Introduction
Tick-borne encephalitis (TBE) is an emerging vector-borne disease in Europe caused by tick-borne encephalitis virus (TBEV) which belongs to the Flaviviridae family [Reference Bogovic and Strle1, Reference Lindquist and Vapalahti2].
There are 3 subtypes of TBEV: European (endemic in rural and forested areas of Central, Eastern and Northern Europe), Far-Eastern (endemic in Far-Eastern Russia and in forested regions of China and Japan) and Siberian subtype (endemic in the Urals region, Siberia and Far-Eastern Russia, and also in some areas in North-Eastern Europe) [3].
The number of TBE cases in Europe varies depending on the country and ranges from a few up to 1000 cases per year [3].
In Poland, TBE morbidity increased several fold in 1993 and has remained constant since then [Reference Pancewicz4]. According to the National Hygiene Institute, 200–300 cases are reported annually [5]. The cases from Podlaskie Voivodeship constitute ca. 46% of all the cases noted in Poland [Reference Czupryna6]. The Department of Infectious Diseases and Neuroinfections of the Medical University of Bialystok is a reference centre for the whole region, so the majority of TBE patients are hospitalised here.
The clinical course of TBE may present as meningitis (M), meningoencephalitis (ME) or meningoencephalomyelitis (MEM) [Reference Kaiser7, Reference Valarcher8]. M is usually mild and patients recover after 2–3 weeks of symptomatic treatment. In patients with ME and MEM, the course of the disease is more severe. The mortality rate is 1–4% and the fatal outcome is usually observed in the elderly or immunodeficient patients, but may also happen in young persons [Reference Zajkowska9].
Although most of patients quickly recover from the disease, some require further neurological and psychiatric treatment due to persisting symptoms. Sometimes the sequelae might be the continuation of symptoms from the acute phase, yet in some cases they may appear after hospitalisation. The aim of the study was to evaluate sequelae in patients hospitalised because of TBE and analyse the potential risk factors predisposing to sequelae development.
Material and methods
A retrospective analysis of medical records of 1072 patients with TBE hospitalised in the Department of Infectious Diseases and Neuroinfections of the Medical University of Bialystok between 1993 and 2014 was performed. All patients had 1-month follow-up appointment after the first hospital discharge. The duration of further observation lasted from 6 months to 10 years and depended on the patients’ clinical status and occurrence of sequelae.
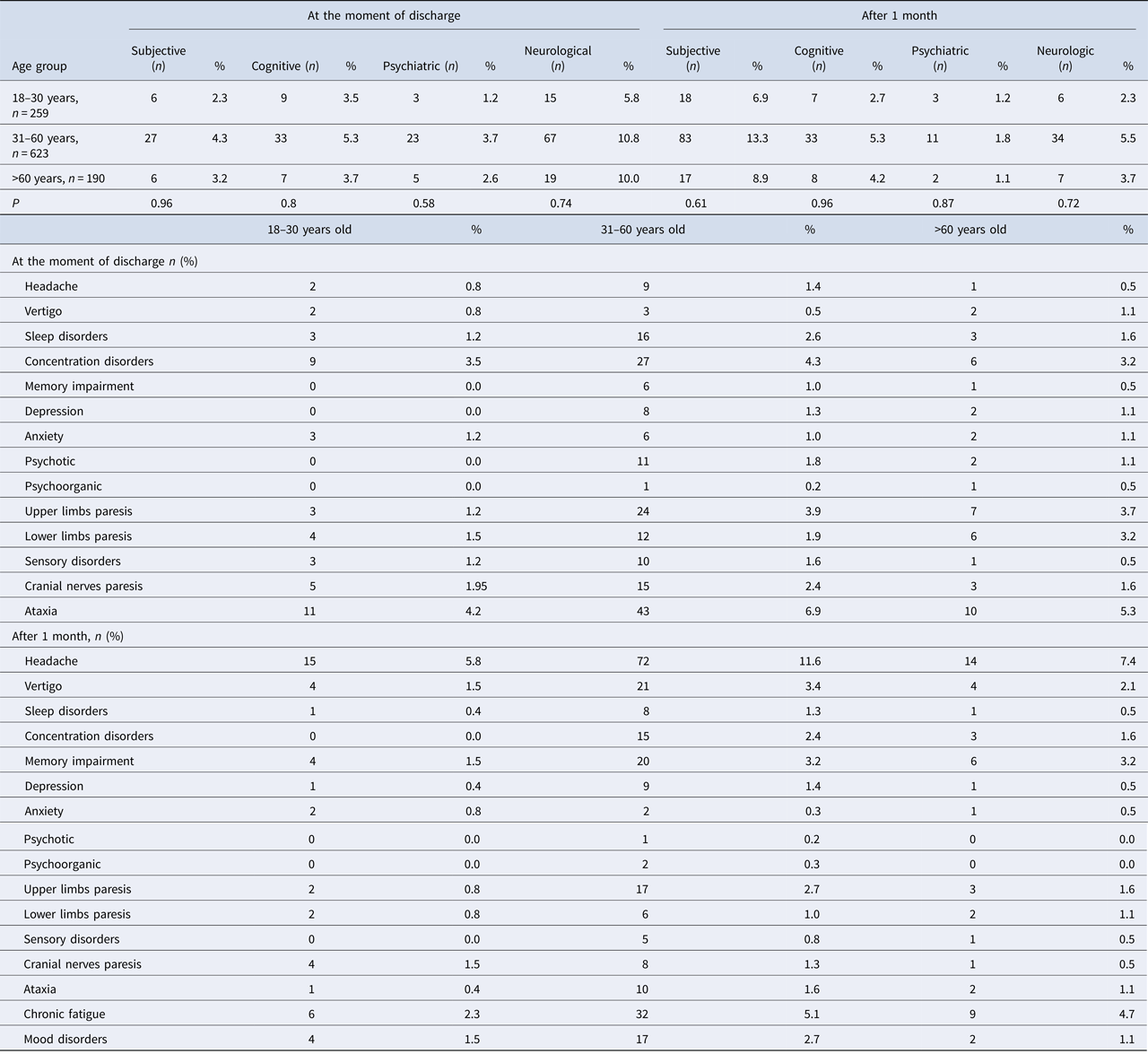
