Importance of comorbidity
Comorbidity refers to disorders and diseases that occur in tandem with likelihoods greater than expected by chance alone.Reference Jakovljevic and Ostojic1 The study of comorbidity has evolved rapidly in recent years since its formal description with respect to chronic disease by Feinstein et al. Reference Feinstein2 Yet the study of comorbidity remains complex and challenging, even as the field of comorbidity medicine continues to progress.Reference Jakovljevic and Ostojic1 Current definitions incorporate concepts such as multi-comorbidity in addition to simple two-diagnosis comorbidity, and the idea of etiological and non-etiological comorbidity, referring to its causal and independent forms, among others.Reference Jakovljevic and Ostojic1 Its applicability to clinical practice is limited and, although common, it remains poorly understood.Reference Jakovljevic and Ostojic1
With respect to cancer, study most frequently focuses on constrained sets of disorders related to a specific type of cancer.Reference Ahern, Horvath-Puho, Spindler, Sorensen, Ording and Erichsen3–Reference Kaul, Veeranki, Rodriguez and Kuo14 Other approaches often focus on proximal or prognostic comorbidity and employ comorbidity indices, such as the Charlson index.Reference Asano, Yamada and Fujii5, Reference Chang, Yin and Wei15–Reference Tian, Xu, Yu, Li and Liu25
Current evidence on the comorbidity of cancer and mental disorders
Recent studies that examined the association between neoplasms, including cancer, and mental disorders focused on the association of mental morbidity in cancer patients and survivors, utilising diagnostic interviews and surveys to assess mental disorder status.Reference Keszte, Danker and Dietz26, Reference Ng, Roder, Koczwara and Vitry27 Others have examined mental health problems and needs for social support post-diagnosis.Reference Faller, Weis and Koch28 While a population study approach is informative, especially when both mental and physical comorbidities are examined, studies are often cross-sectionalReference Ng, Roder, Koczwara and Vitry27 and are biased by recall in interviews to assess mental status in association with cancer.Reference Nakash, Levav and Guilar-Gaxiola29
Population-based study
One recent large population study,Reference Lu, Andersson and Fall30 employing 10 years of data from Sweden's national registry of physician diagnoses, studied temporal data related to mental disorder, finding that mental disorder rates increased 10 months before cancer, peaking immediately after and remaining elevated for the study duration. Findings were strongest with cancers that had a poorer prognosis. The overall long-term co-occurrence in a population of any mental disorder and any cancer has not been reported in the reviewed literature.
While the temporal order of disorder and disease arising in any given patient is identified in terms of the progress of time, the population-based study of the temporal order of comorbid diseases may be bidirectional in time, given the advent of large data-sets, as the 10-year Swedish studyReference Lu, Andersson and Fall30 and othersReference Cawthorpe and Davidson31 have demonstrated. Such studies have formed the groundwork for better understanding the temporal relationship between cancers and mental disorders.
Potential mechanisms
There is a growing body of evidence showing that systemic inflammation mediated by pro-inflammatory cytokines can facilitate tumour growth and metastasis.Reference Mantovani, Allavena, Sica and Balkwill32–Reference Chiang and Massague35 Inflammation-based prognostic scores have been found to be independently associated with survival in cancer, independent of tumour type. Furthermore, antipsychotic medications, in addition to stress and mental disorder, affect central nervous system cell–cell communication, it is possible that they also modulate immunity and inflammation.
Other research has shown that environmental factors can lead to epigenetic changes. Bioactive nutrients and gut microbiota can alter DNA methylation and modulate the ‘gut–brain axis’ via their influence on inflammatory cytokines and production of antimicrobial peptides.Reference Paul, Barnes and Mark-Wahnefried36 It is biologically plausible that similar pathways or conditions drive the development of both cancer and mental illness;Reference Alam, Abdolmaleky and Zhou37 however, the temporal association of such factors remains confounded by treatment and is largely unstudied.
Purpose of present study
The purpose of the current study was to expand our present understanding of comorbidity. Using a 16-year, regional population data-set, we examined, separately for males and females, the relationship between all neoplasms (cancers) before and after any mental disorder and all mental disorders before and after any neoplasm, where these physician-diagnosed main ICD classes co-occurred. We hypothesised that specific mental disorders and neoplasms would arise significantly in proportion before or after the pivot ICD classes of interest (neoplasm or mental disorder).
