Implications
Unlike other B vitamins, vitamin B12 is not present in plants and is produced only by bacteria if cobalt supply is adequate. Therefore, dairy cows rely on synthesis of the vitamin by the bacteria present in rumen to cover their requirements. However, the proportion of cobalt used for these syntheses is low. Providing a preformed part of the vitamin B12 molecule (5,6-dimethylbenzimidazole) increased by 34% apparent synthesis of the vitamin by ruminal bacteria but failed to increase substantially efficiency of cobalt utilization. The results highlight the lack of knowledge on nutritional factors driving ruminal production of vitamin B12.
Introduction
Increasing vitamin B12 supply of dairy cows with an adequate folate status has been reported to improve efficiency of energy metabolism in early lactation (Girard and Matte, Reference Girard and Matte2005; Graulet et al., Reference Graulet, Matte, Desrochers, Doepel, Palin and Girard2007; Preynat et al., Reference Preynat, Lapierre, Thivierge, Palin, Matte, Desrochers and Girard2009). However, as much as 80% of a dietary supplement of cyanocobalamin, the synthetic form of vitamin B12, was catabolized in the rumen (Girard et al., Reference Girard, Santschi, Stabler and Allen2009b). Thus, the use of such supplements in commercial dairy farms is not economically viable. Unlike other B vitamins, vitamin B12 (cobalamin, CBL) is produced only by bacteria and archaebacteria if cobalt supply is adequate (Martens et al., Reference Martens, Barg, Warren and Jahn2002). Even ciliate protozoa present in rumen need vitamin B12, which they obtain by ingestion of these vitamin B12-synthesizing bacteria (Bonhomme et al., Reference Bonhomme, Durand, Quintana and Halpern1982). In addition, bacteria present in the rumen use dietary cobalt to produce molecules chemically related to CBL but devoid of biological activities for the host; these molecules are called vitamin B12 analogs and their presence in rumen content has been reported in previous studies (Ford et al., Reference Ford, Holdsworth, Kon and Porter1953a; Dawbarn et al., Reference Dawbarn, Hine and Smith1957; Gawthorne, Reference Gawthorne1970a; Dryden and Hartman, Reference Dryden and Hartman1971).
The molecule of vitamin B12 is composed of four major parts: the corrin ring, the nucleotide moiety, the aminopropanol residue linking the nucleotide to the corrin ring and the B ligand linked to the cobalt atom within the corrin ring. In CBL, 5,6-dimethylbenzimidazole (5,6-DMB) is the base in the nucleotide moiety. In addition to CBL, six cobamides, molecules in which the 5,6-DMB in the nucleotide moiety is replaced by other bases and one cobinamide (COB), which lacks the nucleotide moiety have been detected in bovine ruminal content, duodenal and ileal digesta, and feces (Dryden and Hartman, Reference Dryden and Hartman1971; Girard et al., Reference Girard, Berthiaume, Stabler and Allen2009a, Reference Girard, Santschi, Stabler and Allen2009b). Increasing the proportion of forages in the diet has frequently been reported to increase CBL in rumen without effect on total vitamin B12 (CBL+analogs; Sutton and Elliot, Reference Sutton and Elliot1972; Walker and Elliot, Reference Walker and Elliot1972; Santschi et al., Reference Santschi, Chiquette, Berthiaume, Martineau, Matte, Mustafa and Girard2005). Moreover, apparent ruminal synthesis of CBL is negatively correlated with rumen pH (Schwab et al., Reference Schwab, Schwab, Shaver, Girard, Putnam and Whitehouse2006), starch disappearance (Sutton and Elliot, Reference Sutton and Elliot1972) or starch intake (Schwab et al., Reference Schwab, Schwab, Shaver, Girard, Putnam and Whitehouse2006) and positively correlated with ADF or NDF intakes (Sutton and Elliot, Reference Sutton and Elliot1972; Schwab et al., Reference Schwab, Schwab, Shaver, Girard, Putnam and Whitehouse2006). Use of probiotics able to maintain a higher rumen pH during experimental induction of subacute ruminal acidosis also prevented the decrease in CBL concentration in rumen content observed at that time (Chiquette et al., Reference Chiquette, Lagrost, Girard, Plaizier and Talbot2012). Seemingly in contradiction with the effects of rumen pH on CBL concentration in rumen, Cannizzo et al. (Reference Cannizzo, Gianesella, Casella, Giudice, Stefani, Coppola and Morgante2012) observed that plasma concentrations of total vitamin B12 increased in cows with a rumen pH smaller than 5.6. However, this observation is in accordance with Walker and Elliot (Reference Walker and Elliot1972) and Sutton and Elliot (Reference Sutton and Elliot1972) who reported that decreasing the proportion of forages in the diet increased serum concentrations of total vitamin B12 (Sutton and Elliot, Reference Sutton and Elliot1972; Walker and Elliot, Reference Walker and Elliot1972) but decreased the proportion of CBL (Sutton and Elliot, Reference Sutton and Elliot1972).