Medical data, such as patients’ age, gender, place of living, subjective complaints (such as headache, vertigo, sleep disorders and fatigue), neurological (such as upper limbs paresis, lower limbs paresis, sensation disorders, cranial nerves paresis and cerebellar syndrome) and psychiatric sequelae (such as irritability, concentration disorders, memory impairment, depression, anxiety, psychotic and psychoorganic symptoms) were analysed twice: at the moment of discharge and at follow-up visits 1 month after discharge (Table 1). Patients were examined by specialists: a neurologist and a psychiatrist.
Table 1. Definition of TBE confirmed case
Psychiatric symptoms were diagnosed by a clinical psychiatrist, therefore we considered these symptoms objective. Among these were memory and concentration impairment. The symptoms reported only by patients but with no clinical confirmation were defined as subjective complaints. The statistical analysis included laboratory parameters such as data from cerebrospinal fluid (CSF) general analysis.
Sequelae were defined as symptoms that persisted or appeared at least 1 month after the first hospitalisation (discharge from hospital) and affected patients’ life.
TBE was confirmed by the detection of specific antibodies with enzyme-linked immunosorbent assay (ELISA) using the kit of Virion/Serion (Wurzburg, Germany) according to the manufacturer's instructions.
TBE antibodies’ titre was measured with an Enzygnost Anti-TBE/FSME Virus (IgG, IgM) Siemens test (enzyme immunoassay for the qualitative detection and quantitative determination of specific antibodies to the TBE virus in the human serum and plasma). The measuring range for the quantitative antibody determination of anti-TBE virus IgG is 7–700 U/ml.
The result of the IgM test was quantified by forming a quotient from the absorbance value of the test sample and the appropriate cut-off as the index. The index for the upper margin for retesting (‘retest index’) was calculated by dividing the value of the upper margin for retesting (cut-off +0.1) by the cut-off. Index 1.4 times higher than the retest index was considered a positive result.
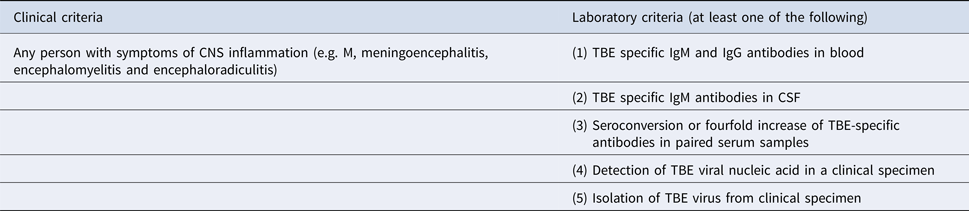
The patients enrolled in the study fulfilled clinical, laboratory and epidemiological criteria of the TBE definition [10] (Table 1).
Confirmed TBE case is diagnosed in any person meeting the clinical and laboratory criteria for case confirmation.
TBE was confirmed in all of the included patients.
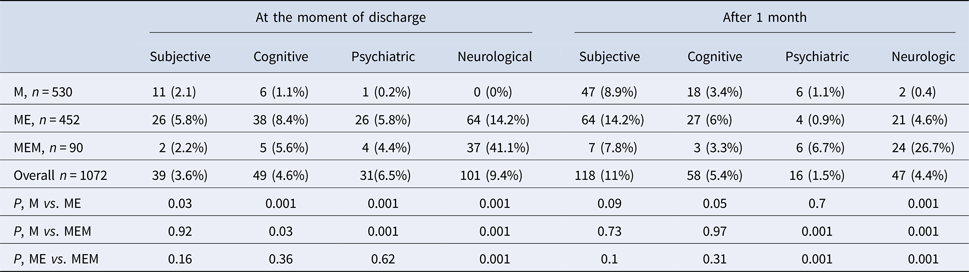
As far as clinical forms of TBE are concerned, M was diagnosed in 530 patients (49.4%), ME in 452 patients (42.2%) and MEM in 90 patients (8.4%) (Table 2).
