Lithium was the first representative of mood stabilisers in the long-term treatment of bipolar disorders and is still considered a prototype drug for the prevention of manic and depressive recurrences.Reference Geddes, Goodwin, Rendell, Azorin, Cipriani and Ostacher 1 About one-third of lithium-treated patients are excellent responders, showing total prevention of manic and depressive episodes.Reference Rybakowski, Chlopocka-Wozniak and Suwalska 2 , Reference Garnham, Munro, Slaney, Macdougall, Passmore and Duffy 3 A number of clinical predictors for treatment response to lithium have been reported, such as age at onset, family history of bipolar disorder, rapid cycling and atypical features.Reference Kleindienst, Engel and Greil 4 – Reference Berghofer, Alda, Adli, Baethge, Bauer and Bschor 6 However, none of these has sufficient sensitivity to be clinically useful.
It has been suggested that lithium-responsive bipolar disorder may have a unique genetic component. The evidence has suggested that individuals who respond to lithium maintenance treatment seem to cluster in families.Reference Rybakowski 7 The first genome-wide association study (GWAS) of response to lithium maintenance treatment in bipolar disorder revealed that positive lithium response might be associated with a region on chromosome 4q32 spanning a gene coding for a glutamate receptor, GRIA2.Reference Perlis, Smoller, Ferreira, McQuillin, Bass and Lawrence 8 Recently, a GWAS conducted by the Taiwan Bipolar Consortium (TBC) has identified glutamate decarboxylase-like protein 1 (GADL1) as a valid genetic marker for response to lithium maintenance treatment in bipolar I (BPI) disorder.Reference Chen, Lee, Lee, Ouyang, Chen and Chong 9 In that study, rs17026688, a representative of three valid variants in GADL1, had a sensitivity of 93% for predicting a response to lithium maintenance therapy in BPI patients.Reference Chen, Lee, Lee, Ouyang, Chen and Chong 9
A meta-analysis of hundreds of empirical medical studies concludes that the average rate of non-adherence to medical recommendations is 24.8%.Reference DiMatteo 10 Rates of adherence to long-term regimens are much lower than those to short-term (≤2 weeks) regimens.Reference Haynes, McDonald and Garg 11 It is well recognised that treatment adherence is poor among psychiatric patients.Reference Osterberg and Blaschke 12 Rates of non-adherence ranged from 20 to 50% in patients with bipolar disorder.Reference Julius, Novitsky and Dubin 13 – Reference Murru, Pacchiarotti, Amann, Nivoli, Vieta and Colom 15 In one study,Reference Connelly, Davenport and Nurnberger 16 non-adherence to lithium prophylactic regimen was reported to be associated with a poor outcome in patients with affective-spectrum disorders.
Medication non-adherence has often been overlooked in pharmacogenetic studies.Reference Malhotra, Zhang and Lencz 17 If a substantial proportion of participants are non-adherent to treatment, it would be very difficult to detect a significant genotype–phenotype relationship regardless of the strength of the effect of the genetic marker. Some researchers have emphasised the importance of design considerations to ensure increased assessment of participants in the earliest phases of the illness and to maximise adherence to treatment in pharmacogenetic studies.Reference Malhotra, Zhang and Lencz 17
In this study, we have examined the individual and joint effects of the genetic marker GADL1 and drug adherence status on the clinical outcome of BPI (assessed by the frequencies of manic and depressive episodes during off-lithium, poor lithium adherence and good lithium adherence periods).
Method
Study participants
Study patients were selected from a total of 1901 BPI patients recruited from 52 psychiatric departments of general hospitals and psychiatric institutions in the TBC up to August 2015. The TBC aims to understand genetic vulnerability of BPI and to conduct pharmacogenetic study of mood stabilisers. Methodology of the study has been described in detail elsewhere.Reference Lee, Chen, Lee, Chen, Chong and Ouyang 18 In brief, unrelated BPI patients with Han Chinese ethnicity aged 20 to 65 were recruited from the psychiatric departments and institutions in the TBC. All of them were diagnosed according to DSM-IV criteria for BPI disorder with recurrent episodes of mania with or without depressive episode(s). 19 Patients with other psychotic and affective disorders were excluded.