Nevertheless, it has been long recognized that the primary factor affecting the amounts of CBL and analogs synthesized by ruminal bacteria is cobalt (Gawthorne, Reference Gawthorne1970a). However, the proportion of dietary cobalt used for these synthetic processes is relatively low in sheep and cows, varying from 3% to 15% (Smith and Marston, Reference Smith and Marston1970; Stemme et al., Reference Stemme, Lebzien, Flachowski and Scholz2008; Girard et al., Reference Girard, Santschi, Stabler and Allen2009b). In vitro studies demonstrated that addition of different bases to the culture media enhanced the synthesis of the corresponding cobamides at the expense of the other forms (Ford et al., Reference Ford, Holdsworth and Kon1955; Gawthorne, Reference Gawthorne1970b). Rickard et al. (Reference Rickard, Bigger and Elliot1975) observed that feeding 5,6-DMB increased production of ‘true vitamin’ B12 (CBL) in rumen of sheep but had no effect on apparent ruminal synthesis of total vitamin B12. Therefore, the main objective of the present study was to determine if, in dairy cows, a daily supplement of 5,6-DMB could increase utilization of dietary cobalt for synthesis of CBL at the expense of vitamin B12 analogs. Effects of the 5,6-DMB supplement on animal performance, ruminal fermentation and protozoa counts, omasal flow of nutrients and nutrient digestibility in rumen were also studied.
Materials and methods
Care and handling of the animals were conducted as outlined in the guidelines of the Canadian Council on Animal Care (2009), and the study was approved by the Institutional Animal Care Committee of the Dairy and Swine Research and Development Centre, Sherbrooke, Québec, Canada.
Animals, experimental design and treatments
Eight ruminally cannulated multiparous Holstein cows averaging (mean±s.d.) 238±21 days in milk and 736±47 kg of BW at the beginning of the experiment were randomly assigned to one of two treatments in a crossover design. Daily treatments were administered intraruminally via gelatin capsules containing or not 1.5 g of 5,6-dimethylbenzimidazole (Sigma-Aldrich, St Louis, MO, USA). Cows were fed twice daily at 0800 and 2000 h a total mixed ration (Table 1). Mineral and vitamin supply was calculated to meet or exceed the National Research Council (2001) recommendations and provided 0.1 mg Co/kg of DM. Orts were collected daily at 0700 h, and the amount of feed offered to the cows was adjusted daily to yield refusals equal to ∼5% to 10% of intake. Cows were housed in a tie stall barn and had free access to water throughout the experiment. Each period lasted 29 days (total of 58 days) and consisted of 21 days for treatment adaptation and 8 days for data and samples collection.
Table 1 Ingredient and nutrient composition of the TMR fed to Holstein cows supplemented or not with 5,6-DMB
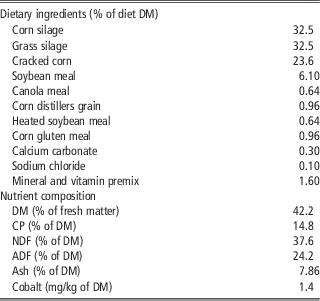
TMR=total mixed ration; 5,6-DMB=5,6 dimethylbenzimidazole; DM=dry matter; CP=crude protein.
