According to the US Census Bureau, over 100 million US residents are now considered to be from a minority group(1). With a population of about 15 million(2), Asian Americans, including the Hmong, are one of the fastest-growing minority populations in the USA. The Hmong, a South-East Asian group originally from Laos, secretly assisted the US military and Central Intelligence Agency during the Vietnamese Conflict (1963–1975)(Reference Yang3). After the Conflict, communists targeted Hmong because of their help to the USA and many Hmong suffered hardships including genocide, poverty, excessive labour, depression and food insecurity, and consequently fled Laos and settled in refugee camps in Thailand(Reference Franzen and Smith4–Reference Lee and Pfeifer8). Conditions of refugee camps varied, but poverty and food insecurity were common, leading to Hmong migration to countries such as Australia, France and the USA(Reference Franzen and Smith4, Reference Fadiman6, Reference Lee and Pfeifer8). Today it is estimated that about 200 000 Hmong live in the USA(2). This number is expected to grow because Hmong tend to have larger families; the average family size of Hmong Americans is 6·51 people, much higher compared with 3·14 people in the average American family(Reference Pfeifer and Lee9).
Immigration to the USA has introduced the once physically active Hmong to an obesogenic American environment. Franzen and Smith(Reference Franzen and Smith4) found that after immigrating to the USA, environmental changes and increased acculturation to American dietary habits have negatively impacted the weight and health status of this population. Increased rates of obesity and obesity-related conditions have been noted among the Hmong(Reference Franzen and Smith4, Reference Franzen and Smith5, Reference Clarkin10–Reference Stang, Kong and Story14). In a sample of adult Hmong refugees (n 448, aged >20 years), Culhane-Pera et al. (Reference Culhane-Pera, Moua and DeFor12) found that 33 % of the sample was overweight and 15 % obese. Further, diabetes rates also seem to be rising in this group(Reference McCarty15) and among Hmong adults in the USA, the rate of diabetes is estimated to be twenty times higher than that of Hmong adults in Thailand(Reference Yang, Xiong and Vang16). Her and Mundt(Reference Her and Mundt17) found among Wisconsin Hmong adults (n 144) that 41 % had casual capillary blood glucose levels ≥140 mg/dl, considered a positive screen test for diabetes. Among children, the Centers for Disease Control and Prevention (CDC)(18) estimated that the rate of obesity-dependent type 2 diabetes is greater than type 1 diabetes among Asian/Pacific Islanders younger than 20 years of age.
The US environment appears to have influenced Hmong dietary and food-related habits. In Laos, traditional Hmong diets were higher in complex carbohydrates, boiled vegetables and seasonal fruits, and water and vegetable/meat broths were the usual beverages of choice(Reference Franzen and Smith4, Reference Goody and Drago13). Additionally, desserts were rarely consumed and snacking was atypical(Reference Franzen and Smith4). After immigration to the USA, an increased consumption of saturated fats, sugars, refined grains and salt has been noted among the Hmong(Reference Franzen and Smith4, Reference Franzen and Smith5, Reference Goody and Drago13).
Little is known about the dietary intake of Hmong adults or children at the nutrient level. In focus group discussions, dietary behaviours and acculturation among Hmong children (9–18 years) were explored, but individual dietary intake was not assessed(Reference Franzen and Smith5). Vue and Reicks(Reference Vue and Reicks19) assessed intake of Ca-rich foods and beverages among 10–13-year-old Hmong girls through questionnaires and parental interviews but did not collect dietary data on other nutrients/food groups.
To our knowledge, comprehensive dietary intake and BMI status for school-aged Hmong children has not been studied and research investigating Hmong dietary practices, current nutritional status and post-migration impact on dietary acculturation is also very limited. Knowing that Hmong are a growing ethnic group in the USA and with increasing rates of obesity and diabetes in this group, it is necessary to investigate the dietary consumption patterns of Hmong children so that appropriate and timely interventions may be planned. Therefore, the purposes of the present study were to: (i) investigate whether time lived in the USA and the degree of acculturation impact the quality and quantity of diet; and (ii) assess differences in food consumption patterns by food groups and nutrient intakes for Hmong children born in the USA compared with those recently immigrated from Thailand or Laos. To the best of our knowledge, ours is the first study which assesses dietary intake among Hmong specifically from an acculturation perspective, incorporating detailed quantitative methodology.
