Heart disease and cancer continue to be the leading causes of death globally, and the total number of deaths from these causes increased by 14·5 and 17·8 %, respectively, between 2006 and 2016( 1 ). Ca, the most abundant mineral in the body, is essential for numerous processes, such as cardiovascular function, neural transmission and various metabolic processes, and is most commonly consumed through foods, such as dairy products or supplements( 2 , 3 ). In the USA, dietary daily Ca reference intake recommendations are 1200 mg for females over 50 years of age, and 1000 mg for males 50–70 years of age, increasing to 1200 mg for males over 70 years( 2 ). Population reference intakes for various European countries range from 700 to 1300 mg/d for adults 50 years and older( 3 ).
Evidence from recent meta-analyses of observational studies of associations of Ca and dairy products with risk of colorectal neoplasia suggests that higher intake, within reasonable limits, may reduce the risk of colorectal cancer (CRC) mortality( Reference Aune, Lau and Chan 4 – Reference Keum, Lee and Greenwood 6 ). A recent meta-analysis of cohort studies of dietary Ca and CVD mortality suggested an inverse association with intake around 800 mg/d, but possible higher risk with higher and lower intakes( Reference Wang, Chen and Ouyang 7 ). Some reports suggested that older postmenopausal women taking Ca supplements may have higher risk of myocardial infarction and related deaths( Reference Bolland, Barber and Doughty 8 , Reference Bolland, Grey and Avenell 9 ). Although previous studies investigated Ca and dairy product intakes, their relation to all-cancer or all-cause mortality remains unclear.
The associations of Ca and dairy product intakes with IHD mortality in the Iowa Women’s Health Study (IWHS) were previously published using data collected through 7 years of follow-up and suggested that Ca, but not dairy products, were inversely associated with IHD mortality( Reference Bostick, Kushi and Wu 10 ). After another 18 years of follow-up, the purpose of this analysis was to update the previous analyses on heart disease mortality and expand them to include all-cause, all-cancer and CRC mortality.
Methods
Study population and design
The study design and methodology of the prospective IWHS was described previously( Reference Folsom, Kaye and Potter 11 ). Briefly, in 1986, 41 836 women randomly selected from 1985 Iowa driver’s license information returned completed questionnaires on demographics, medical history, lifestyle and diet. These participants, aged 55–69 years, were followed for mortality and cancer incidence, with follow-up surveys mailed in 1987, 1989, 1992, 1997 and 2004. Written informed consent was obtained from all participants. The University of Minnesota Institutional Review Board (IRB) approved the study, and the Emory University IRB also approved the present analysis.
Dietary assessment
At baseline, detailed information on demographics, self-measured anthropometrics, lifestyle, medical and family history, diet and other factors was collected. A self-administered, 127-food item Willett FFQ was used to assess the usual food and nutritional supplement intake over the previous 12 months. The validity and reliability of the FFQ in the study population were previously reported( Reference Munger, Folsom and Kushi 12 ). Ca and dairy product intakes were determined by assessing a participant’s usual intake of total, dietary and supplemental Ca, and milk (whole, low-/non-fat), cream, non-dairy products creamer, sour cream, ice cream/ice milk, yogurt, butter, margarine and various cheeses, respectively. Diet was only comprehensively reassessed in 2004, at which time only 68·3 % of the cohort remained alive (therefore, only baseline exposure information was used in the present analyses).
Outcome assessment
Deaths were ascertained through the State Health Registry of Iowa or the National Death Index for participants who were lost to follow-up or moved out of Iowa. In addition to all causes of death, the causes of interest for this analysis were deaths due to all cancers, CRC and CHD. The underlying cause of death was assigned and coded by state vital registries, according to the International Classification of Diseases (ICD). Cancer mortality was defined using ICD-9 codes 140–239 and ICD-10 codes C00–D48, CRC mortality using ICD-9 codes 153–154 and ICD-10 codes C18–C21 and CHD mortality using ICD-9 codes 410–414 and 429·2 and ICD-10 codes I20–I25 and I51·6. Follow-up time was calculated as the time from the date of completion of the baseline questionnaire to the date of death, the last date of follow-up contact or the end of follow up (31 December 2012), whichever was first.
Statistical analyses
After excluding participants with a self-reported history of cancer (other than non-melanoma skin cancer) at baseline (n 3830), those who skipped thirty or more of the FFQ questions (n 2499), or those with implausible self-reported total energy intake (<600 or >5000 kcal/d (<2510 or >20 920 kJ/d); n 286), the final sample size for analysis was 35 221.
Ca and dairy products were categorised according to quintiles of intake. Dietary Ca and milk intakes were highly correlated (Pearson r 0·87) in this study population, so to create a variable to represent the non-Ca component of milk (whole and low-/non-fat), the residuals of milk adjusted for dietary Ca from linear regression models were calculated; this method was modelled following the energy adjustment residual method( Reference Willett, Howe and Kushi 13 ), with the dependent variable being the individual milk variables and the independent variable being dietary Ca. This procedure yielded a zero correlation of the residuals with dietary Ca. Our intent was that the residuals would be an indirect indicator of the non-Ca component of milk, which contains other components, such as insulin-like growth factor-1 (IGF-1). All residuals were also categorised into quintiles.