For the pharmacogenetic study of mood stabilisers, we identified 445 patients who had ever received lithium treatment with good adherence for at least 2 years during their disease course from all the 1901 BPI patients. Among them, 215 patients who also had both period(s) without lithium maintenance treatment and period(s) with poor adherence to lithium treatment were included in this study. Figure 1 shows the case selection profile. The study was approved by the institutional review board at each of all the participating hospitals and at Academia Sinica, Taiwan. All the study participants provided a written informed consent.
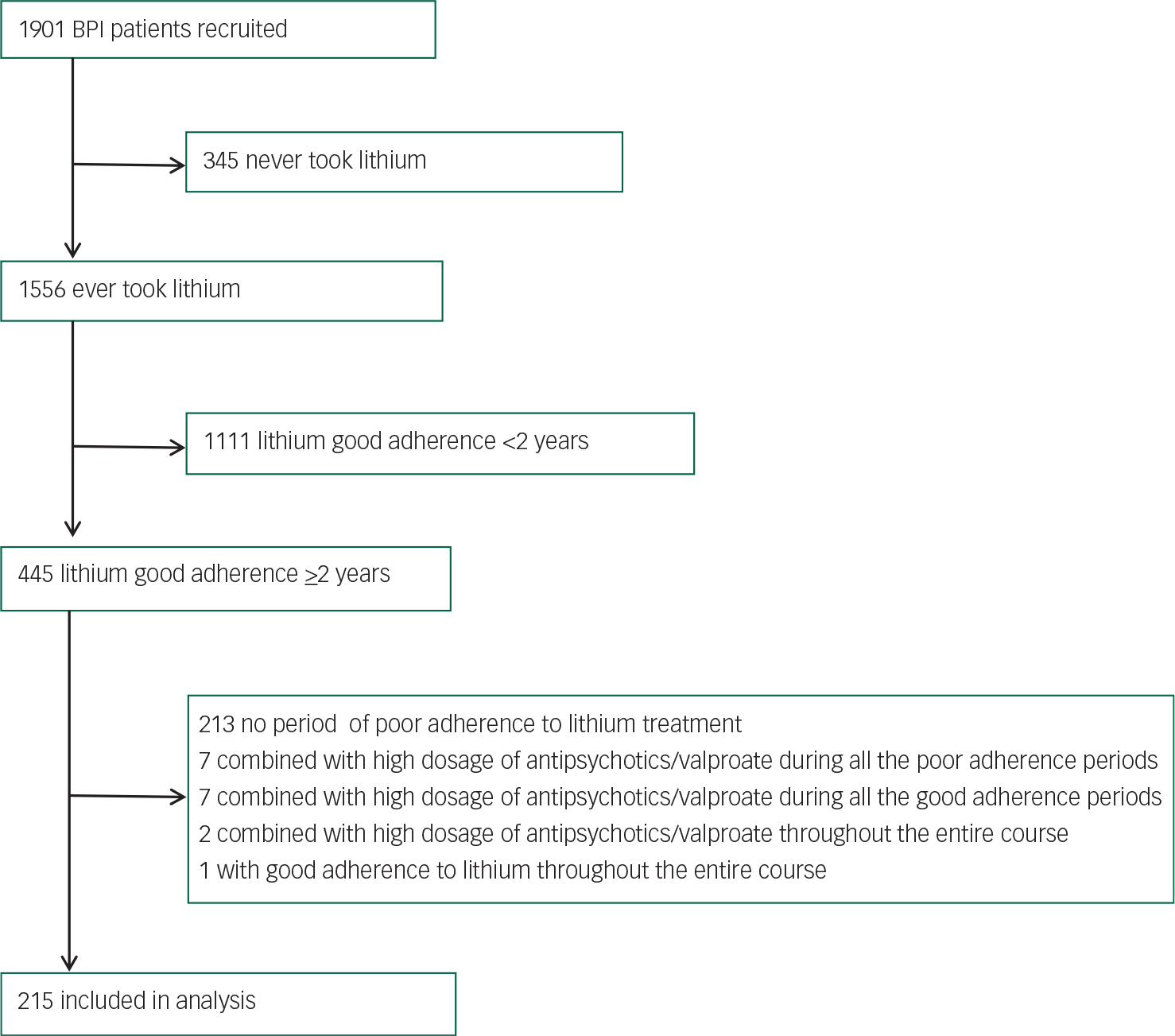
Fig. 1 Case selection profile.