Experimental methods
Participants
Three hundred and thirty-five Hmong children (9–18 years) living in Minneapolis/Saint Paul, Minnesota, participated in the present study. Some children were born and/or raised in the USA (born-US) and were either 9–13 years old (n 144) or 14–18 years old (n 156). A small number were born and/or raised in Thailand/Laos and had been in the USA for <5 years (born-T/L) and were either 9–13 years old (n 21) or 14–18 years old (n 14). Hmong organizations and key informants assisted in recruitment efforts and in total seventeen different sites were visited to maximize diversity within the sample. Children were recruited through activity-based organizations (54 %), Hmong schools (26 %), churches (12 %), and via advertisement in the local Hmong newspaper (8 %). Informed parental consent/child assent was obtained and the University of Minnesota's Institutional Review Board approved this study.
Dietary recall methodology
Two 24 h dietary recalls were collected by trained researchers on non-consecutive days (30 % of the recalls included a weekend day) and averaged in order to better describe each child's usual intake of food and nutrients. While interviewing children, a four-stage, multiple-pass technique was used(Reference Gibson20). During stage 1, a complete list of all foods and beverages consumed by the child was obtained. Stage 2 involved a detailed description of each food and beverage consumed, and cooking methods and food brand names were also asked. An estimated amount of each food and beverage item consumed was obtained in stage 3. Lastly, in stage 4, the recall was reviewed by the researcher with the child to ensure that all items, including dietary supplementation, had been recorded. While the 24 h dietary recall has limitations for individual assessment, it can be useful in comparing groups(21). To evaluate dietary assessment methods used among 5–18-year-olds, McPherson et al. (Reference McPherson, Hoelscher and Alexander22) examined thirty-eight validity and nine reliability studies. Correlations between the dietary method and the validation standard were higher for 24 h recall and food record methodologies than for FFQ. Furthermore, Frank(Reference Frank23) suggested the 24 h recall method to be a reliable tool for ages 9 years and above.
In addition to using the multiple-pass interviewing technique, memory prompts such as colourful food models, measuring cups and food pictures were used as aids to reduce error, improve the quality and accuracy of the intake, and to provide children with models to estimate portion sizes. Food models included foods consumed in both Hmong and American cultures such as rice, stir-fries, soups, bread, milk, pizza and cereal.
Acculturation score
Acculturation level was assessed using ten questions asking about language use, social connections and overall dietary habits. The acculturation tool used in the present project was previously created by Marin et al. (Reference Marin, Sabogal and Marin24) for Hispanics, and has reliability/validity coefficients comparable to other published acculturation tools(Reference Franzen and Smith5). This tool has successfully been used with Hmong adults(Reference Franzen and Smith4). Prior to using the tool with a larger group of children, it was pilot-tested and assessed for ease of use and readability with twenty-two Hmong children; these children reported no difficulties with the tool (Flesch Reading Ease score was 84·3 (easy to read) and Flesch–Kincaid Grade Level was 4th). To measure the reliability of the acculturation tool, children completed the same acculturation assessment at two different times. Paired-samples t tests were then computed to determine whether there were significant differences in children's responses between the two assessments; no significant differences were found, suggesting that this was an appropriate tool for children. Acculturation score was determined by summing the responses to individual questions and a higher score indicated more acculturation to US norms. Sample questions were: (i) ‘What language do you usually speak at home?’ (ii) ‘Your closest friends are?’ (iii) ‘I eat _____ foods’. Possible responses to these questions were: (i) ‘only Hmong’; (ii) ‘more Hmong than American’; (iii) ‘both Hmong and American’; (iv) ‘more American than Hmong’; and (v) 'only American’.
Anthropometric measures
Heights and weights were measured using standard procedures(Reference Frisancho25) without outer heavier clothing and shoes. BMI was calculated as weight in kilograms divided by the square of height in metres, and plotted on the CDC BMI-for-age gender specific growth charts to obtain a percentile, which ranks underweight children as <5th percentile, healthy weight as ≥5th to <85th percentile, overweight as ≥85th to <95th percentile and obese children as ≥95th percentile(26). Stature rankings were <5th percentile for short, ≥5th to <85th percentile for average, and ≥85th percentile for tall children.