Baseline characteristics were compared across quintiles of total Ca intake using the χ 2 test for categorical variables and one-way ANOVA for continuous variables. Cox proportional hazards models were used to calculate multivariable-adjusted hazard ratios (HR) and their 95 % CI to estimate the associations of Ca and dairy product intakes and of dairy product residuals with all-cause and cause-specific mortality. Median values of quintiles were used as a continuous variable for trend tests. Proportional hazards assumption was assessed using log–log survival curves, Schoenfeld residuals and extended Cox models for each exposure and potential covariate.
Potential confounders, selected on the basis of biological plausibility and previous literature, included age (continuous), college education or higher (yes/no), marital status (currently married, yes/no), BMI (continuous), smoking status (current/past/never), alcohol consumption (g/d), self-reported history of hypertension or diabetes (yes/no), physical activity level (low/moderate/high) and hormone replacement therapy (HRT) use (ever/never); intake of total energy (kJ), total and saturated fat (g/d), total vitamin D (μg/d), total Mg (mg/d), meat (total, red and processed; servings/week), dietary fibre (g/d) and total fruits and vegetables (servings/week); and a dietary oxidative balance score (OBS; continuous). The dietary OBS was created using a previously described, equal-weighting method( Reference Dash, Goodman and Flanders 14 ) and is comprised of anti- (carotene, lutein, lycopene, vitamin C and E, n-3 fatty acids, flavonoids) and pro-oxidant (dietary Fe, n-6 fatty acids, saturated fat) nutrients, such that a higher score indicates a greater balance of anti-oxidant relative to pro-oxidant exposures. In models for associations of specific dairy products with mortality, we also considered the remaining dairy products as potential confounders. Of the potential covariates, we included in the final models all established risk factors plus other variables that when included/excluded in the model changed the adjusted HR for the primary exposure ≥10 %. The covariates for the final models are shown in the Tables’ footnotes.
To assess potential effect modification, we stratified the Cox proportional hazards regression analyses by age (</≥65 years), college education or higher, smoking status, alcohol consumption (none/any), physical activity level, HRT use and BMI (</≥30 kg/m2); intake of total energy (</≥ 7184 kJ/d), total (</≥68·5 g/d) and saturated (</≥23·7 g/d) fat, total vitamin D (</≥ 8450 μg/d), total Mg (</≥287·4 mg/d), total meat (</≥12·5 servings/week), red meat (</≥5 servings/week), processed meats (</≥1 serving/week) and total fruits and vegetables (</≥35 servings/week); and the dietary OBS (low/high).
Because some studies suggested that older women who take Ca supplements without concomitant vitamin D supplements may be at higher risk of CVD( Reference Bolland, Barber and Doughty 8 ), we assessed the overall and age wise mortality risks (</≥65 years at baseline), among those who took Ca but not vitamin D supplements, those who took both Ca and vitamin D supplements and those who took vitamin D but not Ca supplements relative to those who took neither Ca nor vitamin D supplements. Circulating concentrations of 25-OH-vitamin D, the best indicator of total vitamin D exposure, were not measured, and dietary intake of vitamin D is now considered to be a poor representation of vitamin D exposure( Reference DeLuca 15 , Reference Holick 16 ). Accordingly, the only variable related to vitamin D intake considered for the present study was supplemental vitamin D intake.
We also conducted several sensitivity analyses. We excluded (1) deaths that occurred within the first 1, 2 and 6 (see below) years after enrolment and (2) participants with self-reported comorbidities (diabetes, heart disease/heart attack or cirrhosis) at baseline to assess the potential influence of pre-morbid health conditions. Information on aspirin and other non-steroidal anti-inflammatory drug (NSAID) was not collected until 1992, after 6 years of follow-up. So, we repeated all analyses using 1992 as the baseline date for follow-up for deaths and included NSAID use as a covariate to assess whether it was a confounding or effect-modifying factor since the use of these medications has been strongly and consistently inversely associated with the risk of colorectal neoplasia( Reference Garcia Rodriguez and Huerta-Alvarez 17 , Reference Chan, Giovannucci and Meyerhardt 18 ). We also tested Fine–Gray competing risk models to assess the potential influence of other causes of death on each specific mortality outcome of interest.
All statistical analyses were performed using SAS version 9.4 software (SAS Institute Inc.). A two-sided P value of <0·05 or a 95 % CI that did not include 1·0 was considered statistically significant.
Results
Selected characteristics of the participants at baseline by total Ca quintiles are summarised in Table 1. At baseline, participants had a mean age of 62 (sd 4·2) years, a mean BMI of 27 (sd 5·1) kg/m2 and 6, 9 and 0·8 % had a self-reported history of diabetes, heart disease/heart attack or cirrhosis, respectively.