Phenotype definition and assessment
Clinical assessment of mania and depression was performed by trained psychiatric nurses and psychiatrists using the Chinese version of the Schedules for Clinical Assessment in Neuropsychiatry (SCAN),Reference Cheng, Tien, Chang, Brugha, Cooper and Lee 20 supplemented by available medical records and reports from key family members and in-charge psychiatrists.
In the previous GWAS conducted by the TBC to identify GADL1,Reference Chen, Lee, Lee, Ouyang, Chen and Chong 9 the assessment of drug adherence and response to lithium maintenance treatment in BPI disorder was based on a life chart with graphical presentation of lifetime clinical course prepared for every patient recruited by the TBC. This life chart has included all manic, hypomanic and depressive episodes with onset year and month, duration and severity, all doses of and duration of treatment with psychotropic drugs and mood stabilisers ever prescribed, drug adherence recorded in medical charts during treatment at out-patient clinics, all recorded blood levels of mood stabilisers and any adverse drug reactions. Mixed episodes were categorised into manic episodes in this study. This graphical life chart was presented on the basis of integrated information gathered from direct interview with patients and their key family members, interview with in-charge psychiatrists and a thorough medical chart review.
In this study, we identified three different types of period throughout the life course of each study patient based on their life charts: one with good adherence to lithium maintenance treatment, one with poor adherence to lithium maintenance treatment and one with no lithium treatment. Since a patient may have several periods of any type, all periods of a specific type were summed to generate a ‘cumulative period’ for that type to compare their clinical outcomes (for a patient initially with 3 years of poor adherence, followed by 5 years of good adherence and another 10 years of poor adherence, the cumulative period of poor adherence is 13 years). This study focused on lithium maintenance treatment. Therefore, periods of hospital admissions for acute treatment were not included in our analyses. Treatment adherence was rated as good when regular visits at out-patient clinics and ≥90% of serum lithium assays remained consistently ≥ 0.50mmol/L,Reference Yang 21 further verified by a close family member, the in-charge psychiatrist as well as the patient. Otherwise, treatment adherence was rated as poor. For example, for those who took lithium just before the blood draw and had serum lithium assays consistently ≥0.50 mmol/L but their family did not confirm the good adherence, we rated their treatment adherence as poor. The main outcomes in this study were the frequencies of recurrent manic, hypomanic and depressive episodes in the above three cumulative periods.
In assessing the relationships between lithium treatment, drug adherence and clinical outcome in individual patients, it is essential to minimise the influence from concomitant use of antipsychotics and/or other mood stabilisers. In this study, we excluded all periods with additional mood stabilisers and/or antipsychotics reaching the therapeutic dose ranges recommended by the expert consensus guideline.Reference Kane, Leucht, Carpenter and Docherty 22
Genotyping
Given the evidence from the previous study that rs17026668 has the highest sensitivity for predicting lithium response and that the other two GADL1 variants (rs17026651 and GADL1 IVS8 +48delG) are in complete or almost complete linkage disequilibrium with rs17026668, we only genotyped rs17026668 in this study. GADL1 rs17026688 genotyping was performed using the TaqManR SNP genotyping assays (ABI: Applied Biosystems Inc. Foster City, CA, USA). The primers and probes for rs17026688 were from the ABI assay on demand (AOD) kit. Reactions were carried out according to the manufacturer's protocols. The probe fluorescence signals were detected using the ABI Prism 7500 Real-Time PCR System.
Statistical analyses
Variables were presented as either mean with standard deviations or frequency (%). The distribution of rs17026688 genotypes was tested for Hardy–Weinberg equilibrium. The chi-square test or t-test was used to compare the difference between rs17026688 T-allele carriers and non-carriers for categorical or continuous variables, respectively.