Method
To be paid for services, physicians must submit forms for each encounter to the Provincial government that include diagnosis (up to three per claim). All regional provincial physician billing records data from the Calgary Health Zone, Alberta, Canada, representing physician-diagnosed ICD-9 classification, age, gender and diagnosis date between Spring 1993 and Fall 2010 were analysed. The sample consisted of 75 944 698 records for 525 439 (55% female) unique individuals over the age of 18. The data represent the majority of the residing population over the period, including those that moved to and away from the region, or were deceased. Additional regional systems (emergency, in-patient, ambulatory) that also record physician diagnosis were not considered to avoid redundancy.
Data were grouped based on Boolean association within the two main ICD classes, with neoplasm and mental disorder independently linked to all associated ICD disorders. For the purpose of comparison between and within groups, odds ratios (OR). In addition to ORs, data were also grouped so that the first date of diagnosis of any disorder for each patient could be compared with the first date of diagnosis by class (any mental disorder or any neoplasm) and by specific main ICD diagnosis within the class (any mental disorder or neoplasm). It was rare for mental disorder and cancer to be diagnosed on the same visit day, and such cases were not included in the analysis (242 males/31 439 diagnoses and 267 females/45 826 diagnoses for all disorders). Age represents a potential confounder. Age (mean and standard deviation) was reported in addition to ORs for each group overall and within age stratification (19–49 years and 50+ years). Additional comorbidities (e.g., obesity and diabetes) while potentially influential, were not considered.
In addition, the duration between the first date of diagnosis for mental disorders associated with neoplasms and cancers was calculated. The total frequency of specific diagnoses arising for each diagnosis within the duration interval before and after the pivot diagnosis (any mental disorder or neoplasm) was also calculated for comparison. The proportions of the diagnostic frequencies for each specific diagnosis were calculated for comparison.
Statistical differences were estimated based on comparison of the 95% confidence intervals (CI). Non-overlapping 95% CIs with z set to 1.96 indicated a statistical difference (P < 0.05).
There are multiple ways to count different groups for the purpose of meaningful comparison: (a) number of unique individuals within defined groups or ICD classes, or individual diagnoses; (b) cumulative frequency of diagnoses for individuals within defined groups or ICD classes, or individual diagnoses; and (c) the temporal order of counts and frequencies comparing counts within defined groups or ICD classes, or individual diagnoses within diagnostic groups of main ICD diagnostic categories (n = 1036). In this analysis, there were two temporal groupings for each gender based on two pivot classes of ICD diagnoses (neoplasm and mental disorder), wherein counts of unique individuals, frequencies and average durations arising before and after each pivot diagnosis (before and after any neoplasm; before and after any mental disorder) were calculated overall for age and gender, and for each independent diagnosis by age and gender temporal grouping. Further, the base rate of mental disorder in the present sample was 54% over 16 years.Reference Cawthorpe and Davidson31
After a general description of the sample and the overall relationships within and between groups, we examined the specific neoplasms arising before or after any mental disorder, and the specific mental disorders arising before or after any neoplasm. Specific examples are detailed (e.g., specific diagnoses within the pivot diagnosis account for the observed significance). The data were analysed using Stata 14. Each table result was verified using two independent algorithms: ‘contract’ v. ‘unique’ to verify counts of unique individuals within groupings, and ‘collapse’ for calculating cumulative frequency and duration within diagnoses by temporal grouping.
Results
Table 1 provides the unique individual counts and the associated cumulative frequencies of diagnoses within each condition. Note the increased counts under the condition of mental disorder before cancer for both genders. The proportion of those with any mental disorder before cancer was higher for males and females in both age groups. For example, for 58% of males and 63% of females aged 19–49 years, any mental disorder preceded any cancer. For 69% of males and 66% of females over 50 years of age, any mental disorder preceded any cancer.