Feed sampling and analyses
Forage samples were collected twice a week and were immediately analyzed by near infrared reflectance spectrometry (Agri-Analyse Laboratoire Agricole, Sherbrooke, Québec, Canada), to adjust, when necessary, the amounts of energy and protein supplements in the total mixed ration to maintain as much as possible a consistent dietary nutrient composition throughout the experimental period. Samples of diet ingredients, total mixed ration and orts were collected in the last 3 days (days 27 to 29) of each period, dried at 55°C for 48 h, and ground to pass through a 1-mm screen (Wiley mill, Arthur H. Thomas, Philadelphia, PA, USA) before analytical procedures. Samples were analyzed for analytical DM (method 930.15; Association of Official Analytical Chemists, 2006) and ash with a thermogravimetric analyzer (model TGA-601; Leco Corporation, St Joseph, MI, USA); total nitrogen (N) using micro-Kjeldahl analysis (Kjeltec 2400 instrument; Foss Analytical, Hillerød, Denmark; method 976.06; Association of Official Analytical Chemists, 2006); NDF and ADF with the Ankom200 fiber analyzer (Ankom Technology, Fairport, NY, USA) using heat-stable α-amylase and sodium sulfite (Van Soest et al., Reference Van Soest, Robertson and Lewis1991); and cobalt by atomic absorption spectrometry (Varian Vista AX-CCD, Varian Instruments, Mulgrave, Australia).
Animal performance, milk and plasma sampling and analyses
Cows were milked twice daily at ∼0800 and 2000 h, and milk yield was recorded at each milking. Milk samples from a.m. and p.m. milkings were collected from day 27 (p.m.) to day 29 (a.m.) of each experimental period, preserved in tubes containing 2-bromo-2-nitropropane 1,3 diol, and kept at 4°C until shipped for determination of fat, protein, lactose and milk urea N by mid-IR reflectance spectroscopy (Valacta, Sainte-Anne-de-Bellevue, Québec, Canada). Concentrations and yields of milk components and milk urea N were computed as the weighted means from a.m. and p.m. milk yields on each test day.
On day 27, blood samples were collected by venipuncture of the coccygeal vein <1 h after the morning feeding using Vacutainer tubes (Becton, Dickinson and Co., Franklin Lakes, NJ, USA) containing EDTA for vitamin B12 determinations. All tubes were immediately placed on ice and centrifuged at 4ºC for 15 min at 3300×g. Plasma was kept frozen at −20ºC until further analysis. Plasma concentrations of vitamin B12 were measured in duplicate by radioassay for two replicate samples (SimulTRAC-S Radioasssay kit, Vitamin B12 (57Co)/Folate (125I), MP Biomedicals, Diagnostics Division, Orangeburg, NY, USA).
Omasal sampling and analyses
Spot samples of omasal digesta leaving the rumen were collected through the reticulo-omasal orifice from the eight ruminally cannulated cows using the omasal sampling technique developed by Huhtanen et al. (Reference Huhtanen, Brotz and Satter1997) and Ahvenjärvi et al. (Reference Ahvenjärvi, Vanhatalo, Huhtanen and Varvikko2000), as adapted by Reynal et al. (Reference Reynal, Broderick, Ahvenjärvi and Huhtanen2003). The following omasal digesta markers were used: indigestible NDF (Huhtanen et al., Reference Huhtanen, Kaustell and Jaakkola1994) for the large particle phase (LP), YbCl3 (modified from Siddons et al., Reference Siddons, Paradine, Beever and Cornell1985) for the small particle phase (SP) and Cr-EDTA (Udén et al., Reference Udén, Colucci and Van Soest1980) for the fluid phase (FP). A marker solution containing YbCl3, Cr-EDTA and 15NH4SO4 with 10 atom percentage excess 15N (Isotec, Miamisburg, OH, USA) as a bacterial marker was prepared as described by Reynal and Broderick (Reference Reynal and Broderick2005). A sample of 500 ml of omasal digesta (background) was taken from each cow before the beginning of markers infusion on day 22 of each period to determine the natural abundance of 15N. Cows were then pulse-dosed with 3.0 l of the same markers solution used during the continuous infusion. The external markers Cr-EDTA, YbCl3 and 15NH4SO4 were continuously infused into the rumen from days 23 to 28 (mean=125 h of infusion) using four peristaltic pumps (Masterflex L/S model no. 7523−50, Cole-Parmer Instrument Co., Barrington, IL, USA) at an average constant rate of 3.02 kg/day providing daily 2.56 g of Cr, 2.34 g of Yb and 0.24 g of 15N. Omasal sampling was initiated ∼72 h