Table 2. Comparison of sequelae prevalence in patients with M, ME and MEM
M was diagnosed on the basis of inflammatory parameters in CSF with no focal neurological symptoms. ME was diagnosed when the inflammatory parameters in CSF, altered consciousness and focal neurological symptoms were present. MEM was diagnosed when ME symptoms were accompanied by flaccid paralyses of the limbs.
The patients were asked about vaccination status against Flaviviruses, including TBE and yellow fever. All patients were local inhabitants and did not report recent journeys to abroad, therefore there was no need for cross-reactive exclusion reaction with other Flaviviridae. There has been no confirmed human case of West-Nile Virus or Yellow Fever Virus infection reported in Poland so far. The patients declared no vaccination against YFV, JEV or TBE.
Statistical analysis
Statistical analysis was performed using Statistica 12. Groups were compared by Kruskal–Wallis test and Mann–Whitney U test. Positive outcome (defined as no death or ICU treatment) in groups with different clinical forms of TBE was calculated by the Kaplan–Meier method and compared with the use of the log-rank test. Hazard ratio for sequelae occurrence was calculated with logistic regression. Receiver operating characteristic (ROC) curve analysis was performed for protein concentration in CSF.
Results
Clinical symptoms on discharge
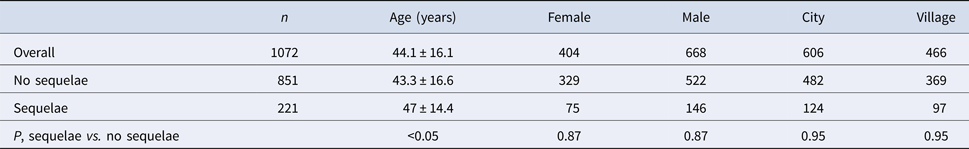
168 out of 1072 (15.7%) analysed patients were discharged with the remaining symptoms (Table 3). Among them there were 65 men (38.7%) and 103 women (61.3%). 93 subjects were inhabitants of towns and cities (55.4%) and 75 of villages (44.6%).
Table 3. Prevalence of symptoms in TBE patients (n = 1072) at the moment of discharge from hospital and after 1 month
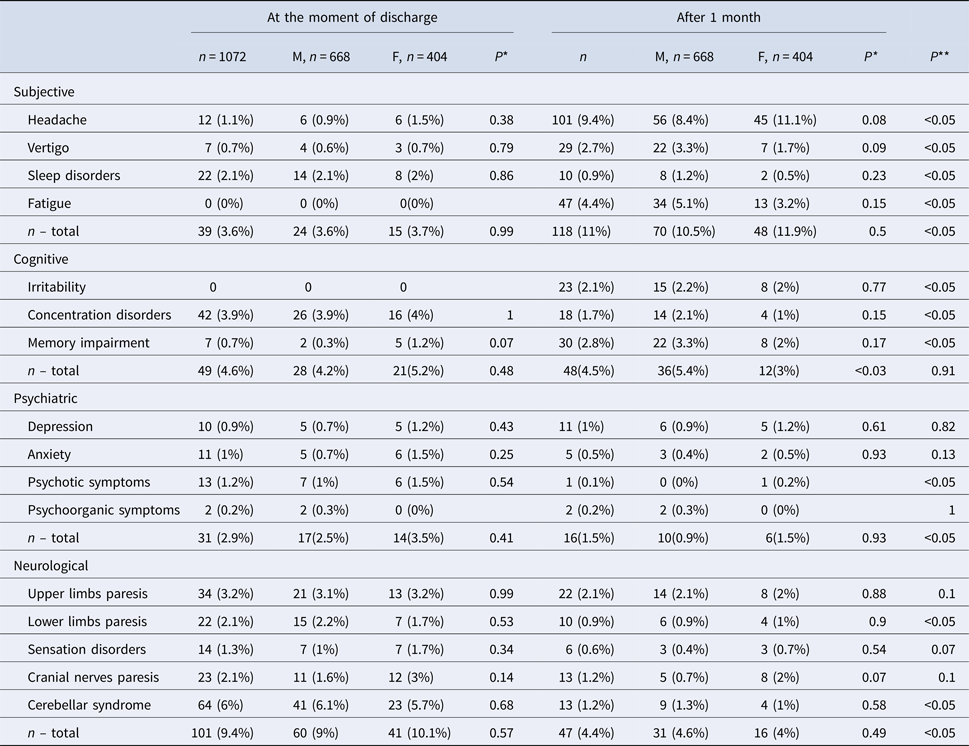
*Comparison between males and females.
**Comparison of the overall number of symptoms at the moment of discharge and during the follow-up.
The most frequent subjective complaints were sleep disorders, cognitive symptoms – concentration disorders, psychiatric–psychotic symptoms and neurologic–cerebellar syndrome (Table 3).
Clinical symptoms during follow-up (sequelae)
221 out of 1072 (20.6%) analysed patients developed sequelae (Table 4). Among them there were 146 men (66.1%) and 75 women (33.9%). 124 were inhabitants of towns and cities (56.1%) and 97 of villages (43.9%). The most frequent subjective sequelae were headaches, cognitive sequelae – memory impairment, psychiatric sequelae – depression and neurological sequelae – upper limbs paresis (Table 3).
