hendriks, Meire, Blicher, Marbà and Sejr2015; Wahl et al., Reference Wahl, Schneider Covachã, Saderne, Hiebenthal, Müller, Pansch and Sawall2018). These patterns occur in response to daylight availability for photosynthesis. In some cases, diurnal variability in pH can be as high as 1.2–1.5 units depending on the density of macroalgal growth and resultant levels of productivity (Krause-Jensen et al., Reference Krause-Jensen, Duarte, Hendriks, Meire, Blicher, Marbà and Sejr2015; Wahl et al., Reference Wahl, Saderne and Sawall2015). The extent of diurnal variability can also be influenced by physical factors, such as tidal processes or seawater exchange. Shallow areas with less mixing and higher water retention times usually experience high diurnal pH variability (Middelboe and Hansen, Reference Middelboe and Hansen2007; Hurd, Reference Hurd2015). Diurnal fluctuations in pH can benefit associated organisms by providing cyclical or periodic relief from OA. Wahl et al. (Reference Wahl, Schneider Covachã, Saderne, Hiebenthal, Müller, Pansch and Sawall2018) found that the brown algae F. vesiculosus in the Kiel Fjord increased overall mean pH of seawater by 0.01–0.2 units, with diurnal variability of 1.2 units. Mussels (Mytilus edulis) benefited from this autotrophic pH modulation as they were able to maintain calcification rates even at low pH levels (7.7–8.1 depending on algal density) by shifting their calcification process to daytime periods to fall in with the periods of higher pH provided by the vegetation (Wahl et al., Reference Wahl, Schneider Covachã, Saderne, Hiebenthal, Müller, Pansch and Sawall2018). Other studies have found similar benefits for growth, development and physiology in other bivalve species (summarised in Table 1) (Frieder et al., Reference Frieder, Gonzalez, Bockmon, Navarro and Levin2014; Young and Gobler, Reference Young and Gobler2018; Jiang et al., Reference Jiang, Jiang, Rastrick, Wang, Fang, Du, Gao, Mao, Strand and Fang2022; Young et al., Reference Young, Sylvers, Tomasetti, Lundstrom, Schenone, Doall and Gobler2022). However, there is also evidence to suggest that not all species are capable of adapting their calcification process to benefit from diurnal pH variability (Cornwall et al., Reference Cornwall, Hepburn, McGraw, Currie, Pilditch, Hunter, Boyd and Hurd2013) and, as such, may still be negatively affected by corrosive conditions at night.
Table 1. Examples of studies assessing the ocean acidification (OA) refuge provision by macroalgal species for various coastal organisms

Spatial trends
Macroalgae can serve as OA refugia over a range of spatial scales as they can form habitats ranging in size from large stands and canopies to low-level algal crusts (Hepburn et al., Reference Hepburn, Pritchard, Cornwall, McLeod, Beardall, Raven and Hurd2011). Macroalgae also occur at a vast range of locations, such as in shallow coastal areas, rock pools, estuaries or deeper subtidal locations (Layton et al., Reference Layton, Coleman, Marzinelli, Steinberg, Swearer, Vergés, Wernberg and Johnson2020; Falkenberg et al., Reference Falkenberg, Scanes, Ducker and Ross2021). The distribution and structure of macroalgal growth will offer refuge from OA at different spatial extents. On a small scale, pH fluctuation related to biological activity can occur at spatial scales as small as centimetres, for example, in the diffusive boundary layer at the thalli surface (Noisette and Hurd, Reference Noisette and Hurd2018; Guy-Haim et al., Reference Guy-Haim, Silverman, Wahl, Aguirre, Noisette and Rilov2020). Conversely, in large- and dense-algal aggregations, biological activity can have an influence at an extent of metres to kilometres (Krause-Jensen et al., Reference Krause-Jensen, Duarte, Hendriks, Meire, Blicher, Marbà and Sejr2015).