after beginning the markers infusion with samples taken six times daily at 1-h intervals during 2 consecutive days in each period to represent a 12-h feeding cycle as follows: 0, 1, 2, 3, 4 and 5 h on day 27 and 6, 7, 8, 9, 10 and 11 h on day 28 with 0 h representing the time of the morning meal. The omasal sampling tube was kept inserted into the reticulo-omasal orifice for the entire collection of omasal digesta, which lasted ∼7 h/day. Before each sampling point it was necessary to confirm the location of the sampling tube and occasionally it had to be repositioned into the omasal canal. It was also necessary a few times to unplug the holes in the end of the sampling tube because of the presence of coarse digesta. At each of the six daily sampling times, a 470-ml spot sample of omasal digesta was collected and split under continuous mechanical agitation into two subsamples of 70 and 400 ml. The six daily 70-ml subsamples were pooled and stored on ice inside a refrigerator (4°C) for the duration of the daily omasal digesta collection. These six daily 70-ml subsamples were pooled into a single composite of 420 ml per cow and transported to the laboratory for bacterial isolation. The six daily 400-ml subsamples were stored at −20°C and pooled over 2 days to obtain a single 4.8-l composite from each cow in each period for later separation into the three omasal digesta phases (LP, SP and FP).
The fluid-associated bacteria (FAB) and particle-associated bacteria (PAB) were isolated from the daily 420-ml composites from each cow on each of the 2 sampling days using filtration and differential centrifugation as described earlier in detail (Brito et al., Reference Brito, Tremblay, Lapierre, Bertrand, Castonguay, Bélanger, Michaud, Benchaar, Ouellet and Berthiaume2009). The resulting FAB and PAB pellets were stored at −20°C, lyophilized, ground with a mortar and pestle, and finally pooled by cow per period by mixing equal amounts of dry matter for later analysis. The 4.8-l pooled omasal composites were thawed at room temperature, separated into the three omasal phases (LP, SP and FP) as described by Brito et al. (Reference Brito, Tremblay, Lapierre, Bertrand, Castonguay, Bélanger, Michaud, Benchaar, Ouellet and Berthiaume2009), and stored at −20°C until lyophilized. After lyophilization, these samples were ground through a 1-mm screen before analyses of digesta markers.
Concentrations of Cr, Yb and indigestible NDF in LP and SP and Cr and Yb in FP were determined using the methods detailed earlier (Brito et al., Reference Brito, Tremblay, Lapierre, Bertrand, Castonguay, Bélanger, Michaud, Benchaar, Ouellet and Berthiaume2009). Marker concentrations were used to physically recombine DM from the lyophilized FP, SP and LP in the correct proportions to reconstitute the omasal true digesta (OTD) flowing out of the rumen using the triple-marker method of France and Siddons (Reference France and Siddons1986). Concentrations of Cr, Yb and indigestible NDF were distinctly greater in, respectively, the FP, SP and LP, thus allowing for successful application of the triple marker method. On a dry matter basis, SP and LP subsamples were mixed in the correct proportions based on the digesta markers to yield a 2-g sample that was sequentially ground through a 1-mm screen and a 0.5-mm screen (Marathon Electric mill, Wausau, WI, USA) and defined as particle phase (PF).
Reconstituted OTD samples were analyzed for analytical DM, ash, total N, NDF and ADF as described previously for diet ingredients, total mixed rations and orts. Extracts from OTD samples were prepared and analyzed for ammonia-N (NH3-N) as described by Brito et al. (Reference Brito, Tremblay, Lapierre, Bertrand, Castonguay, Bélanger, Michaud, Benchaar, Ouellet and Berthiaume2009).
Samples of FAB, PAB, PF and background omasal digesta were prepared for non-ammonia nitrogen (NAN) and 15N analyses as described in detail elsewhere (Brito et al., Reference Brito, Tremblay, Lapierre, Bertrand, Castonguay, Bélanger, Michaud, Benchaar, Ouellet and Berthiaume2009). Both NAN and 15N were analyzed with a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon Ltd, Cheshire, UK) at the Stable Isotope Facility of the University of California-Davis, USA. Bacterial samples (PAB and FAB) were also analyzed for analytical dry matter (overnight at 105°C) and ash (16 h at 550°C).