Table 1 Selected baseline characteristics of participants by quintiles (Q) of total calcium intake in the Iowa Women’s Health Study (Mean values and standard deviations; proportions)
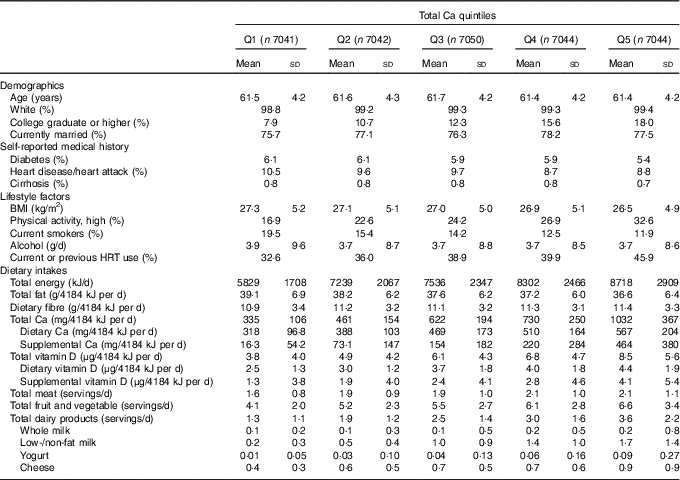
HRT, hormone replacement therapy.
Women in the highest total Ca quintile were more likely to have a college education or higher, be physically active and currently use or have previously used HRT. They also, on average, consumed more energy; more dietary fibre and total, dietary, and supplemental Ca and vitamin D and less total fat adjusted for energy; and more total meat, fruits and vegetables and dairy products.
Upon comparison of the highest with the lowest quintiles of intake (Table 2), in multivariable-adjusted models, total Ca was associated with statistically significant 12 % lower risk of all-cause mortality, 40 % lower risk of CRC mortality and 27 % lower risk of CHD mortality, whereas it was estimated to be associated with 9 % lower risk of all-cancer mortality, a finding that was not statistically significant. Dietary Ca was not statistically significantly associated with mortality risks, although the estimated risk of CRC mortality for those in the highest relative to the lowest quintile was HR 0·77 (95 % CI 0·55, 1·08). However, the estimated inverse association for supplemental Ca was similar to but slightly more attenuated than that for total Ca.
Table 2 Associations of calcium intakes with all-cause, all-cancer, colorectal cancer and CHD mortality among Iowa Women’s Health Study participants, 1986–2012 (Hazard ratios (HR) and 95 % confidence intervals)
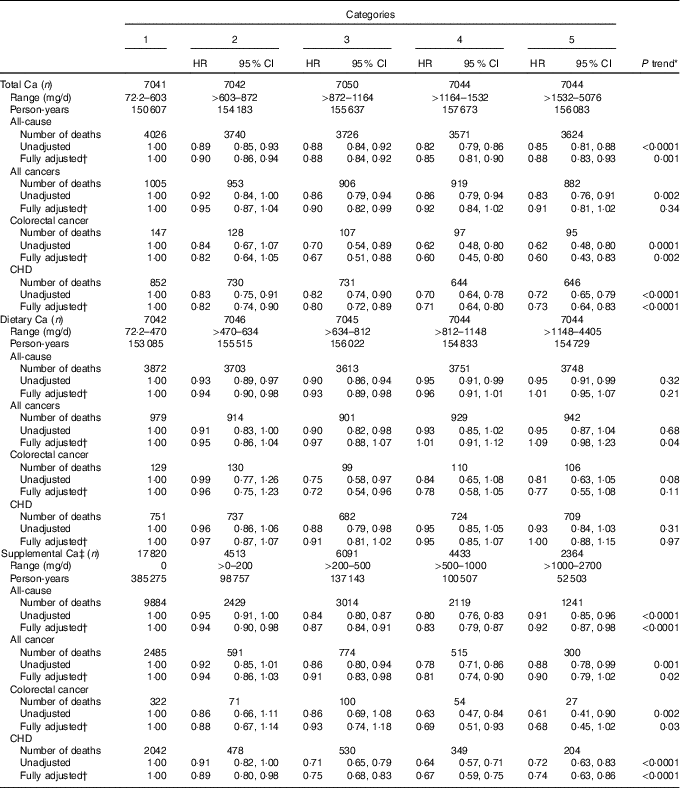
* P trend calculated using medians of each quantile.
† Adjusted for age, family history of colorectal cancer, BMI, smoking, alcohol, physical activity, hormone replacement therapy use, total energy intake, vitamin D, fruit and vegetable intake, red and processed meat intake and dietary oxidative balance score (a score combining anti- and pro-oxidant dietary exposures; see text).
‡ Supplemental Ca analysed as five categories of intake, with no intake as the reference category.
The associations of dairy products with mortality are shown in Table 3. For those in the upper relative to the lowest quintiles of total dairy product and total milk intakes, risks of CRC mortality were statistically significantly approximately 25 % lower. For those in the highest category of whole milk intake relative to those who did not consume whole milk, risks of all-cause and all-cancer mortality were statistically significantly approximately 20 % higher; however, the corresponding findings for high-fat dairy products were close to the null. The estimated risks among those with higher low-fat dairy products and low-/non-fat milk intake for all-cause and cause-specific mortality tended to be slightly lower (8–15 % lower for those in the upper relative to the lowest quintiles of intake), but only the estimated 8 % lower risk of all-cause mortality was statistically significant. Otherwise, the remaining findings for total and specific dairy products, including those other than milk (data not shown), were close to the null.