Table 1 Gender and age for unique individuals over the age of 18 years
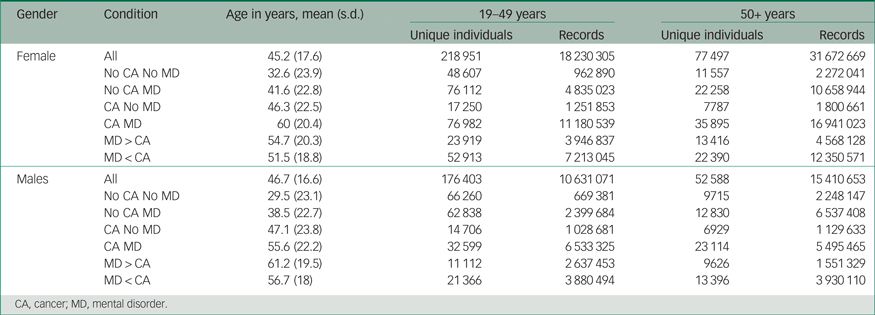
CA, cancer; MD, mental disorder.
Table 2 shows the overall ORs for each category by gender for the two age strata. Males and females were more likely to have both mental disorder and cancer compared to either one or neither, and for both genders, it was more likely that any neoplasm would follow any mental disorder. This was the case for each age category. When stratified by age, the ORs for the 19–49-year-old group are consistent within the age strata and slightly greater than those for the group over 50 years of age, even though the proportions of those with both mental disorder and neoplasm are greater in the over 50 years of age strata for both genders (Table 1).
Table 2 Odds ratios by condition by gender by age strata
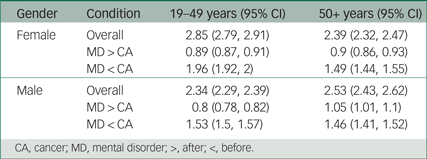
CA, cancer; MD, mental disorder; >, after; <, before.
Table 3 provides a comparison of average age, cumulative diagnostic frequency, average duration, and frequency proportions by pivot diagnosis by independent diagnoses. Examples of serious diagnoses for each pivot category for males and females include schizophrenic disorders (295), affective psychoses (296) and other nonorganic psychoses (298), representing ICD diagnoses that arise with considerable frequency – potentially reflecting intensity – within these patients. Note that this table represents the summed counts or frequency of diagnoses within each ICD main diagnosis under the condition of arising before and after each ICD pivot. ICD disorder 306 (psychological distress) was the only disorder arising significantly after neoplasm in both females and males. Only in males was there one non-significant disorder, ICD disorder 313 (emotional distress). Table 3 identifies the specific neoplasm and mental disorders underpinning the overall ORs shown in Table 2.
Table 3 Comparison of ICD diagnoses (frequency and average duration) by group arising before and after pivot diagnoses

As shown in Table 3, for females, when any cancer was the pivot diagnosis, the average age was 51 years, the average time cancer arose before the specified mental disorders was 2191 days, and the average time after the specified mental disorders was 1378 days. For females, when any mental disorder was the pivot diagnosis, the average age was 59 years, the average time cancer arose before the specified mental disorders was 2311 days, and the average time after the specified mental disorders was 885 days. The specific mental disorder in females with the shortest duration (1423 days) before cancer was psycho-physiological gastro-intestinal disorder (306), and that with the longest duration (2894 days) was intellectual disability not otherwise specified (319). The specific cancer in females with the shortest duration (302 days) arising after any mental disorder was malignant neoplasm – pancreas (157), and that with the longest duration (1549 days) arising after any mental disorder was malignant neoplasm – major salivary (142).
As shown in Table 3, for males, when any cancer was the pivot diagnosis, the average age was 50 years, the average time cancer arose before the specified mental disorders was 2045 days, and the average time after the specified mental disorders was 1420 days. For males, when any mental disorder was the pivot diagnosis, the average age was 61 years, the average time cancer arose before the specified mental disorders was 2114 days, and the average time after the specified mental disorders was 868 days. The specific mental disorder in males with the shortest duration before cancer was senile/presenile psychosis (290), and that with the longest duration was other mental retardation (318). The specific cancer in males with the shortest duration arising after any mental disorder was malignant neoplasm of lip, oral cavity, and pharynx (140), and that with the longest duration arising after any mental disorder was haemangioma/lymphangioma (228).
Table 3 also shows a wide range of frequencies or counts of each diagnosis before or after the pivot diagnosis, as well as the frequency proportions of the total for each specific diagnosis that arose before and after each pivot diagnosis.
Summary of results
The results shown in the tables illustrate in detail the overall and temporal relationships between ICD mental disorders and cancers as categories of diagnosis. Table 3 provides more depth of temporal information about the specific temporal relationships of the ICD diagnoses associated with each pivot diagnosis (any cancer or mental disorder), where both mental disorder and cancer arose in the same person at different times. Mental disorders preceded cancers, independent of age, for both genders. Table 3 provides a basis for examining the relative intensity and the average durations before and after each pivot diagnosis to provide an index of sequence or order.