The cumulative periods of poor adherence observed in 37 patients and those of good adherence observed in 2 patients were shorter than the average intervals of affective episodes during their cumulative off-lithium periods. These could lead to an inflation of frequencies of affective episodes during these cumulative on-lithium periods when durations of observation were served as the denominator. To adjust this bias, the frequencies of affective episodes and psychiatric admissions during these short cumulative on-lithium periods were treated as missing values.
The frequencies of affective episodes and psychiatric admissions were defined as numbers of episodes divided by durations of observation. The distribution of affective frequency was linear but non-normal. In consideration of efficacy for handling missing data, non-normal distribution of target variables and non-independence of observations, the generalised linear mixed models were applied to analyse the frequencies of affective episodes and psychiatric admissions by adherence (during cumulative periods of off-lithium/poor lithium adherence/good lithium adherence) and rs17026688 polymorphism (T allele carriers/non-carriers). Potential two-way interactions of rs17026688 polymorphism and lithium adherence status on dependent variables were examined. Duration of lithium prophylaxis treatment was treated as a covariate. The planned comparisons were frequencies of manic and depressive episodes. Complementary comparisons included frequencies of all affective episodes and frequencies of psychiatric admissions. The post hoc analyses comparing mean frequencies of affective episodes between rs17026688 T and non-T allele carriers were adjusted for multiple comparisons using sequential Bonferroni correction. The statistical software package IBM SPSS Statistics version 21.0 (IBM Co., Armonk, NY, USA) was utilised for statistical analyses. All tests were two-tailed and P-values <0.05 were considered significant.
Results
Genotypic frequencies of rs17026688 in all 215 study patients were 47, 41.9 and 11.2% for CC, CT and TT alleles, respectively. The genotypic distribution of the rs17026688 polymorphism was in Hardy–Weinberg equilibrium (P=0.561). Demographic and clinical characteristics between the effective T allele carriers and non-carriers are shown in Table 1. The mean age was 49.5 (s.d.=11.0) years, and males and females were similar in proportion. No significant difference was found between T carriers and non-T carriers for all characteristics, except the duration of lithium maintenance treatment. Patients carrying the T allele tended to have a longer period of lithium maintenance treatment than their non-T carrier counterparts (P=0.003). The median duration of lithium therapy among study patients was 10 years (ranging from 2 to 35 years). The mean frequency of manic and depressive episodes during off-lithium periods was 0.8 (s.d.=0.5) per year and 0.3 (s.d.=0.4) per year, respectively. The mean interval between episodes was 13.8 (s.d.=8.0) months for affective episodes and 21.5 (s.d.=20.7) months for manic episodes during off-lithium periods.
Table 1 Demographic and clinical characteristics of study patients: GADL1 T allele carriers and non-T carriers
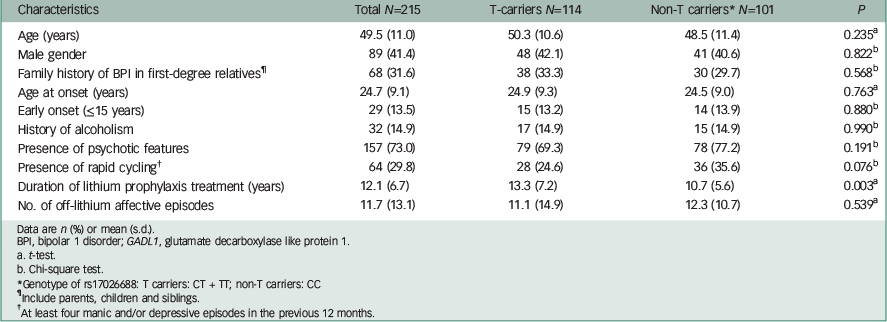
| Characteristics | Total N=215 | T-carriers N=114 | Non-T carriers* N=101 | P |
|---|---|---|---|---|
| Age (years) | 49.5 (11.0) | 50.3 (10.6) | 48.5 (11.4) | 0.235 a |
| Male gender | 89 (41.4) | 48 (42.1) | 41 (40.6) | 0.822 b |
| Family history of BPI in first-degree relatives ¶ | 68 (31.6) | 38 (33.3) | 30 (29.7) | 0.568 b |
| Age at onset (years) | 24.7 (9.1) | 24.9 (9.3) | 24.5 (9.0) | 0.763 a |
| Early onset (≤15 years) | 29 (13.5) | 15 (13.2) | 14 (13.9) | 0.880 b |
| History of alcoholism | 32 (14.9) | 17 (14.9) | 15 (14.9) | 0.990 b |
| Presence of psychotic features | 157 (73.0) | 79 (69.3) | 78 (77.2) | 0.191 b |
| Presence of rapid cycling † | 64 (29.8) | 28 (24.6) | 36 (35.6) | 0.076 b |
| Duration of lithium prophylaxis treatment (years) | 12.1 (6.7) | 13.3 (7.2) | 10.7 (5.6) | 0.003 a |
| No. of off-lithium affective episodes | 11.7 (13.1) | 11.1 (14.9) | 12.3 (10.7) | 0.539 a |
Data are n (%) or mean (s.d.).