Table 3 Associations of dairy product intakes with all-cause, all-cancer, colorectal cancer and CHD mortality among Iowa Women’s Health Study participants, 1986–2012 (Hazard ratios (HR) and 95 % confidence intervals)
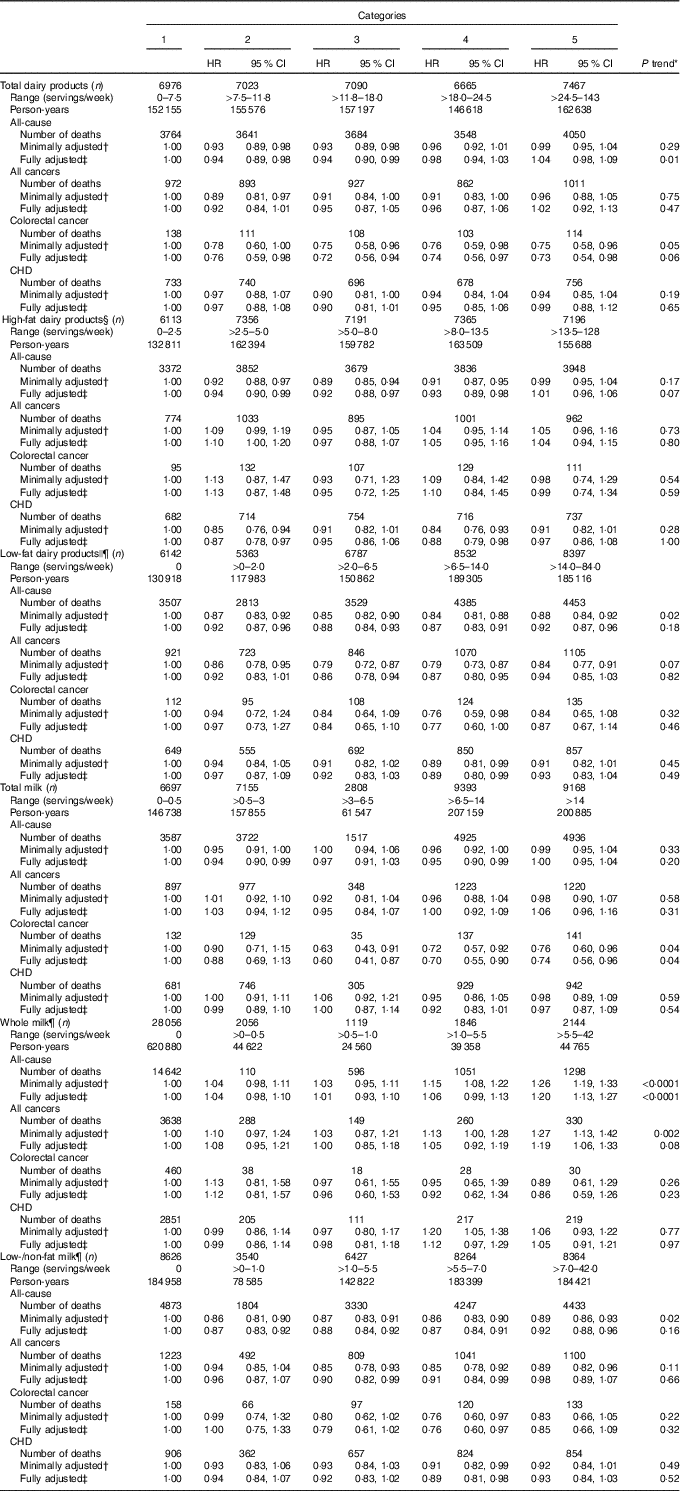
* P trend calculated using medians of each quantile.
† Adjusted for supplemental Ca.
‡ Adjusted for age, family history of colorectal cancer, BMI, smoking, alcohol, physical activity, hormone replacement therapy use, total energy intake, vitamin D, fruit and vegetable intake, red and processed meat intake, dietary oxidative balance score (a score combining anti- and pro-oxidant dietary exposures; see text), and supplemental Ca.
§ High-fat dairy products include whole milk, cream, yogurt, ice cream, cream cheese, cottage cheese, other cheese, sour cream and butter.
|| Low-fat dairy products include low-/non-fat milk and ice milk/sherbet.
¶ Low-fat dairy products and whole and low-/non-fat milks analysed as five categories with no intake as the reference category.
The associations of dietary Ca-adjusted dairy product and milk residuals with mortality are shown in Table 4. Those in the highest relative to those in the lowest quintiles of whole milk residuals had statistically significant 20 % higher risk of all-cause mortality (HR 1·20; 95 % CI 1·13, 1·28; P trend=0·0002). After additional adjustment for total fat intake to account for possible confounding by the fat content of whole milk, the association was not materially different (HR 1·22; 95 % CI 1·14, 1·30; P trend <0·0001). In contrast, those in the highest relative to the lowest quintile of low-/non-fat milk residuals had statistically significant 9 % lower risk of all-cause mortality. Otherwise, the findings for the associations of various dairy product residuals with mortality were close to the null.