Discussion
Our findings support the study of comorbid disorders, advancing the field by comprehensively examining the relationship between neoplasms, including cancer, and mental disorder, information that until now was not available. The results represent an important advance detailing the 16-year relationship between all cancers and all mental disorders in a large population, supporting future laboratory research on plausible biological mechanisms potentially linking a variety of disorders – for example, the effect of antipsychotics on cadherin-mediated cell–cell adhesion.Reference Batlle and Wilkinson38
The results of the present study are most comparable to those of a Swedish national study of mental disorder and cancer.Reference Lu, Andersson and Fall30, Reference Zhu, Fang, Sjolander, Fall, Adami and Valdimarsdottir39 Yet, the results of the present study are materially different. The Swedish study examined the relationship between cancer and mental disorder, noting an increase in relative risk from 2 years before the onset of cancer and finding that cancer predisposed people to psychological distress and mental disorder from 2 years before to after the onset of cancer for the study duration.Reference Lu, Andersson and Fall30 Our results are the opposite of the Swedish study results, showing that, if random, cancer and mental disorders had equal odds of arising either before or after one another, according to the null hypothesis. We observed a strong relationship between mental disorder and cancer, with mental disorder preceding cancer. The differences in findings may be due to the 16-year time-based approach, or the different analytical methods employed, or both.
The strength of the temporal relationship between mental disorder and cancer illustrated in this paper is similar to that shown by another study based on this data sample. In that study, the temporal relationship between mental disorder and ulcerative colitisReference Cawthorpe and Davidson31 pointed to a potential mechanism for the onset of anxiety and depression preceding the onset of ulcerative colitis. This effect might be related to one or more of the principal medications used to treat these disorders, such as selective serotonin reuptake inhibitors. For example, there was no relationship with thought disorders or psychosis, for which a different class of medications is used in treatment. Psychotropic drugs probably do not only modulate cell signalling in the central nervous system, but also affect communication across all cellular systems, such as immune system regulation.Reference Trakhtenberg and Goldberg40
Age represented a potential confounder of the results; hence, age was stratified into two groups, with the finding that the overall and temporal relationships of cancer and mental disorder were independent of age. This strengthens the idea that there may be a mechanism operating separately from the process of simple functional decline.Reference Kenis, Decoster and Bastin41–Reference Lee46
In summary, this paper provides a description of the overall relationship between cancer and mental disorder in a large North American population. More directly related to clinical utility are descriptions of the frequencies and the average times in days with which specific diagnoses arise either following or in advance of cancer and mental disorder. For example, frequencies of specific cancers or mental disorders arising before or after the pivot class may represent an index of that specific disorder's intensity in patients, and may indicate the need for further screening or investigation.
Limitations, conclusion and next steps
We recognise that only cancer and mental disorder were considered in this paper. The relationships within and between the remaining classes of ICD disorders are also important and warrant more detailed study. Although the present work remains illuminating, and we contend that it is congruent with developments in laboratory and biological research, a complete list of disorders leading to and from a given pivot diagnosis is a worthy pursuit. Notwithstanding the complexity of conducting a metabolomics study at a population level, the main limitation of this contribution is the need to await the development of the algorithms required to develop the temporal roadmaps within and between the thousands of diagnoses underpinning the 18 main ICD classes of disorder. In such algorithms, the native patterns of the relationships arising in sequence among related disorders within patients would be preserved. Comparison of the average durations by specific diagnosis only provides a crude index of sequence; however, these calculations are independent for each specific diagnosis arising either before or after the pivot class diagnosis.
Another main limitation is that the present paper cannot resolve a clear understanding of the mechanism underpinning the temporal relationship between mental disorder and cancer. The results mainly introduce the additional confounder of psychiatric treatment. The relationship between brain function, the effects of antipsychotics and immune function is appealing in its simplicity in terms of basic research models testable in laboratory settings. Regardless, a great deal of work remains to design and implement studies that might resolve mechanistic issues such as treatment with antipsychotics and common constitutional and epigenetic vulnerability, as well as the influence of environmental and sociocultural factors.






eLetters
No eLetters have been published for this article.