BPI, bipolar 1 disorder; GADL1, glutamate decarboxylase like protein 1.
a t-test.
b Chi-square test.
* Genotype of rs17026688: T carriers: CT + TT; non-T carriers: CC
¶ Include parents, children and siblings.
† At least four manic and/or depressive episodes in the previous 12 months.
Table 2 shows the main and interactive effects of adherence and rs17026688 polymorphism on response to lithium treatment using generalised linear mixed models, with duration of lithium treatment as a covariate. There were significant interactions between lithium adherence and rs17026688 polymorphism for manic, depressive, all affective episodes and psychiatric admissions.
Table 2 Effect of medication adherence and GADL1 rs17026688 polymorphisms on response to lithium maintenance treatment (n=215)*
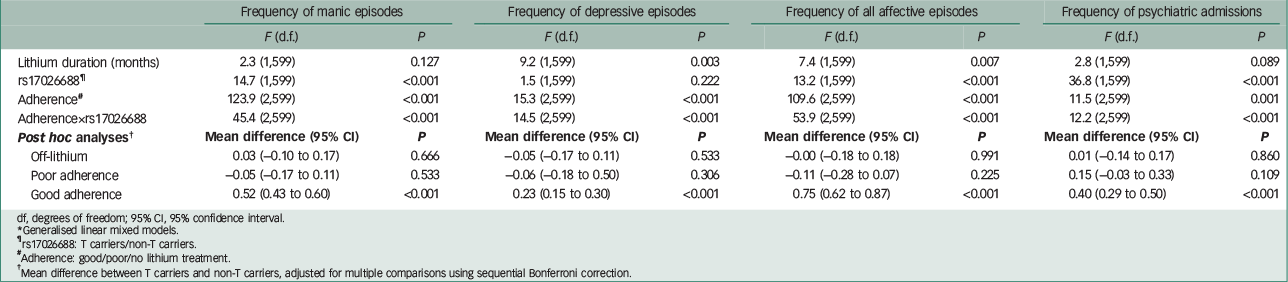
| Frequency of manic episodes | Frequency of depressive episodes | Frequency of all affective episodes | Frequency of psychiatric admissions | |||||
|---|---|---|---|---|---|---|---|---|
| F (d.f.) | P | F (d.f.) | P | F (d.f.) | P | F (d.f.) | P | |
| Lithium duration (months) | 2.3 (1,599) | 0.127 | 9.2 (1,599) | 0.003 | 7.4 (1,599) | 0.007 | 2.8 (1,599) | 0.089 |
| rs17026688 ¶ | 14.7 (1,599) | <0.001 | 1.5 (1,599) | 0.222 | 13.2 (1,599) | <0.001 | 36.8 (1,599) | <0.001 |
| Adherence # | 123.9 (2,599) | <0.001 | 15.3 (2,599) | <0.001 | 109.6 (2,599) | <0.001 | 11.5 (2,599) | 0.001 |
| Adherence×rs17026688 | 45.4 (2,599) | <0.001 | 14.5 (2,599) | <0.001 | 53.9 (2,599) | <0.001 | 12.2 (2,599) | <0.001 |
| Post hoc analyses † | Mean difference (95% CI) | P | Mean difference (95% CI) | P | Mean difference (95% CI) | P | Mean difference (95% CI) | P |
| Off-lithium | 0.03 (−0.10 to 0.17) | 0.666 | −0.05 (−0.17 to 0.11) | 0.533 | −0.00 (−0.18 to 0.18) | 0.991 | 0.01 (−0.14 to 0.17) | 0.860 |
| Poor adherence | −0.05 (−0.17 to 0.11) | 0.533 | −0.06 (−0.18 to 0.50) | 0.306 | −0.11 (−0.28 to 0.07) | 0.225 | 0.15 (−0.03 to 0.33) | 0.109 |
| Good adherence | 0.52 (0.43 to 0.60) | <0.001 | 0.23 (0.15 to 0.30) | <0.001 | 0.75 (0.62 to 0.87) | <0.001 | 0.40 (0.29 to 0.50) | <0.001 |
df, degrees of freedom; 95% CI, 95% confidence interval.