Table 4 Associations of dietary calcium-adjusted dairy products and milk residuals with all-cause, all-cancer, colorectal cancer and CHD mortality among Iowa Women’s Health Study participants, 1986–2012 (Hazard ratios (HR) and 95 % confidence intervals)
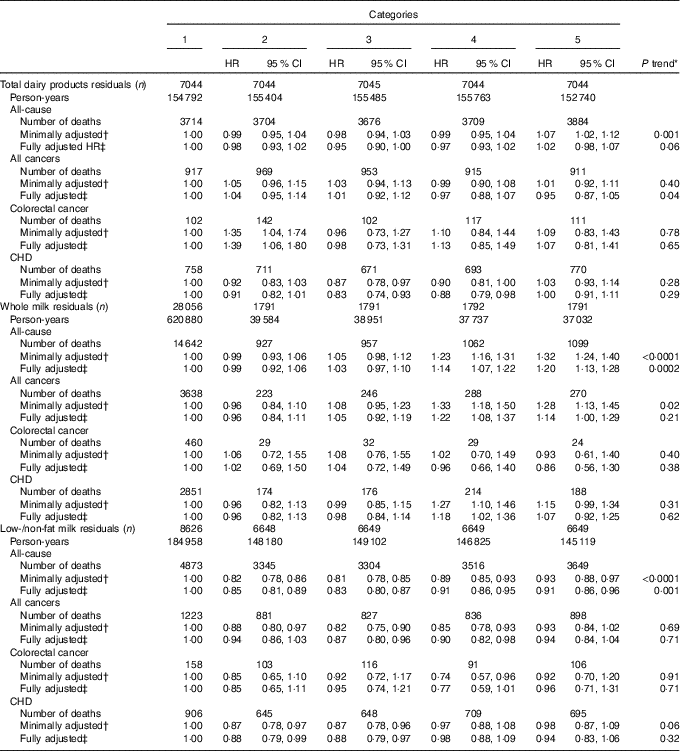
* P trend calculated using medians of each quantile.
† Adjusted for dietary Ca.
‡ Adjusted for age, family history of colorectal cancer, BMI, smoking, alcohol, physical activity, hormone replacement therapy use, total energy intake, vitamin D, Mg, fruit and vegetable intake, red and processed meat intake, dietary oxidative balance score (a score combining anti- and pro-oxidant dietary exposures; see text), and dietary Ca.
Because our results suggested that the associations for all-cancer mortality may have been largely driven by CRC mortality, we conducted secondary analyses to assess the association of the various exposures with all non-CRC cancers combined. These findings generally were close to the null (data not shown). As examples, for those in the highest relative to the lowest quintiles of total Ca and total dairy product intakes, the multivariable-adjusted HR were 0·97 (95 % CI 0·86, 1·09) and 1·07 (95 % CI 0·96, 1·20), respectively.
Associations of supplemental Ca and vitamin D use with mortality are shown in Table 5. Those who took Ca supplements, with or without vitamin D supplements, relative to those who took neither supplement, were at statistically significant lower risk of all-cancer, all-cause and CHD mortality. The estimated lower risks ranged from 6 % for the association of supplemental Ca alone with all-cause mortality, to 23 % for the association of Ca supplements alone with CHD mortality. For these analyses, the number of CRC deaths among supplement users was small; however, the estimated risks of those who took Ca with or without vitamin D supplements were approximately 15 % lower and close to statistically significant. The estimated risks of all-cause and cause-specific mortality among those who took vitamin D but not Ca supplements were close to null. When we stratified these analyses by <65 v. ≥65 years of age at baseline, the findings were similar across the age strata but slightly more attenuated among the older women for all outcomes, including for CHD mortality (data not shown).
Table 5 Associations of supplemental calcium and vitamin D use with all-cause, all-cancer, colorectal cancer and CHD mortality among Iowa Women’s Health Study participants, 1986–2012 (Hazard ratios (HR) and 95 % confidence intervals)
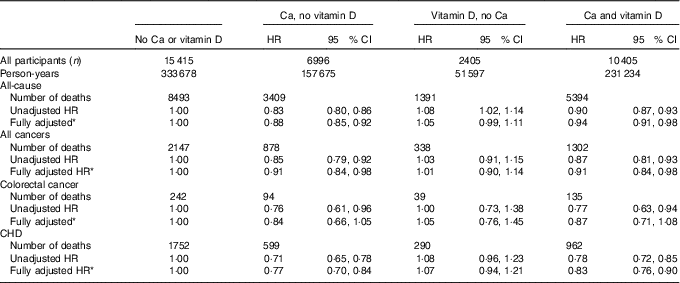
* Adjusted for age, BMI, smoking, alcohol, physical activity, hormone replacement therapy use, total energy intake, red and processed meat intake, fruit and vegetable intake, and dietary oxidative balance score (a score combining anti- and pro-oxidant dietary exposures; see text).