Table 4. Demographic analysis of patients with/without sequelae
Sequelae were observed in 67 patients with M (12.6%), 115 patients with ME (25.4%) and 39 patients with MEM (43.3%) (Table 2).
Comparison of clinical results between the analysed groups
Patients with sequelae were significantly older than patients with no sequelae (Table 2). Sequelae were most prevalent in patients aged 31–60 years old (Table 5).
Table 5. Comparison of sequelae prevalence with regard to the patients’ age groups
Statistical analysis showed no difference in the prevalence of all complications between males and females (Table 4).
Subjective sequelae reported during the follow-up after 1 month post-hospitalisation were more frequent than subjective complaints during the hospitalisation (118 patients vs. 39 patients P < 0.001), while objective neurological symptoms during the hospitalisation were more frequent than neurological sequelae (101 patients vs. 47 patients P < 0.001) (Table 3). No significant differences between psychiatric symptoms during hospitalisation and sequelae were observed (70 patients vs. 56 patients P = 0.15).
There were no significant differences in sequelae between the patients with biphasic and monophasic courses of the disease.
Comparison of laboratory test results between the analysed groups
The mean pleocytosis and protein concentration in CSF in the sequelae group were 114.85 ± 136.49 cells/μl and 71.45 ± 30.16 mg/dl while in the non-sequelae group – 109.44 ± 109.29 cells/μl and 65.41 ± 24.8 mg/dl (difference in protein concentration was statistically significant, P < 0.05).
The mean pleocytosis and protein concentration in CSF in patients with neurological sequelae were 178.95 ± 228.42 cells/μl (normal <5 cells/μl) and 76.67 ± 29.62 mg/dl (normal <45 mg/dl) while in the rest of the patients – 107.77 ± 108.24 cells/μl and 66.45 ± 26.09 mg/dl (difference in protein concentration was statistically significant, P < 0.05).
Logistic regression, ROC curve and Kaplan–Meier curve analysis results
Patients’ age and protein concentration in CSF were independent risk factors of the sequelae development. Logistic regression analysis confirmed that the risk of sequelae was increased in patients with higher protein concentration in CSF (r = 8.9; P = 0.002) and in older patients (r = 8.2; P = 0.004).
Logistic regression analysis showed that the risk of neurological complications persistence was increased in patients with higher protein concentration in CSF (r = 5.8; P = 0.01).
Additionally, ROC curve analysis showed that protein concentration in CSF differentiated patients with neurological sequelae and patients without neurological sequelae (AUC = 0.607 at cut-off 83.8 mg/dl; the sensitivity was 41.46% and specificity was 80.37%) (Fig.1).
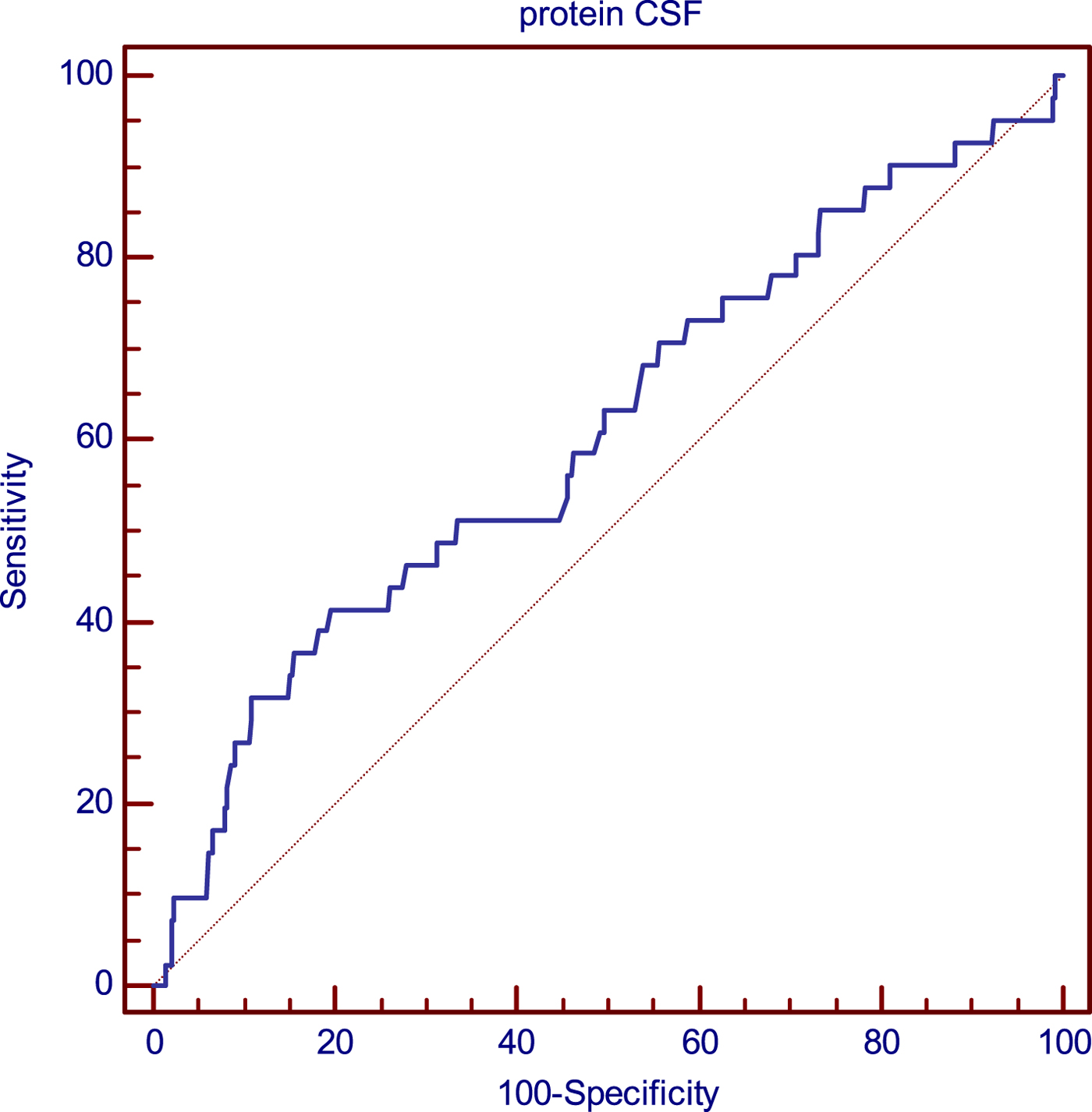
Fig. 1. Analysis of the relationship of CSF protein concentration and probability of late neurological sequelae occurrence using the ROC curves.