* Generalised linear mixed models.
¶ rs17026688: T carriers/non-T carriers.
# Adherence: good/poor/no lithium treatment.
† Mean difference between T carriers and non-T carriers, adjusted for multiple comparisons using sequential Bonferroni correction.
Table 2 also shows post hoc comparisons of mean frequencies of affective episodes and psychiatric admissions between rs17026688 T carriers and non-T carriers during off-lithium, poor adherence and good adherence cumulative periods adjusted for multiple comparisons using sequential Bonferroni correction. During off-lithium and poor adherence periods, there was no significant difference in frequencies of recurrent manic/depressive/all episodes. However, during good adherence periods, T carriers had significantly lower frequencies of recurrent affective episodes than non-T carriers (P<0.001 for manic, depressive and all episodes, respectively).
Figure 2 demonstrates interactive effects of rs17026688 polymorphism and lithium adherence on response to lithium maintenance treatment. These interactions indicated that frequencies of affective episodes decreased more for those with rs17026688 T carriers and good adherence to lithium treatment.
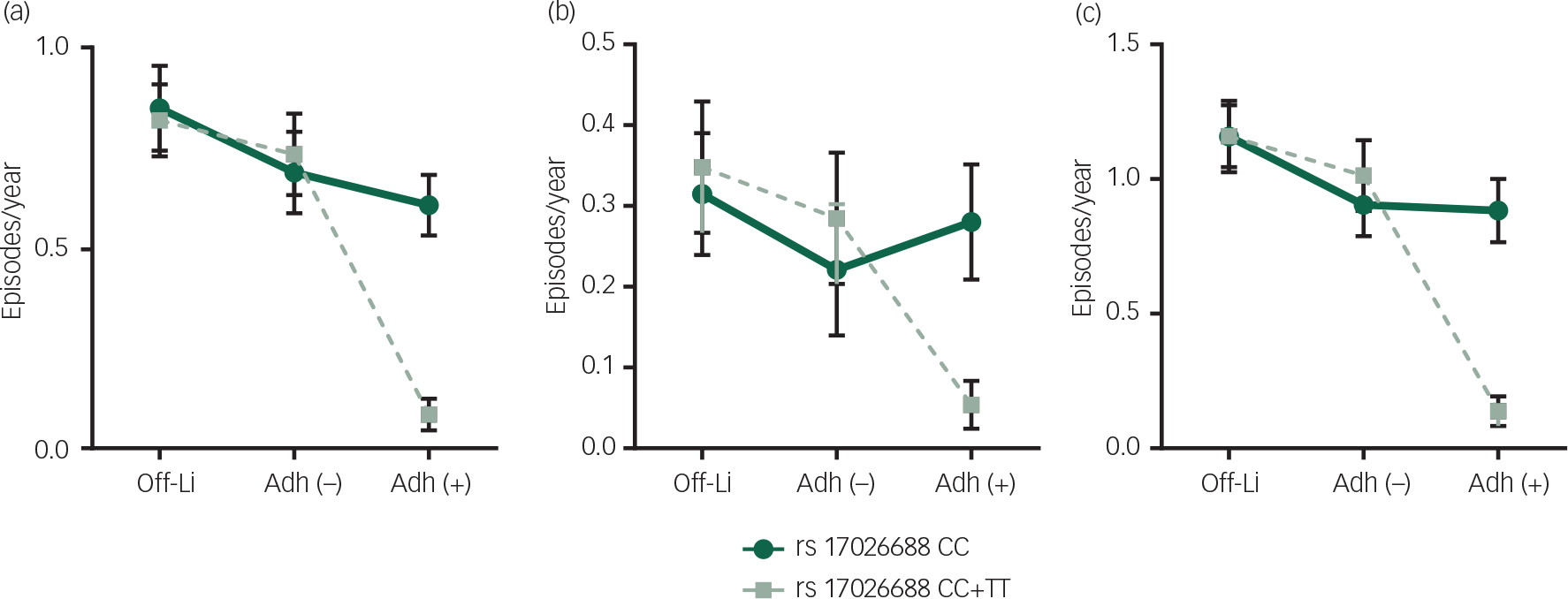
Fig. 2 Changes in mean frequencies of affective episodes during cumulative periods of off-lithium, poor lithium adherence and good lithium adherence between different SNP rs17026688 polymorphism (T carriers and non-T carriers). The X-axis is the cumulative periods of off-lithium, poor lithium adherence and good lithium adherence. The Y-axis is the mean frequencies of affective episodes. Panel (a) shows the changes in mean frequencies of manic episodes. Panel (b) shows the changes in mean frequencies of depressive episodes. Panel (c) shows the changes in mean frequencies of all affective episodes. Error bars represent 95% confidence interval of the means. T carriers had significantly lower frequencies of affective