Data analysis
Data were first checked for normality and analysed using the Predictive Analytics SoftWare (PASW) statistical software package version 17 (formerly SPSS; IBM Corporation, Armonk, NY, USA). Descriptive statistics computed means, standard deviations and frequencies (Table 1). The 24 h dietary recalls were analysed using the ESHA Food Processor® SQL Software version 10·4·0 (ESHA Research, Salem, OR, USA), which computed nutrient and MyPyramid intakes. The 2010 Dietary Reference Intakes (DRI) were used as a reference for each nutrient recommended within a specific age group (9–13 years and 14–18 years) and gender(27) (Tables 2 and 3). MyPyramid guidelines were used to compute servings of grains, vegetables, fruits, milk, meat and beans, and fats, oils and sweets(28, 29). A serving of fat was the number of grams in 1 tbsp of fat for butter, margarine, oils and shortening(28, 29). For meats, an additional fat serving was reported as a multiple of the fat standard for the specific meat, and for milk products and mixed foods, an additional fat serving was reported as a multiple of 12·8 g, the weight of 1 tbsp of shortening(29). A serving of sugar was defined as the number of grams in 1 tsp of sugar (4 g)(28, 29). The results from 24 h data and MyPyramid analysis were then imported into PASW (version 17) for further analysis. Per MyPyramid guidelines(30), children <12 years of age have lower serving suggestions for fruits and meat and beans than older children, and were therefore compared separately (Table 4). The independent-samples t test was used to compare differences in nutrient and food group intakes between children born-US v. born-T/L. The associations among years lived in the USA, acculturation scores, BMI and nutrients consumed were calculated by Pearson correlation (r). The significance level was set at P < 0·05.
Table 1 Sample characteristics of Hmong children aged 9–18 years from Twin Cities, Minnesota, USA
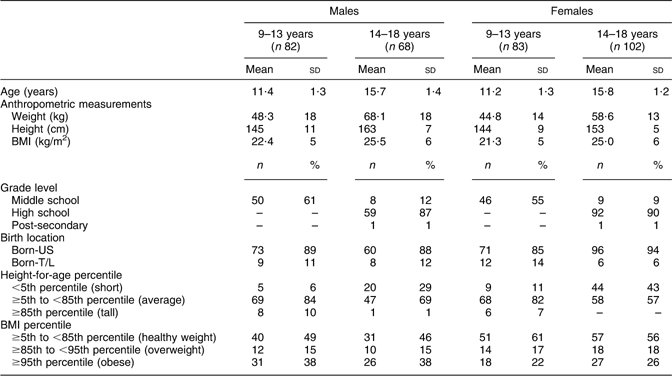
Born-T/L, born and/or raised in Thailand or Laos and had been living in the USA for <5 years; born-US, born in the USA.
Dashes indicate no values.
Table 2 Dietary intakes by birth location for 9–13-year-old Hmong children from Twin Cities, Minnesota, USA, compared with DRI

DRI refer to the Dietary Reference Intakes(27), including Recommended Dietary Allowances and Adequate Intakes (dashes indicate that values have not been determined).
PBD (percentage below DRI): for a specific nutrient, PBD refers to the percentage of children who consumed a nutrient below the DRI.
*Within a specific age group and gender, this superscript indicates significant differences in nutrient consumption between children born in the USA v. those born in Thailand/Laos (T/L; P < 0·05).
†Adequate Intakes.
Table 3 Dietary intakes by birth location for 14–18-year-old Hmong children from Twin Cities, Minnesota, USA, compared with DRI

DRI refer to the Dietary Reference Intakes(27), including Recommended Dietary Allowances and Adequate Intakes (dashes indicate that values have not been determined).
PBD (percentage below DRI): for a specific nutrient, PBD refers to the percentage of children who consumed a nutrient below the DRI.
*Within a specific age group and gender, this superscript indicates significant differences in nutrient consumption between children born in the USA v. those born in Thailand/Laos (T/L; P < 0·05).
†Adequate Intakes.
Table 4 MyPyramid food group analysis of dietary intake among Hmong children from Twin Cities, Minnesota, USA

Born-T/L, born and/or raised in Thailand or Laos and had been living in the USA for <5 years; born-US, born in the USA.
PBS (percentage below suggested): percentage of children who consumed a food group below the suggested serving size.
*Within a specific age group and gender, this superscript indicates significant differences in dietary consumption between children born in the USA v. those born in Thailand/Laos (P < 0·05).
†Values indicate total fats, oils and sweet intake. No suggested MyPyramid serving for fats, oils and sweets. Generally, such items are classified under ‘discretionary calories’ and are recommended to be used sparingly.