Ruminal sampling and analyses
Samples of whole ruminal contents (about 200 g) were taken from the ventral sac of the eight ruminally cannulated cows at 0 (pre-feeding), 1, 2, 3, 4, 5, 6, 8 and 10 h after the morning feeding on day 29 of each period. Ruminal digesta samples were strained through two layers of cheesecloth and pH was measured immediately (pH/temp meter 199 Model No. 3D, Fisher Scientific, Pittsburgh, PA, USA). Two 10-ml samples were then preserved by addition of 0.2 ml of 50% H2SO4 and stored at −20°C until analysis. Samples were thawed at room temperature, centrifuged (25 200×g, 15 min, 4°C), and supernatants analyzed for NH3-N as previously described and for volatile fatty acids (VFA) with a GLC equipment (Hewlett-Packard 6890N, Hewlett-Packard Inc., Montreal, QC, Canada) equipped with a flame-ionization detector and a 7683B model autosampler as described by Chiquette et al. (Reference Chiquette, Allison and Rasmussen2008). Five hundred ml of ruminal fluid and 250 g of solid digesta were collected from each ruminally cannulated cow at 0 (pre-feeding), 3, 6 and 9 h after the morning feeding, blended and strained through two layers of cheesecloth. A 3-ml portion of the strained ruminal fluid was preserved using 3 ml of methyl green formalin-saline solution for protozoa enumeration (Ogimoto and Imai, Reference Ogimoto and Imai1981). Protozoa samples were stored at room temperature in the dark until counting. Protozoa were enumerated microscopically using a Levy-Hausser counting chamber (Hausser Scientific, Horsham, PA, USA). Each sample was counted twice, and if the average of the duplicates differed by more than 10%, the counting was repeated.
Analyses of vitamin B12 and analogs in feeds and OTD
Analyses of CBL and its analogs were performed on feeds and OTD samples. Sample preparation and analysis of CBL and its analogs by liquid chromatography–MS (LC–MS) were performed as described by Allen and Stabler (Reference Allen and Stabler2008) using the following standards: CBL, the biologically active form in mammals; COB, a CBL without the base, ribose and phosphate groups or substitution of 5,6-DMB by adenine, ADE; benzimidazole, BZA; cresol, CRE; 2-CH3-adenine, MADE; 5-CH3-benzimidazole, MBZA; 5-CH3O-benzimidazole, MOMZA; 5-CH3O, 6-CH3-benzimidazole, MOMBZA; 2-CH3-S-adenine, MSADE; napthimidazole, NZA; 5-OH-benzimidazole, OHBZA and phenol, PHE. All data are presented as CN-CBL equivalents.
Both ‘true’ (CBL) and total (CBL+analogs) vitamin B12 were also measured by radioassays using either pig intrinsic factor (Sigma) or cow saliva as binding proteins as described by Santschi et al. (Reference Santschi, Chiquette, Berthiaume, Martineau, Matte, Mustafa and Girard2005) except that 5 µl of NaCN 1 M per ml of solution was added during the extraction process. Intrinsic factor binds preferentially CBL, whereas haptocorrin present in saliva binds most corrinoids (Combs, Reference Combs2012). Concentration of analogs was obtained by difference between total (haptocorrin) and true vitamin B12 (intrinsic factor).
Omasal flow calculations
Feed intake was measured throughout the duration of the experiment. It is important to note, however, that dry matter intake reported on Table 2 was calculated by averaging daily intakes from days 23 to 29 (i.e. total sampling week), whereas dry matter and other nutrient intakes reported on Tables 3 and 4 were calculated by averaging daily intakes during omasal sampling (days 26 to 28). Omasal flow of NAN was determined by difference between total N and NH3-N flows. Total NAN flowing past the omasal canal was assumed to be composed by PAB NAN, FAB NAN and non-NH3 non-bacterial N (NANBN). The natural abundance of 15N in samples from background omasal digesta averaged 0.36086 (s.d. 0.002083) atom %. Enrichment of 15N was defined as 15N atom % excess above the natural abundance of 15N measured in the background omasal digesta samples.
Table 2 Dry matter intake, milk production and composition and ruminal variables in Holstein cows supplemented or not with 5,6-DMB

5,6-DMB=5,6-dimethylbenzimidazole.