episodes than non-T carriers during good adherence periods. There was no significant difference in frequencies of manic/depressive/all episodes between T carriers and non-T carriers during off-lithium and poor adherence periods. Off-Li: off-lithium; Adh (−): poor adherence; Adh (+): good adherence.
Discussion
This study revealed that both GADL1 variant and medication adherence are equally important in predicting the outcome of maintenance treatment with lithium in BPI. To the best of our knowledge, this is the first study in psychiatry, and probably the first in all medical fields, to demonstrate interactive effects of genetic markers and medication adherence in predicting treatment outcome.
Two major clinical issues have led to profound difficulties in evaluating the long-term maintenance effect of lithium. First, the lifelong clinical course of bipolar disorder varies widely from patient to patient, and profound recall bias leads to difficulties in accurate comparison between on- and off-lithium periods when relying simply on interviewing patients. Since our data showed that the mean interval between episodes was 13.8 (s.d.=8.0) months for affective episodes and 21.5 (s.d.=20.7) months for manic episodes during off-lithium periods, it is reasonable to choose a 2-year cut-point of lithium therapy with good adherence in the recruitment of study patients for assessing response to lithium maintenance treatment. Second, drug adherence to lithium and other mood stabilisers is often difficult to examine accurately in the long term.Reference Malhotra, Zhang and Lencz 17 These two problems are likely to generate a considerable proportion of misclassification in responders and non-responders in pharmacogenetic study of response to lithium and other mood stabilisers. We believe that the preparation of a life chart for every patient in our pharmacogenetic study of mood stabilisers has overcome these two difficulties to a great extent.