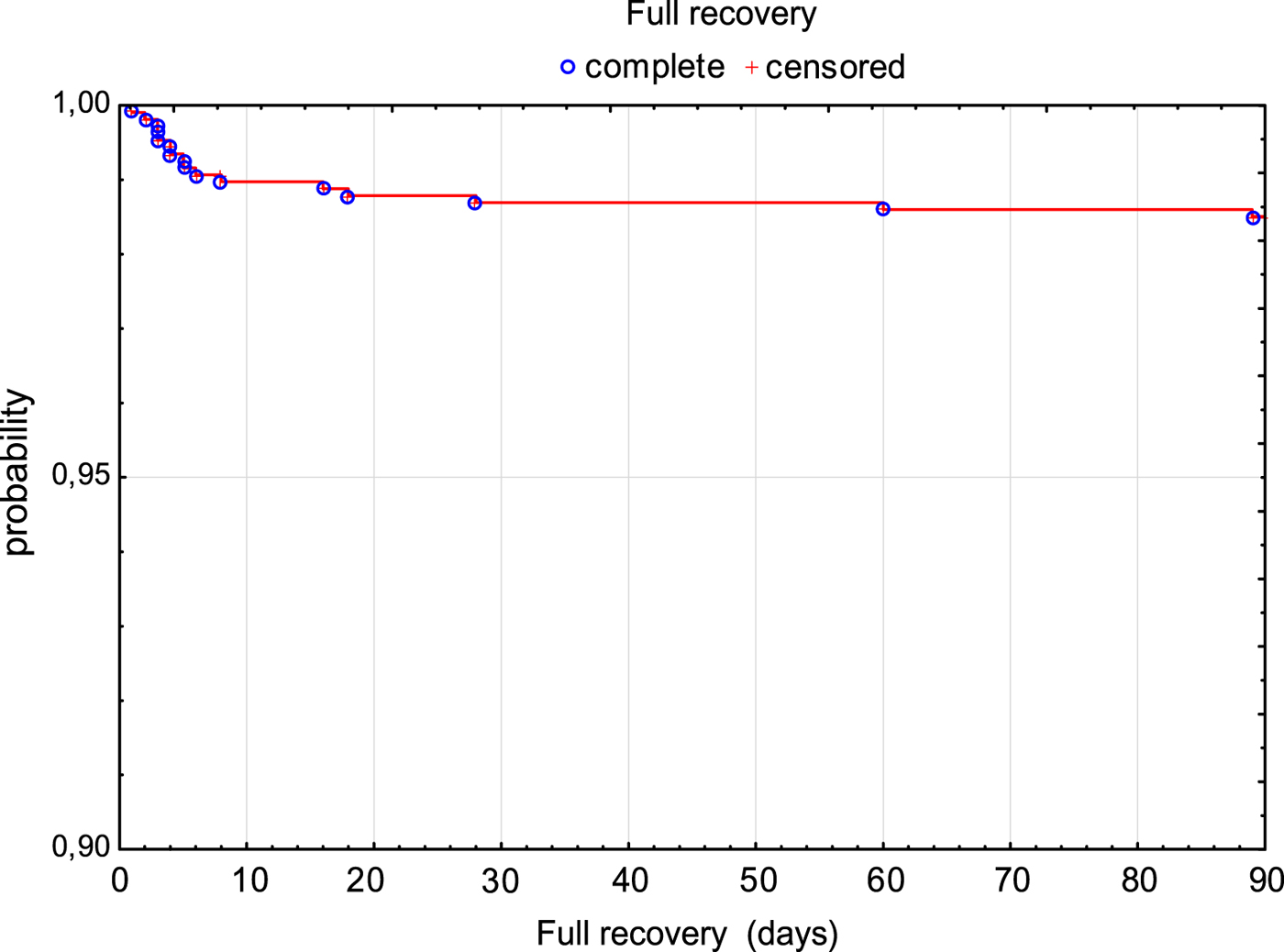
The risk of fatal outcome or hospitalisation in ICU was assessed by Kaplan–Meier curves in all patients (Fig. 2). Additionally, patients with milder course of the disease (M and ME) and more severe course of the disease (MEM) were compared. Risk of unfavourable outcome of the disease was significantly higher in MEM patients (P < 0.001) (Fig. 3).