Table 3 Intake, omasal flow and apparent ruminal synthesis of corrinoids in Holstein cows supplemented or not with 5,6-DMB

5,6-DMB=5,6-dimethylbenzimidazole; LC–MS=liquid chromatography–MS.
1 Corrinoids measured by LC–MS: cobamides with substitution of 5,6-DMB by 5-OH-benzimidazole, OHBZA; 2-CH3-adenine, MADE; adenine, ADE; 2-CH3-S-adenine, MSADE; summation of these forms=total analogs; cobalamin, CBL. All data are presented as CN-Cbl equivalents.
2 Corrinoids measured by radioassays using intrinsic factor (true vitamin B12) or haptocorrin from cow saliva (total vitamin B12). Vitamin B12 analogs were obtained by differences between total and true vitamin B12.
3 In total mixed ration, total vitamin B12 determined with a radioassay using haptocorrin as binding protein was smaller than true vitamin B12 measured with a radioassay using intrinsic factor as binding protein.
Table 4 Intake, omasal flow and ruminal digestibility of DM, OM, NDF, ADF and nitrogenous fractions in Holstein cows supplemented or not with 5,6-DMB

DM=dry matter; OM=organic matter; 5,6-DMB=5,6-dimethylbenzimidazole; NAN=non-ammonia nitrogen; NANBN=non-ammonia non-bacterial nitrogen; FAB=fluid-associated bacteria; PAB=particle-associated bacteria; OMADR=organic matter apparently digested in the rumen; OMTDR=organic matter truly digested in the rumen.
Assuming that FAB and PAB were representative of bacteria flowing with the FP and the PF, respectively, omasal flows of FAB NAN, PAB NAN, total bacterial NAN, NANBN and OM truly digested in the rumen (OMTDR) were calculated as follows:
Omasal flows of NANBN and RUP, RDP supply and OM TDR were calculated as follows:
where flows and intakes are in grams per day or kilograms per day and NAN concentrations are in grams per gram of OM.
Statistical analyses
Data were analyzed using the MIXED procedure of SAS (Version 9.3; SAS Institute Inc., Cary, NC, USA) according to a crossover design. The following model was fitted for all variables with no repeated measures over time:
where: Y ijk is the dependent variable; μ the overall mean; S i the mean effect of the i th crossover sequence group, C j (S) i the mean effect of j th cow nested within i th sequence, T k the mean effect of the l th treatment, ST ik the interaction between i th crossover sequence group and k th treatment (same as period effect) and E ijk the random residual variation. All terms were considered fixed except C j (S) i and E ijk that were considered random.
The following model was fitted for variables with repeated measures over time (ruminal pH, ruminal concentrations of NH3-N and VFA, and ruminal protozoal count):
where Y ijkl is the dependent variable, μ the overall mean, S i the mean effect of the i th crossover sequence group, C j (S) i the mean effect of j th cow nested within i th sequence, T k the mean effect of k th treatment, ST ik the interaction between i th crossover sequence group and k th treatment (same as period effect), E1 ijk the whole-plot random residual variation, H l the mean effect of l th hour postfeeding analyzed as repeated measures, TH kl the interaction between k th treatment and l th hour postfeeding and E2 ijkl the subplot random residual variation. The spatial covariance structure with the lowest Akaike information criterion was retained in the final model. The subject of the repeated measurements was defined as cow (period). All terms were considered fixed, except C j (S) i , E1 ijk and E2 ijkl , which were considered random.
All reported values are least square means and standard error of the mean. Differences were considered significant at P⩽0.05 and trends were declared at 0.05<P⩽0.10.