There were no substantial differences in the observed Ca and dairy product associations with mortality in the analyses stratified by categories of the various selected risk factors (data not shown). In the sensitivity analyses, exclusion of those who died during the first 1, 2 or 6 years of follow-up did not materially change the association of any of the exposures with all-cause or cause-specific mortality (data not shown). Exclusion of participants with self-reported comorbidities (history of diabetes, heart disease/heart attack or cirrhosis) at baseline also did not materially change any of the associations. In addition, there was no evidence of confounding or effect modification by aspirin or NSAID use when we repeated the analyses using 1992 as the baseline date (data not shown). Competing risk models yielded no material differences from the estimated associations using Cox proportional hazards models (data not shown).
Discussion
The results of this study suggest that higher Ca intake may be associated with lower risk of all-cause and CRC- (but not non-CRC-) and CHD-specific mortality among older women. Our results also suggest that total dairy product consumption may be associated with lower risk of CRC mortality, whole milk consumption may be associated with higher risk of all-cause mortality, and consumption of lower fat milk may be associated with lower risk of all-cause mortality. Our results are also consistent with the Ca content of milk/milk products being of primary importance in the dairy product and CRC association, but they also suggest that whole and low-/non-fat milk product components other than Ca may contribute to the association of milk with mortality risks. Finally, our results suggest that taking supplemental Ca, alone or with vitamin D, may be associated with lower risk of all-cause, all-cancer and CHD mortality among women, but we also found that taking vitamin D supplements without taking Ca supplements was not associated with mortality in our study population.
Calcium, dairy products and CRC aetiology and survival
The plausibility of Ca reducing risk of CRC is strong and well established( Reference Bostick 19 , Reference Chan and Giovannucci 20 ). The plausibility for dairy products reducing CRC risk primarily involves their Ca content, and the epidemiological support for an inverse dairy product and CRC association, though less extensive, is similar to that for Ca( Reference Aune, Lau and Chan 4 ). If consumed in amounts greater than required for maintaining normal serum concentrations, Ca binds secondary bile acids in the gut, decreasing their cytotoxic/mitogenic and mutagenic effects( Reference Govers, Termont and Lapre 21 , Reference Bartram, Kasper and Dusel 22 ), and binding to the Ca sensing receptor in the gut epithelium decreases proliferation and promotes differentiation and apoptosis( Reference Lamprecht and Lipkin 23 – Reference Fedirko, Bostick and Flanders 26 ). These mechanisms may also be relevant to post-diagnosis survival( Reference Chakrabarty, Radjendirane and Appelman 27 , Reference Momen-Heravi, Masugi and Qian 28 ).
Our findings of inverse associations of total and supplemental Ca with CRC mortality are supported by consistent evidence for modest inverse associations of Ca and dairy products with the risk of colorectal neoplasms( Reference Aune, Lau and Chan 4 – Reference Keum, Lee and Greenwood 6 ). A meta-analysis of fifteen cohort studies reported 8 % lower risk of incident CRC per 300 mg daily increase in total Ca intake( Reference Keum, Aune and Greenwood 5 ). A similar meta-analysis of eight prospective studies reported 5 % lower risk of colorectal adenomas per 300 mg daily increase in total Ca intake( Reference Keum, Lee and Greenwood 6 ).
Additional evidence from three, large clinical trials supports a protective effect of Ca supplementation against colorectal adenoma recurrence( Reference Hofstad, Almendingen and Vatn 29 – Reference Bonithon-Kopp, Kronborg and Giacosa 31 ). A fourth large clinical trial of Ca and adenoma recurrence did not find an overall treatment effect( Reference Baron, Barry and Mott 32 ) but recurrence was reduced among those with a normal BMI or certain vitamin D receptor genotypes( Reference Barry, Peacock and Rees 33 ). A fifth clinical trial of supplemental Ca and vitamin D found no effect on invasive CRC incidence; however, only 60 % of participants reported taking at least 80 % of their treatment medications, the Ca (1000 mg/d) and vitamin D (400 IU/d) doses were relatively low, and the follow-up time may have been inadequate to assess CRC development( Reference Wactawski-Wende, Kotchen and Anderson 34 ).
Although Ca may have similar roles in CRC prevention and survival, few studies reported associations of Ca with CRC survival or mortality. Four cohort studies found no association of pre-diagnostic dietary Ca intake with CRC survival( Reference Slattery, French and Egger 35 – Reference Yang, McCullough and Gapstur 38 ). Of these, one study investigated pre- and post-diagnosis Ca and dairy product intakes, but only post-diagnosis total Ca intake was inversely associated with CRC mortality (relative risk 0·59; 95 % CI 0·33, 1·05; P trend=0·01)( Reference Yang, McCullough and Gapstur 38 ). A second study investigated dairy product intake, finding no association of total and specific dairy products with CRC mortality( Reference Dik, Murphy and Siersema 37 ).