On a micro-scale (centimetres) macroalgal metabolism can create favourable micro-zones of higher pH within their diffusive boundary layer (Noisette and Hurd, Reference Noisette and Hurd2018; Guy-Haim et al., Reference Guy-Haim, Silverman, Wahl, Aguirre, Noisette and Rilov2020), where pH can be up to 1–3 units higher than surrounding seawater (Wahl et al., Reference Wahl, Saderne and Sawall2015). Marine organisms that live on the surface of macroalgae can benefit from this chemical refuge, especially during the daytime when photosynthetic activity is highest (Noisette and Hurd, Reference Noisette and Hurd2018) (Table 1). For example, Saderne and Wahl (Reference Saderne and Wahl2013) found that both calcifying (Electra pilosa) and non-calcifying (Alcyonidium hirsutum) Bryozoan species were tolerant of high pCO2 levels (1,193 ± 166 μatm) associated with upwelling events in the Western Baltic Sea, and they attributed this tolerance to the potential periodic relief from OA provided by the brown macroalgal species Fucus serratus on which these species live. Similarly, Doo et al. (Reference Doo, Leplastrier, Graba-Landry, Harianto, Coleman and Byrne2020) found that an epiphytic foraminifera, Marginopora vertebralis, showed higher tolerance to end-of-the-century temperature (±3 °C) and pCO2 (~1,000 μatm) when kept experimentally under these treatments with its red macroalgal host, Laurencia intricata. Similar beneficial symbiotic relationships that facilitate micro-refugia from OA exist between calcifying and non-calcifying algae. For example, Guy-Haim et al. (Reference Guy-Haim, Silverman, Wahl, Aguirre, Noisette and Rilov2020) and Short et al. (Reference Short, Kendrick, Falter and McCulloch2014) found that the coralline algae Ellisolandia elongata and Hydrolithoideae spp. are less susceptible to the effects of OA, evidenced by higher calcification rates at low pH (7.8 and 7.7, respectively), when associated with non-calcifying epiphytic algae. There are also examples of species, such as urchin larvae (Pseudechinus huttoni) that are well adapted to pH variability and therefore show no positive or negative response to the variable pH in their settlement habitats (Houlihan et al., Reference Houlihan, Espinel-Velasco, Cornwall, Pilditch and Lamare2020).
The effect of photosynthesis on surrounding seawater carbonate chemistry can extend further than the immediate vicinity of the algal growth. Macroalgal assemblages that form complex communities can provide refuge from OA over much larger spatial scales from metres (e.g., in rock pools or within canopies) to kilometres (e.g., extensive algal beds in temperate areas) due to higher and more concentrated levels of metabolic activity (Björk et al., Reference Björk, Axelsson and Beer2004; Middelboe and Hansen, Reference Middelboe and Hansen2007; Duarte et al., Reference Duarte, Hendriks, Moore, Olsen, Steckbauer, Ramajo, Carstensen, Trotter and McCulloch2013; Krause-Jensen et al., Reference Krause-Jensen, Duarte, Hendriks, Meire, Blicher, Marbà and Sejr2015). The refuge potential is especially significant in macroalgal assemblages with dense growth and complex canopy structure with the additional benefit of reduced seawater flow, which increases water residence time (Hendriks et al., Reference Hendriks, Olsen, Ramajo, Basso, Steckbauer, Moore, Howard and Duarte2014; Hurd, Reference Hurd2015).
Macroalgae have been reported to increase the average pH (to levels >8.5 pH units) in rock pools (Björk et al., Reference Björk, Axelsson and Beer2004), lagoons (Menéndez et al., Reference Menéndez, Martı́nez and Comı́n2001) and bays (Buapet et al., Reference Buapet, Gullström and Björk2013). For example, Krause-Jensen et al. (Reference Krause-Jensen, Duarte, Hendriks, Meire, Blicher, Marbà and Sejr2015) found pH variability of 0.1–0.3 units occurs within the scale of 1 m2 in response to location within the canopy of a large kelp forest in the Kobbefjord in southwest Greenland. At a slightly larger scale, Buapet et al. (Reference Buapet, Gullström and Björk2013) compared the pH conditions in different vegetation types (mixed macroalgae and seagrass beds) to non-vegetated areas in six shallow coastal bays in temperate Sweden. Their results showed that vegetation can influence the conditions at the scale of an entire bay as evidenced by higher pH and lower DIC concentrations relative to the adjacent seawater outside the bay, even in areas of the bay where vegetation did not occur (Buapet et al., Reference Buapet, Gullström and Björk2013).
Impacts of ocean acidification on macroalgae
It is important to consider the potential negative impacts that OA may have on macroalgae in order to determine their provision as OA refugia under future acidified conditions. Most studies that have assessed the response of macroalgae to changes in seawater carbonate chemistry associated with OA have shown that macroalgal species from all algal groups (red, brown and green) are generally physiologically tolerant to predicted OA and even show enhanced growth under these conditions (see reviews by Porzio et al., Reference Porzio, Buia and Hall-Spencer2011 and Koch et al., Reference Koch, Bowes, Ross and Zhang2013). This tolerance is attributed to the ability of most macroalgal species to efficiently assimilate DIC in various forms for photosynthesis thus allowing them to benefit from increased availability of DIC under acidified conditions (Fernández et al., Reference Fernández, Roleda and Hurd2015; Cornwall and Hurd, Reference Cornwall and Hurd2019). Of course, there are exceptions with some species sensitive to OA, with sensitivity likely linked to mechanisms of carbon uptake and calcification (Cornwall et al., Reference Cornwall, Hepburn, Pritchard, Currie, McGraw, Hunter and Hurd2012; Hofmann and Bischof, Reference Hofmann and Bischof2014) as well as the scale of exposure (e.g., extreme low pH conditions associated with coastal acidification induced by mariculture activities as shown by Narvarte et al., Reference Narvarte, Nelson and Roleda2020). Of all the algal groups, sensitivity to OA is most often reported for calcifying species (see the section “Calcification”), with this group facing the threat of being outcompeted by the more tolerant non-calcifying algae (Hofmann and Bischof, Reference Hofmann and Bischof2014).