Results
Five corrinoids, CBL and four cobamides, ADE, MADE, MSADE and OHBZA were detected in the total mixed ration and the omasal digesta (Table 3). The supplement of 5,6-DMB increased (P=0.02) the apparent ruminal synthesis of CBL by 34% but had no effect (P>0.1) on apparent ruminal synthesis of the four analogs, OHBZA, MADE, ADE and MSADE (Table 3). When analyzed by radioassays using intrinsic factor and cow saliva (haptocorin) as binding proteins, the supplement of 5,6-DMB had no effect (P>0.1) on apparent ruminal synthesis of total vitamin B12 and analogs but increased (P=0.01) synthesis of ‘true vitamin B12’ by 38% (Table 3). The correlation between CBL measured directly using the LC–MS method or as ‘true vitamin B12’ by radioassay using intrinsic factor as binding protein was high (r=0.97, P⩽0.0001). Total corrinoids (summation of the forms quantified using the LC–MS method) and total vitamin B12 measured by radioassay using cow saliva (haptocorrin as binding protein) were also correlated (r=0.67; P=0.004) but the correlation between the two methods to estimate the analogs (summation of the forms other than CBL measured by the LC–MS method or difference between the two radioassays) was not significant (r=0.40; P=0.13).
Plasma concentrations of vitamin B12 (P=0.98) were not affected by treatments, averaging 201 and 202 (s.e.m. 10) pg/ml for control and treated cows, respectively.
A daily dose of 5,6-DMB had no effect (P>0.1) on DMI, milk production and composition (Table 2). Ruminal pH and concentrations of VFA and NH3-N in ruminal content were also unaffected (P>0.1; Table 2) by the 5,6-DMB supplement. Similarly, protozoal count did not differ (P=0.98) between treatments; averaging 585 631 and 584 312 (s.e.m. 44 745) protozoa/ml of ruminal fluid, for the control and treated groups, respectively. The supplement had also no effect (P⩾0.2) on intakes, omasal flows as well as apparent ruminal digestibility of DM, OM, NDF, ADF and nitrogenous fractions (Table 4). Although omasal flow of total bacterial NAN (i.e. FAB NAN+PAB NAN flows) did not differ (P=0.96) between treatments, feeding 5,6-DMB tended (P=0.08) to increase (+10%) FAB NAN flow and decreased (−9%; P=0.02) PAB NAN flow.
Discussion
Cyanocobalamin, pseudovitamin B12 (ADE), factor A (MADE), factor B (COB) and factor C have been detected in silage, although the plant species was not mentioned (Ford et al., Reference Ford, Holdsworth, Kon and Porter1953b). In the present study, CBL and four cobamides, ADE, MADE, MSADE and OHBZA were quantified in the total mixed ration, whereas only CBL and COB were detected in total mixed rations used by Girard et al. (Reference Girard, Berthiaume, Stabler and Allen2009a and 2009b), even if those rations contained some ingredients similar to those used in the present study, such hay, grass silage or corn silage. Nevertheless, dietary concentrations of corrinoids (CBL and analogs; 11 µg/kg of DM) and CBL (6 µg/kg of DM) were similar to values observed by Girard et al. (Reference Girard, Berthiaume, Stabler and Allen2009a and 2009b). The total amount of corrinoids in the total mixed ration (0.25 mg/day) represented only 0.3% of the omasal flow of total corrinoids. Vitamin B12 is not synthesized by plants; therefore, the small amounts detected in feedstuffs are likely due to bacterial contamination from soil or during silage fermentation process (Martens et al., Reference Martens, Barg, Warren and Jahn2002; Combs, Reference Combs2012). In the present study, CBL and four cobamides, ADE, MADE, MSADE and OHBZA, were quantified in omasal digesta, whereas COB and two supplementary cobamides were also detected in duodenal digesta of dairy cows fed different diets (Girard et al., Reference Girard, Berthiaume, Stabler and Allen2009a and Reference Girard, Santschi, Stabler and Allen2009b).
Radioassays using intrinsic factor from pig stomach are available commercially and allow for rapid determination of ‘true vitamin B12’ in a large number of samples. Both intrinsic factor and haptocorrin have a high affinity for CBL but haptocorrin has a greater affinity for analogs than intrinsic factor (Kolhouse and Allen, Reference Kolhouse and Allen1977; Adjalla et al., Reference Adjalla, Benhayoun, Nicolas, Guéant and Lambert1993). Measurements of CBL in feed and omasal digesta by LC–MS or by a radioassay using intrinsic factor as binding protein were highly correlated. However, quantification of analogs by difference between the two radioassays was less accurate than by the LC–MS method, possibly because the affinity of haptocorrin for corrinoids varies among the different cobamides.