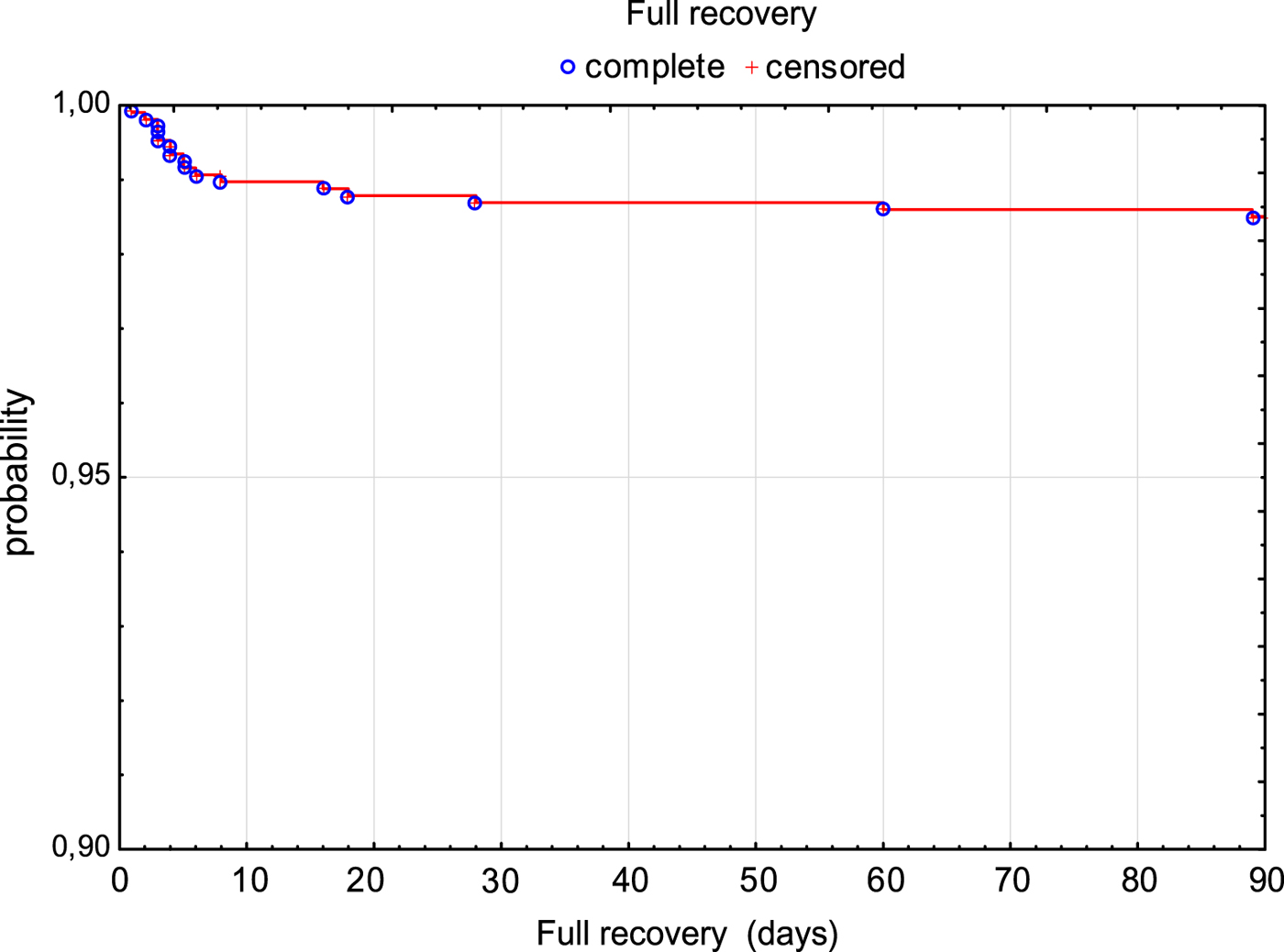
Fig. 2. Survival rate (3 months without death or need for ICU treatment) in TBE patients.