Calcium, dairy products and other cancers
The plausibility for how Ca could reduce the risk of or survival from other cancers is unclear. Whereas normal colorectal epithelium is directly exposed to variable levels of Ca in the gut, other tissues are exposed to Ca from the circulation, where Ca concentrations are tightly regulated within a very narrow range( Reference Mundy and Guise 39 , Reference Fijorek, Puskulluoglu and Tomaszewska 40 ). This suggests that, at best, circulating Ca concentrations may have limited effects on the risk of other cancers. However, Ca in the gut may have systemic effects. Ca binding of bile and free fatty acids increases their excretion and thus fat absorption( Reference Lupton, Steinbach and Chang 41 ). Also, by reducing the cytotoxic effects of the bile acids, oxidative stress and inflammation is reduced( Reference Fedirko, Bostick and Long 42 , Reference Hopkins, Owen and Ahearn 43 ). This may result in decreased transport of biomarkers of oxidative stress and inflammation into the circulation and thus their transport to other tissues. This, perhaps combined with the effects on the gut microbiome( Reference Bovee-Oudenhoven, Lettink-Wissink and Van Doesburg 44 ), may also result in stronger gut barrier function against other factors in the gut lumen.
Ca and dairy products were not associated with all-cancer mortality in our study, findings apparently driven by their null associations with non-CRC mortality. Also, residuals of total and specific dairy products, representing the non-Ca components of dairy products, were not associated with CRC mortality, thus suggesting that Ca is of primary importance in the dairy product and CRC association. A meta-analysis of two observational studies of total Ca association with all-cancer mortality found no association( Reference Asemi, Saneei and Sabihi 45 ). Another meta-analysis of ten cohort studies reported no association of total dairy products with all-cancer mortality( Reference Lu, Chen and Niu 46 ). A meta-analysis of five randomised clinical trials of Ca supplementation also reported no evidence of an effect of supplemental Ca on all-cancer mortality( Reference Bristow, Bolland and MacLennan 47 ). However, a meta-analysis of twelve cohort studies that investigated total dairy product and total milk intakes with CRC risk reported 19 and 18% lower risk, respectively, among those in the highest relative to the lowest intake categories( Reference Aune, Lau and Chan 4 ).
Calcium, dairy products and CVD
Both protective and harmful effects of Ca on CVD risk have been proposed. Though Ca is needed for normal cardiac and vascular smooth muscle function, high intake may increase vascular calcification, especially among those with renal insufficiency( Reference Rubin, Rundek and McMahon 48 ). In contrast, Ca and dairy products may favourably affect blood lipids and blood pressure, though the evidence is limited( Reference Chai, Cooney and Franke 49 – Reference Reid, Ames and Mason 53 ). Our findings of inverse associations of total and supplemental Ca with CHD mortality are plausible and consistent with the findings in this study population in 1994( Reference Bostick, Kushi and Wu 10 ), but the current existing evidence is inconsistent. In a meta-analysis of eight observational studies, total, dietary and supplemental Ca intakes were not associated with CVD mortality( Reference Asemi, Saneei and Sabihi 45 ). An older meta-analysis of prospective cohort studies suggested that the dietary Ca and CVD mortality association may be U shaped, with higher risk among those with intake <800 and >900 mg/d( Reference Wang, Chen and Ouyang 7 ). However, we did not observe consistent patterns in our findings for total, dietary, or supplemental Ca to support a U-shaped association; and when examined in non-linear models, the P values were not materially different from the P for trend values.
Because a 2010 meta-analysis of five randomised, controlled trials of Ca, conducted primarily among older women in relation to various a priori end points (mostly osteoporosis), found statistically significant higher risk of CVD events among those randomised to supplemental Ca without concomitant vitamin D( Reference Bolland, Avenell and Baron 54 ), we investigated the association of taking supplemental Ca, with and without supplemental vitamin D, with CHD mortality. We observed inverse associations overall and stratified by age; these findings are consistent with a previous analysis among IWHS participants (using data collected through 2004) that found Ca supplement use to be associated with lower risk for all-cause, all-cancer and CVD mortality( Reference Mursu, Robien and Harnack 55 ). In support of our findings is a 2015 meta-analysis of clinical trials conducted between 1992 and 2012, regarding Ca supplementation with or without vitamin D among postmenopausal women( Reference Lewis, Radavelli-Bagatini and Rejnmark 56 ). Analysis of seventeen trials revealed no evidence of an effect of supplemental Ca on all-cause mortality, and analysis of five trials contributing data on CHD outcomes also revealed no evidence of an effect( Reference Lewis, Radavelli-Bagatini and Rejnmark 56 ).
Dairy product components other than calcium and chronic disease risk and mortality
Although dairy products are the major food source of Ca in the USA and many European countries( 2 , 3 ), they also contain other nutrients and factors, such as fat and IGF-1, that may affect the risk of chronic diseases and mortality. As part of fat digestion processes, bile acids are produced in the gut and, as previously noted, have mitogenic and mutagenic effects on the colorectal epithelium( Reference Bernstein, Bernstein and Payne 57 ). IGF-1 may plausibly affect both cancer and CVD mortality since overproduction was observed in tumour cells( Reference Yu and Rohan 58 ), and it has a role in regulating vascular smooth muscle cell function through cell apoptosis and migration( Reference Arnqvist, Bornfeldt and Chen 59 ). Dairy products may also alter the gut microbiome through promoting beneficial SCFA-producing bacteria( Reference Rios-Covian, Ruas-Madiedo and Margolles 60 ) and decrease circulating inflammation biomarkers( Reference Zemel and Sun 61 , Reference van Meijl and Mensink 62 ), which may decrease the risk of various cancers and CVD.