Considering that some macroalgal species have heteromorphic life cycles (e.g., many kelp species), it is also important to consider the impact of OA on the different stages, where the early life stages (unicellular and microscopic stages) are usually deemed more sensitive to environmental change (Roleda et al., Reference Roleda, Wiencke, Hanelt and Bischof2007). Although the early life stages of some macroalgal species have been shown to be more sensitive to other environmental factors (e.g., UV radiation; Roleda et al., Reference Roleda, Wiencke, Hanelt and Bischof2007), there is evidence to suggest that early life stages are not affected by OA conditions (Roleda et al., Reference Roleda, Morris, McGraw and Hurd2012b, Reference Roleda, Cornwall, Feng, McGraw, Smith and Hurd2015; Leal et al., Reference Leal, Hurd and Fernández2017). This provides positive evidence for the persistence of macroalgal habitats in future.
Conclusion and relevance
Macroalgae form important components of shallow coastal marine ecosystems and have been identified as potentially beneficial habitats for coastal species facing ongoing OA by provision of higher average pH conditions (Kapsenberg and Cyronak, Reference Kapsenberg and Cyronak2019). Being a dynamic process, autotrophic pH modulation exposes organisms to higher variability in pH in space and time, and therefore alternating periods of stress and recovery (Wahl et al., Reference Wahl, Schneider Covachã, Saderne, Hiebenthal, Müller, Pansch and Sawall2018). As such, the species that occur in vegetated environments, like macroalgal ecosystems, although benefitting from periodic relief from the physiological stress incurred by OA, still require the physiological capacity to withstand high pH variability (Falkenberg et al., Reference Falkenberg, Scanes, Ducker and Ross2021). Despite short-term fluctuations in pH conditions, autotrophic biological activity likely provides associated organisms with long-term relief from ongoing OA (Hurd, Reference Hurd2015; Koweek et al., Reference Koweek, Zimmerman, Hewett, Gaylord, Giddings, Nickols, Ruesink, Stachowicz, Takeshita and Caldeira2018; Pacella et al., Reference Pacella, Brown, Waldbusser, Labiosa and Hales2018). Considering the apparent tolerance of most photosynthetic macroalgae to future OA conditions, and particularly those taxa that are known to form large stands and aggregations, it is likely these habitats will continue to provide an important refuge for the many marine species associated with them.
The research highlighted in this review provides important evidence for the potential OA refuge function of brown, red and green macroalgae by mitigating the negative effects of OA on growth and calcification of mainly bivalves but also bryozoans, foraminifera and coralline algae (Table 1). However, research conducted over large spatial scales, across biogeographic regions, and for many macroalgae-associated species (such as other calcifying organisms and fish) is lacking and needs to be addressed in future research. The role of macroalgal habitats as refugia should be considered for local OA management and protected area management for conservation efforts (Morelli et al., Reference Morelli, Daly, Dobrowski, Dulen, Ebersole, Jackson, Lundquist, Millar, Maher and Monahan2016; Kapsenberg and Cyronak, Reference Kapsenberg and Cyronak2019), especially in productive coastal marine environments where these habitats already provide important nursery areas for many marine species (James and Whitfield, Reference James and Whitfield2022).
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/cft.2023.9.
Author contribution
C.E. conceptualised the review, reviewed the literature, wrote sections of the manuscript and edited the final version. P.-P.S. reviewed the literature, wrote sections of the manuscript and edited the final version. N.C.J. conceptualised the review, structured the manuscript and edited the final version.
Financial support
This review forms part of a broader research project on South African nursery seascapes funded by the National Research Foundation (NRF) coastal and marine research grants (Grant Number 136489).
Competing interest
All authors declare that they have no competing interest to disclose.






Comments
The Editor in Chief: Coastal Futures
31 August 2022
Herewith please find our invited review entitled “The role of macroalgal habitats as ocean acidification refugia within coastal seascapes” by Carla Edworthy, Paul-Pierre Steyn and Nicola James for consideration in Coastal Futures.
Yours sincerely,
Dr Carla Edworthy