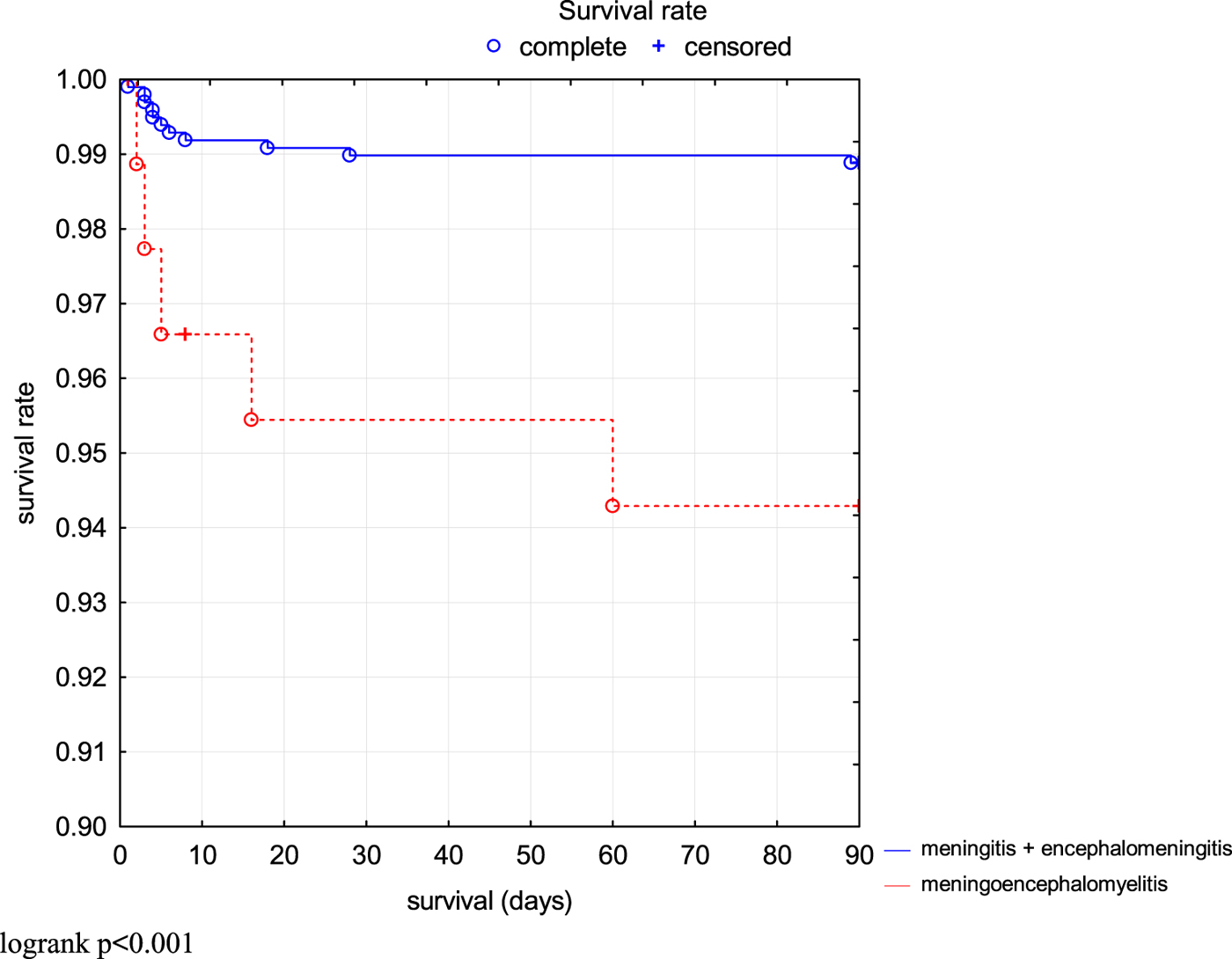
Fig. 3. Comparison of survival rate (3 months without death or ICU treatment) in TBE patients depending on the clinical form.
Discussion
There are many reports concerning the acute phase of TBE, yet only a few presented long-term outcomes of the disease. So called post-encephalitic TBE syndrome was described in 35–58% of the patients. It may result in long-term morbidity that often affects patient's quality of life and forces a change in lifestyle. The most commonly reported symptoms were cognitive or neuropsychiatric complaints (reduced stress tolerance and impaired ability to memorise), balance disorders, headache, dysphasia, hearing defects and limb paresis [Reference Kaiser7, Reference Kaiser11–Reference Laursen and Knudsen14]. The results of our research indicate that 1 month after discharge from hospital 20.6% of patients with TBE suffered from various sequelae. This is less than it was reported in other studies. Kaiser [Reference Kaiser11] observed sequelae in 27% patients. Mickiene et al. [Reference Mickiene15] described sequelae in 77 out of 250 patients 12 weeks after the onset of the disease – 49 (63.6%) had only neuropsychiatric and cognitive complaints, whereas the remaining patients had objective neurological signs. Also Veje et al. [Reference Veje16] reported that patients with a history of TBE scored significantly lower than controls in the dimensions of memory/learning, executive functions, vigilance and physical impairments when a standardised questionnaire, the ESGQ 2000, specifically designed for sequelae after encephalitis was used.
One of the reasons for the difference between our results and those reported by other researchers is the distribution of clinical forms of TBE. In our study, M was the dominant form of the disease, while in other studies moderate and severe forms (ME and MEM) were more frequent. This obviously increased the rate of sequelae.
The other reason would be the relatively strict criteria we used in defining the sequelae (we excluded symptoms that were mild and did not affect patient's life), which prioritised neurological rather than cognitive sequelae. Upon comparison of the number of cognitive sequelae in our study and other reports, it seems that this problem might be underrepresented in our report.