Results
Sample characteristics
Sample characteristics of Hmong children are shown in Table 1. Mean age of participants was 13·6 (sd 2·6) years. Some 45·1 % attended high school, 33·7 % middle school and 20·6 % elementary schools. Sixteen per cent of the children were overweight (BMI-for-age ≥85th to <95th percentile) and 30 % were obese (BMI-for-age ≥95th percentile) for their ages. Further, 23·3 % were short statured (height-for-age <5th percentile) and 4·5 % were tall for their ages (height-for-age ≥85th percentile). Compared with born-T/L, born-US children were significantly heavier (mean (sd): 55·8 (18·1) kg v. 44·2 (11·2) kg), taller (mean (sd): 151·3 (10·9) cm v. 146·9 (11·1) cm) and had a higher BMI (mean (sd): 23·9 (5·8) kg/m2v. 20·2 (3·0) kg/m2; P < 0·05 for all comparisons).
24 h dietary recall
The 24 h dietary recall results are shown in Tables 2 and 3. In general, diets of most children were below the DRI levels for fibre, vitamins A, D and E, Ca, Mg and K. Among 9–13 year-old males, born-US consumed significantly more energy, carbohydrates, fat, saturated fat, Na and fluoride than born-T/L ones (P < 0·05). Among 9–13-year-old females, born-US consumed higher amounts of trans fatty acids and Na than their born-T/L counterparts (P < 0·05; Table 2). Approximately one-third of 9–13-year-old females did not meet DRI recommendations for Fe. Among 14–18-year-old males, born-US consumed more energy, carbohydrates, vitamins C and E, Cu, Na and fluoride than born-T/L ones (P < 0·05). Further, among 14–18-year-old females, those born-US consumed more trans fatty acids and Na, and less cholesterol, than their born-T/L counterparts (P < 0·05; Table 3). About two-thirds of 14–18-year-old females did not meet DRI recommendations for Fe.
MyPyramid analysis
MyPyramid analysis indicated that most 9–11-year-olds consumed less vegetables and milk than the suggested servings (Table 4). Further, the majority of 12–18-year-olds consumed less fruits, vegetables and milk than recommended. Born-US males consumed significantly higher amounts of fats, oils and sweets than born-T/L males (P < 0·05), while no significant differences were observed among females in this regard (Table 4). Among 12–18-year-old females, born-US consumed less fruits than the born-T/L ones (P = 0·04). Among 12–18–year-old males, born-US consumed more grains than the born-T/L ones (P = 0·01).
Dietary associations
Among all children, acculturation was positively associated with consumption of carbohydrates (r = 0·12, P = 0·03), saturated fat (r = 0·13, P = 0·02), trans fatty acids (r = 0·13, P = 0·02), Ca (r = 0·16, P = 0·005), Na (r = 0·21, P < 0·001), and fats, oils and sweets (r = 0·11, P = 0·04). Additionally, more acculturated children had a higher BMI-for-age compared with less acculturated ones (r = 0·16, P = 0·005). Likewise, the number of years lived in the USA was positively associated with consumption of energy (r = 0·23, P < 0·001), saturated fat (r = 0·18, P = 0·001), trans fatty acids (r = 0·17, P = 0·003), fibre (r = 0·13, P = 0·02), Na (r = 0·29, P < 0·001), fats, oils and sweets (r = 0·17, P = 0·002) and BMI-for-age (r = 0·39, P < 0·001). Higher BMI was also associated with a significantly higher consumption of Na (r = 0·16, P = 0·004), and a significantly lower consumption of fruits (r = −0·21, P < 0·001) and milk (r = −0·16, P = 0·004).
Discussion
The results of our study suggest that diets of Hmong children are low in nutrients such as Ca, Fe, vitamins A and D, P and fibre, and high in Na and fats, oils and sweets. This is reflected in low consumption levels of vegetables and milk, and high consumption of energy-dense foods. In general, US-born children consumed more energy, carbohydrates, saturated fat and Na, and had a higher BMI, than those born in Thailand/Laos (and had been in the USA for <5 years), suggesting that an obesogenic US environment is a probable reason for poor dietary habits among Hmong children. Additionally, the 24 h dietary recalls of most US-born children included items such as muffins, cakes, chips, soda, chocolate milk, pizza, burgers and fried meats, and most US-born children reported using high-Na sauces as added seasonings (results not shown). Most Thailand/Laos-born children consumed boiled meats, cooked vegetables, steamed rice, candy and cookies (results not shown). No significant differences in rice consumption were noted between the two groups (mean (sd): 1·2 (0·92) cups/d for born-US v. 1·3 (0·61) cups/d for born-T/L).