Since the identification of GADL1 as a valid genetic marker for response to lithium maintenance treatment in BPI disorder via GWAS,Reference Chen, Lee, Lee, Ouyang, Chen and Chong 9 a few studies have failed to replicate this finding.Reference Hou, Heilbronner, Rietschel, Kato, Kuo and McMahon 23 – Reference Cruceanu, Alda, Dion, Turecki and Rouleau 26 There are two possible explanations for this discrepancy. One is the failure of these replicative studies to duplicate the methods in phenotype definition and assessment used in the TBC study. In this study, we have applied a stringent phenotype definition for adherence to lithium maintenance treatment which was similar to the criteria used by Gonzalez-Pinto et al.Reference Gonzalez-Pinto, Mosquera, Alonso, López, Ramírez and Vieta 27 Good treatment adherence was defined when ≥90% of serum lithium assays remained consistently ≥0.50 mmol/L.Reference Yang 21 The other explanation is the extremely low frequencies of GADL1 rs17026688 alleles in persons of European and African ancestry. 28 , 29
In this study, the significant association between rs17026688 polymorphism and lithium prophylaxis response can only be observed during good adherence periods. The sample size in this study is probably not sufficient for detecting small genetic effects. However, the genetic marker GADL1 identified in our previous studyReference Chen, Lee, Lee, Ouyang, Chen and Chong 9 has a quite large effect which we trust is enough to examine its interaction with drug adherence in response to lithium maintenance treatment in this study. Our findings support that a more careful and accurate phenotype assessment and clinical evaluation on drug adherence is most important in assessing the long-term maintenance treatment effect of mood stabilisers. To improve and ensure drug adherence during the long term, maintenance treatment of mood stabilisers is essential.
In this study, we did not simply categorise our study patients into responders and non-responders to lithium treatment. In order to investigate the effect of drug adherence on treatment response at different periods under lithium therapy, we classified the entire illness course into three types of periods: off-lithium, on-lithium with poor adherence and on-lithium with good adherence. The significant association between medication adherence and response to lithium maintenance treatment found in our study has lent strong support to those in previous reports.Reference Connelly, Davenport and Nurnberger 16 , Reference Gonzalez-Pinto, Mosquera, Alonso, López, Ramírez and Vieta 27
The participants in this study were BPI patients who had received lithium treatment with good adherence for at least 2 years during their disease course. One could question about selection bias or representativeness of the results in this study by assuming that a lack of drug efficacy may lead to premature termination of medication and good efficacy may contribute to good adherence. However, one cannot assess the effect of lithium in patients with poor adherence or premature termination, which may involve factors related to patients, psychiatrists, treatment and social environment. In this study, rs17026688 T carriers had a significantly longer duration of lithium prophylaxis treatment than non-T carriers. It is difficult to determine the causal relations between duration of lithium prophylaxis treatment and lithium response. Nevertheless, duration of lithium prophylaxis treatment was used as a covariate when interactions of rs17026688 polymorphism and lithium adherence status on dependent variables were examined.
Limitations
There are at least three major limitations in this study. First, the low frequencies of rs17026688 T allele in Caucasians and Africans reduces the applicability of rs17026688 as a genetic marker to examine its effect in predicting response to lithium therapy jointly with medication adherence among these ethnic populations. Second, the frequency of psychiatric admissions assessed in this study may not be only related to pharmacotherapeutic effect. Social factors including healthcare accessibility and possibly different standards for admissions among the investigators were not included in these analyses. Third, this study employed a retrospective design that cannot escape from recall bias, though all efforts had been made to minimise this. The most important is that we have constructed a detailed life chart for individual patients over their long-term illness course to minimise any recall bias.
Implications
We studied the individual and joint effects of GADL1 and medication adherence in predicting response to lithium maintenance treatment in Han Chinese BPI patients. We have demonstrated that the interaction between rs17026688 polymorphism and lithium adherence had a significant effect on reducing manic and depressive episodes. This is the first study of its kind in psychiatry and probably the first in all medical fields. The results of this study suggest that GADL1 rs17026688 and medication adherence can jointly predict response to lithium maintenance treatment in Han Chinese BPI patients. This information may explain at least in part the ambiguous results of recent replication studies on GADL1 valiant and response to lithium in BPI disorder. The evidence currently available also suggests the importance of design considerations to maximise medication adherence in pharmacogenetic studies.
Funding
The study was supported by a grant (AS 23-23, 52102310023C) from Academia Sinica and grants (NSC100-2325-B-001-026, NSC101-2325-B-001-023 and NSC102-2325-B-001-023) from the Ministry of Science and Technology, Taiwan. C.K.C. was supported in this work by Chang Gung Memorial Hospital, Keelung, Taiwan, (BMRP769) and a grant (MOST103-2314-B-182-011-MY3).








eLetters
No eLetters have been published for this article.