The frequency of sequelae differed depending on the clinical form of TBE. The patients with ME more often developed subjective complications, while the patients with MEM suffered from neurological sequelae. The probability of unfavourable outcome (death or need for an intensive care treatment) was also significantly higher in the MEM group. This remains in accordance with the results reported by Lenhard et al. [Reference Lenhard17].
The neurological symptoms prevailed on discharge, while subjective symptoms dominated during the follow-up. The TBE virus usually affects cerebellum, thalamus and caudate nucleus [Reference Kaiser11, Reference Czupryna18]. In our previous study, ataxia was a dominant neurological symptom during the acute phase of TBE and was present in 14.1% of patients [Reference Czupryna6]. In this study, ataxia was also the most frequent early complication of TBE (6%). However, it was persistent only in 1.2% of patients. The most of the sequelae were mild, although influencing the quality of life, subjective symptoms such as headache or vertigo. In some patients these symptoms were present even 10 years after the acute phase of the disease (they reported it during the later follow-up). It is hard to explain why the subjective symptoms were more common during the follow-up than on discharge. No pathophysiological mechanism has been reported for the cognitive symptoms and these sequelae cannot be explained purely by the affinity of the virus to specific parts of the brain as in the case of neurological sequelae.
Age and protein concentration in CSF were the independent risk factors predisposing to sequelae development. Statistical analysis confirmed that the CSF protein concentration was the independent risk factor for the persistence of neurological symptoms.
The risk of late neurological complication persistence was increased in patients with higher protein concentration in CSF. This is similar to Kaiser [Reference Kaiser11], who reported that patients with persistent sequelae presented with higher protein concentration in CSF than the rest of TBE patients. The protein concentration in acute neuroinfection may be elevated due to: blood brain barrier (BBB) dysfunction, abnormal CSF flow and impairment of protein absorption, increased intrathecal immunoglobulin production and elevation of acute inflammatory mediators including cytokines [Reference Blakeley, Irani and Irani19]. Grygorczuk et al. [Reference Grygorczuk20] indicates that the increase of albumin quotient and protein concentration in TBE patients suggests that increased BBB permeability is an important pathogenetic mechanism of TBE, but its dynamics is suggestive rather of a protracted BBB impairment during CNS inflammation and healing than of an early disruption preceding neuroinvasion. In the mouse TBE model described by Růžek et al. [Reference Růžek21], the BBB dysfunction does not occur during systemic infection, but only after the establishment of encephalitis, simultaneously with the up-regulated intrathecal TNFα and IL-6 synthesis. Such course of events does not contradict the pathogenetic role of BBB permeability, but postpones it to the later, neurological phase of the disease.
Czupryna et al. reported that in TBE patients with paresis, albumin quotient on admission was higher than in the rest of the group. This is consistent with the role of an early BBB impairment in the neuropathology and hints for a possibility of specific pathogenesis of particular neurological complications [Reference Czupryna18].
As the treatment of TBE is only symptomatic, the only way to prevent sequelae development is to avoid the infection. The best example of vaccine effectiveness is Austria with its mandatory vaccinations and the number of annual TBE cases dropping from 300–700 to 50–100 [Reference Kunze and Böhm22].
The vaccination against TBE was introduced in Poland over 20 years ago. Since then it has been mandatory for forestry workers, which resulted in a great significant decrease in TBE incidence among this group. The most at-risk populations are farmers and people who frequently visit forests (mushroom or berry picking and leisure activity). The vaccination coverage in this group is very low due to the unawareness of the disease or high cost of the vaccine [Reference Czupryna6, Reference Godfrey and Randolph23]. Despite TBE being endemic in some regions of Poland, only 0.34% of the population was vaccinated between 1999 and 2007 [Reference Kollaritsch24, Reference Zavadska25].
The limitation of our study is its retrospective character. Some information relevant to the study may have not been recorded in the medical files. The retrospective character of our study may be the reason for underrepresentation of cognitive symptoms. It seems that in prospective studies concentrating on cognitive symptoms with the usage of specialised questionnaires the frequency of these sequelae is higher than in our study. Therefore, those reports may be superior in this field.
In conclusion, it appears very disappointing that although the vaccine is theoretically available for Polish population, TBE still remains a serious clinical and epidemiological problem, with long-lasting complications, which has not changed over the past few years. We observe that there is a necessity for a better vaccination program, which would effectively prevent the sequelae development.
Conclusions
(1) Although TBE seems to be a mild and preventable disease, serious complications may occur, with the sequelae affecting 20.6% of TBE patients. Neurological symptoms, such as upper limbs paresis, lower limbs paresis, sensation disorders, cranial nerves paresis and cerebellar syndrome are the most common group of early clinical signs, while subjective symptoms, such as headache, vertigo, sleep disorder and fatigue are the most common sequelae. Patients with MEM are predisposed to neurological complications, while subjective symptoms are more common in ME.
(2) Age and protein concentration in CSF were independent risk factors for sequelae development. The risk of late neurological complication persistence was increased in patients with higher protein concentration in CSF.
(3) There is a need for better vaccination program, which would effectively prevent the TBE sequelae development.