In our study, whole milk and whole-milk residuals were associated with higher risk of all-cause mortality, even after adjusting for total fat, while low-/non-fat milk and low-/non-fat milk residuals were associated with lower risk. While this may suggest that the fat or other non-Ca components of milk are associated with mortality, we cannot rule out chance or uncontrolled confounding, particularly since whole milk consumption was low in this study population. In addition, though the fat content of whole milk and other high-fat dairy products was previously hypothesised to increase adiposity and CVD risk, a systematic review reported that greater consumption of high-fat dairy products was generally not associated with adiposity or metabolic dysfunction, and the findings from studies that investigated the risk of IHD, CHD and overall CVD were inconsistent( Reference Kratz, Baars and Guyenet 63 ). Although SFA are hypothesised to independently elicit a pro-inflammatory response, potentially via the gut microbiome( Reference Fritsche 64 ), evidence from cross-sectional studies suggests that dairy products may be inversely associated with circulating inflammation biomarkers( Reference Esmaillzadeh and Azadbakht 65 , Reference Panagiotakos, Pitsavos and Zampelas 66 ), while a review of intervention trials reported inconsistent findings( Reference Ballard and Bruno 67 ). These mixed results may be attributable to differences in the composition of the dairy products tested, the different circulating biomarkers measured and the different lengths of the interventions.
Our results for milk residuals may also be attributable to IGF-1 in milk, though the current limited evidence is unclear. Two previous observational studies suggested that circulating IGF-1 concentrations are directly associated with milk intake( Reference Holmes, Pollak and Willett 68 , Reference Giovannucci, Pollak and Liu 69 ), though it is uncertain whether this is directly attributable to milk IGF-1 or other components( Reference Katsumata, Kawakami and Kaji 70 , Reference Wan, Wang and Xu 71 ). A meta-analysis of twenty-one observational studies reported that higher circulating IGF-1 concentrations were associated with 83 % higher risk of prostate cancer, 93 % higher risk of premenopausal breast cancer and 58 % higher risk of CRC( Reference Renehan, Zwahlen and Minder 72 ). Interestingly, both low and high circulating IGF-1 concentrations were previously associated with CVD mortality. In a Danish prospective cohort study, those in the lowest relative to the highest baseline IGF-1 concentration quartile had 94 % higher risk of IHD( Reference Juul, Scheike and Davidsen 73 ). However, in the Swedish cohort of the multicentre, prospective Osteoporotic Fractures in Men Study, a U-shaped association of baseline circulating IGF-1 concentrations with CVD events was observed( Reference Carlzon, Svensson and Petzold 74 ). Relative to quintiles 2–4, those in the lowest IGF-1 quintile had 26 % higher risk of CVD events, and those in the highest quintile had 27 % higher risk.
Study strengths and limitations
Our study had several limitations and strengths. We did not measure circulating 25-OH-vitamin D concentrations, which is considered the best indicator of vitamin D exposure( Reference DeLuca 15 , Reference Holick 16 , Reference Kennel, Drake and Hurley 75 ). This precluded meaningful analyses of vitamin D and Ca–vitamin D interactions beyond assessing supplement use. Semi-quantitative FFQ have well-described limitations (e.g. measurement error). Dietary and other key covariate data were only available at baseline, so we could not account for potential temporal changes in dietary intake. Our study population was limited to older, white women, so our results may not be generalisable to other populations. In addition, we recognise that further research is needed on dairy product–dietary Ca linear regression residuals as an estimate of the non-Ca component of dairy products. Strengths of our study include the prospective design, long-term follow-up, large sample size and number of deaths and our novel approach of estimating dairy product–dietary Ca residuals to investigate the non-Ca component of dairy products.
Conclusions
Taken together with previous literature, our findings suggest that, among older women, (1) total and supplemental Ca intakes may be associated with lower risk of all-cause and CRC- and CHD-specific mortality, (2) total dairy product consumption may be associated with lower risk of CRC mortality, (3) whole milk consumption may be associated with higher risk of all-cause mortality and (4) consumption of lower fat milk may be associated with lower risk of all-cause mortality. Our results are also consistent with the Ca content of milk being of primary importance in the dairy product and CRC association, but they also suggest that non-Ca components of milk products may contribute to the association of milk with mortality risk, though further investigation is needed. Finally, our findings suggest that the use of Ca supplements, with or without vitamin D, may be associated with lower risk of all-cause, all-cancer and CHD mortality among postmenopausal women. Because Ca intake, particularly among older adults, is estimated to be largely below the estimated average requirements( Reference McGuire 76 ), our findings support consuming adequate amounts of Ca and dairy products to reduce chronic disease-related mortality risk.
Acknowledgements
The IWHS was supported by the National Cancer Institute of the National Institutes of Health (grant R01 CA039742). The National Cancer Institute had no role in the design, analysis or writing of this article.
The authors’ responsibilities are as follows: C. Y. U. and R. M. B. designed the research; A. P., C.-P. H. and D. L. conducted the research; C. Y. U. analysed the data; C. Y. U. and R. M. B. wrote the manuscript and had primary responsibility for the final content. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.