Children who were more acculturated to US norms including language use, social connections and dietary habits had a higher BMI-for-age compared with their less acculturated counterparts. About half of our participants were either overweight or obese. Research has indicated that obesity during childhood and adolescent years is a risk factor for developing CHD, hypertension, dyslipidaemia, type 2 diabetes, and even results in premature mortality in adulthood(Reference Bibbins-Domingo, Coxson and Pletcher31, Reference Engeland, Bjorge and Tverdal32), suggesting that most Hmong children in our sample may be at a risk for developing such conditions in their near future.
Dietary status of Hmong children
More than 90 % of the children in our sample did not meet the MyPyramid recommendations for the dairy food group including milk, yoghurt and cheese. This observation is similar to national trends in dairy consumption among children, with more than half of children aged 2–8 years and three-quarters of children aged 9–19 years not consuming recommended dairy servings(33). Nutrients such as Ca, vitamin D, P and protein are found in the dairy food group and are required to support growth and development during childhood and adolescent years, including reaching peak bone mass. It is believed that about 85–90 % of the final adult bone mass is acquired by the age of 18–20 years(Reference Heaney, Abrams and Dawson-Hughes34), necessitating the inclusion of bone-building nutrients during childhood. Possible reasons for low dairy consumption in our sample may be related to high lactose intolerance found among Asians(35), and not consuming milk because of taste preferences and/or cultural reasons(Reference Whitney and Rolfes36). Inadequate intakes of Ca and vitamin D during developmental years may increase the risk for osteoporosis later in life(Reference Kirby and Danner37, Reference Tan, Ji and Tsai38). To decrease future cases of osteoporosis, schools should be encouraged to increase Ca intake among children either by encouraging milk and yoghurt consumption among non-lactose intolerant children or by providing non-dairy fortified foods such as juices, cereals and grains to those with lactose intolerance.
The mean Fe consumption was below the DRI for approximately 67 % of 14–18-year-old females (mean 13·6 (sd 8·4) mg; DRI = 15 mg/d). Similar to our results, the National Health and Nutrition Examination Survey (1999–2000) estimated an average Fe intake of 13·4 mg/d among females aged 12–19 years(Reference Wright, Wang and Kennedy-Stephenson39). Fe deficiency affects 2·4 million children in the USA, and it is one of the most common nutritional deficiencies among menstruating adolescents and women(Reference Brotanek, Gosz and Weitzman40). Fe deficiency limits the delivery of oxygen to cells, resulting in decreased immunity, increased fatigue, poor work performance and, among pregnant women, delivery of low-birth-weight infants(Reference Kirchengast and Hartmann41, Reference Grondin, Ruivard and Perreve42). Our results indicated lower consumption levels of Fe among Hmong children and oral supplementation might be a potential source of Fe for this group. Research suggests that long-term oral Fe supplementation can improve cognitive abilities including attention span and the ability to concentrate(Reference Haas and Brownlie43, Reference Falkingham, Abdelhamid and Curtis44).
The diets of most Hmong children were below the recommendations for fibre; the mean fibre intake was about 10 g/d. Further, the mean vegetable intake among all children was less than 1 cup/d. Within the USA, it is estimated that only 39 % of children within the 2–17 years age range meet the US Department of Agriculture's dietary recommendations for fibre(45). As reported in the literature, Hmong-American diets tend to be low in fibre-rich foods such as whole grains, fruits and vegetables, partly because of acculturation to US dietary norms(Reference Goody and Drago13, Reference Yang and Mills46). We found no significant differences in fibre consumption between children consuming traditional diets and those consuming more Americanized diets. Franzen and Smith(Reference Franzen and Smith4) reported low intake levels of fruits among Hmong because fruits were considered as luxury items and consumed sparingly, often as a dessert. Also, fruits that were preferred and easily available in Thailand/Laos, such as jackfruit, mango, guava, papaya and pineapple, are either hard to find or too expensive to purchase in the USA, further decreasing fruit consumption(Reference Goody and Drago13). While a diet rich in fibre has many health benefits such as lowering LDL cholesterol, decreasing the incidence of CVD and diabetes, preventing obesity, limiting total energy intake and providing other important micronutrients(Reference Marlett and Slavin47), it will be a challenge to health-care professionals to create ways to increase fibre in this Asian subgroup. Our results suggest that Hmong children would benefit from early education about the benefits of fibre and foods rich in fibre, with emphasis on the consumption of whole grains, fruits and vegetables. This might be best accomplished at school through the National School Lunch Program by including whole grain food choices, fresh fruits and salads on the school menu. Further, involving parents in educational and/or physical activity programmes with their children could improve activity levels, although this has not been evaluated among Hmong. Parental participation will be important because Hmong parents (specifically the recently immigrated ones) might perceive losing weight as a negative health condition, because being heavy is generally perceived as being beautiful and healthy in traditional Hmong culture(Reference Mulasi-Pokhriyal and Smith48). One such intervention could be incorporating gardening projects in school curricula/community programmes, with parents and children planting seasonal fruits and vegetables as a family.