As observed in vitro (Ford et al., Reference Ford, Holdsworth and Kon1955; Gawthorne, Reference Gawthorne1970b) and in vivo (Rickard et al., Reference Rickard, Bigger and Elliot1975), in studies done with sheep, supplementing 5,6-DMB to dairy cows increased apparent synthesis of CBL in rumen by 34% (5 mg/day) but had no effect on the amounts of analogs produced. The authors of both studies concurred that, when cobalt supply is adequate, 5,6-DMB availability may become limiting (Gawthorne, Reference Gawthorne1970b; Rickard et al., Reference Rickard, Bigger and Elliot1975), although the metabolic pathway for anaerobic synthesis of 5,6-DMB by ruminal bacteria is not fully elucidated (Renz et al., Reference Renz, Endres, Kurz and Marquart1993; Roth et al., Reference Roth, Lawrence and Bobik1996). On a weight basis, cobalt represents 4.4% of the CBL molecule, thus, in the present study 0.64 and 0.86 mg of Co were incorporated into CBL for control and 5,6-DMB supplemented diets, respectively. With a basal diet providing 1.4 mg of Co/kg of DM, efficiency of cobalt utilization for CBL synthesis represented 2.0% and 2.7% for control and 5,6-DMB supplemented diets, respectively. Efficiency of cobalt utilization in the present study was lower than expected. Indeed, in sheep, efficiency of cobalt utilization decreased as the dietary concentration of cobalt increased (Smith and Marston, Reference Smith and Marston1970). In cows fed different diets, efficiency of cobalt utilization for CBL synthesis in the rumen has been reported to be 7.1%, 9.5% and 4.4% for diets providing 0.17, 0.29 and 2.5 mg of Co/kg of DM, respectively (Stemme et al., Reference Stemme, Lebzien, Flachowski and Scholz2008; Girard et al., Reference Girard, Berthiaume, Stabler and Allen2009a). Therefore, it is likely that the efficiency of bacterial utilization of cobalt for CBL synthesis is influenced by the ingredients and chemical compositions of diets and their effects on microflora and fermentation in rumen.
Already in 1953, Ford et al. (Reference Ford, Holdsworth, Kon and Porter1953a) reported that the relative proportions of vitamin B12 and its analogs produced in rumen depend not only on the composition of the microflora but also on the nature of the diet. Only four species out of the 21 studied species of microorganisms present in rumen, Selenomonas ruminantium, Megasphaera elsdenii, Butyrivibrio fibrisolvens and an unnamed species, were able to synthesize corrinoids with the first two species producing the greatest amounts of vitamin B12 and analogs (Dryden et al., Reference Dryden, Hartman, Bryant, Robinson and Moore1962). Moreover, changes in culture media, such as energy sources or precursors of the nucleotide base, affect the proportions of CBL and analogs (Dryden et al., Reference Dryden, Hartman, Bryant, Robinson and Moore1962). Many bacterial species producing analogs (cobamides) in pure culture preferentially use 5,6-DMB when it is available in the culture medium (Hazra et al., Reference Hazra, Tran, Crofts and Taga2013). These authors reported to have detected free 5,6-DMB in microbial communities and hypothesized that bacterial species not able to synthesize 5,6-DMB could produce CBL if the nucleotide base is available in their environment. In the present study, there appears to be a shift in bacterial population occurring with the addition of 5,6-DMB, which favored the growth of FAB, this fraction comprising the known vitamin B12-synthesizing bacteria, at the expense of PAB. Nevertheless, this shift in the order of 10% for both bacterial NAN flows was not sufficient to impact on rumen fermentation parameters or protozoa counts.
In conclusion, despite the significant increase in the apparent ruminal synthesis of CBL in response to intraruminal supplementation of 5,6-DMB, this improvement was not large enough to enhance markedly the efficiency of cobalt utilization for CBL synthesis. The current results highlight the lack of knowledge on the nutritional factors driving ruminal production of vitamin B12 in presence of a sufficient cobalt supply as well as on the interactions among bacterial populations for preferential production of CBL over analogs.
Acknowledgments
The authors would like to thank Chrystiane Plante, Véronique Roy, Karoline Lauzon, Maude Jolicoeur and Étienne Viens for their technical support, the barn staff for animal care and Steve Méthot for statistical advice. Appreciation is extended to Agriculture and Agri-Food Canada for financial support.