Acculturation and dietary intake
Number of years lived in the USA and acculturation to US dietary habits were associated with a higher consumption of energy, trans fatty acids, saturated fats, sugars and Na, and may partly explain why about half of our sample was overweight/obese. In a sample of low-income Puerto Rican women living in the USA, Himmelgreen et al.(Reference Himmelgreen, Perez-Escamilla and Martinez49) found significant increases in BMI with the length of time stayed in the USA. Research also indicates that after immigrating and acculturating to the US environment, sedentary habits, busy lifestyle and physical inactivity have led to increases in overweight and obesity in the Hmong population(Reference Franzen and Smith4, Reference Franzen and Smith5, Reference Goody and Drago13). Stang et al.(Reference Stang, Kong and Story14) found that when compared with white adolescents, Hmong adolescents reported less physical activity and were at an increased risk for obesity. Most Hmong migrated from areas where they worked hard in farm fields; manual labour was the primary economic source. Post-migration, many Hmong adopt a sedentary lifestyle, and have less time for being physically active(Reference Franzen and Smith4, Reference Kim, Harrison and Kagawa-Singer50). Further, many still associate physical activity with occupation and are usually not interested in exercising during leisure time(Reference Franzen and Smith4). Further, Asian Americans appear to be genetically susceptible to develop abdominal obesity and insulin resistance and the risk of type 2 diabetes among Asians starts at a lower BMI(Reference Chan, Malik and Jia51), emphasizing the importance of a healthy diet and physical activity among Hmong from an earlier age.
Results from the present study also indicate that children who were born in the USA consumed significantly more Na than their Thailand/Laos-born counterparts. Research has shown that number of years lived in the USA and acculturation to US dietary patterns are associated with increased Na consumption and consequently a higher prevalence of hypertension among immigrant populations(Reference Moran, Roux and Jackson52). Because the children in our study are in their preadolescent to adolescent years, consuming high-Na diets makes them susceptible to develop hypertension and associated conditions such as CVD if measures to educate them about healthy lifestyle are not taken soon.
Limitations and conclusions
Although our study is the first one to demonstrate a detailed, descriptive quantitative analysis of Hmong diets from an acculturation perspective, nevertheless it has some limitations. Some participants may have under/over-reported their food intake. Earlier research has found that overweight/obese respondents, women and weight-conscious people tend to under-report their food intake because of social desirability, probably leading to respondent bias during data collection(Reference Gibson20, Reference Harnack, Jeffery and Boutelle53, Reference Briefel, Sempos and McDowell54). Second, interviewer bias is a common form of error within 24 h dietary recalls(Reference Gibson20), and some participant dietary information might have been missed, misunderstood or incorrectly recorded by the researchers. However, we believe that using the multiple-pass interview technique and incorporating memory prompts such as food models and measuring cups/spoons during the interviews minimized this problem. Third, the born-T/L sample was smaller in size than the born-US one, making the comparisons between these two groups somewhat difficult. However, statistical tests including t tests adjust for the sample size and some results were found to be significant while comparing born-US children with the born-T/L ones. Finally, although we recruited a representative sample of Hmong children in Minnesota, our results cannot be generalized to all Hmong children living in the USA. Given that the Hmong are a fast-growing Asian ethnic subgroup in the USA(2, Reference Pfeifer55), it is important to learn more about their nutritional status and needs from a health-care perspective. Our findings indicate high intakes of fats, sweets and Na among young Hmong and suggest a need for dietary education and intervention among Hmong children towards eating healthier foods.
Acknowledgements
The present study was funded by the University of Minnesota Agricultural Experiment Station. No author has a conflict of interest. This manuscript is an original contribution and has not been published elsewhere; all authors collected data and contributed to the interpretation of the data, the writing of the manuscript, and read and approved this version of the manuscript. U.M.-P. collected, entered and analysed data, and took the lead in the writing of the manuscript. C.S., project Principal Investigator, conceptualized the design of the project, collected data, supervised the data analyses, and assisted in the writing and editing of the manuscript. L.F.-C. collected, entered and analysed the data, and read through and edited the manuscript.






