INTRODUCTION
Hand, foot, and mouth disease (HFMD) is a worldwide childhood infectious disease mainly caused by coxsackie virusA16 (CV-A16) and enterovirus 71 (EV71) [Reference Gopalkrishna1, Reference Xing2]. The main clinical symptoms of HFMD include mouth ulcers, fever, and vesicles on the hands, feet, and mouth [Reference Jiang3]. In most cases, the disease is mild and self-limiting. But some patients may develop serious cardiopulmonary and neurological complications, and such cases are mainly caused by EV71 [Reference Ooi4].
In recent decades, Asia-Pacific countries have experienced an increasing trend of HFMD outbreaks [Reference Ang5–Reference Ni8]. In particular, the epidemic situation of HFMD in China is quite serious. In 2014, for example, over 2·7 million HFMD cases were reported to the China Center for Disease Control and Prevention, including 501 fatal cases. The public health threat caused by HFMD pushes the need to identify environmental predictors, which have significant impacts on HFMD.
It is well accepted that meteorological factors play important roles in the transmission of many communicable diseases [Reference Huang9–Reference Xu13]. Epidemiologists have conducted a large amount of researchers to quantify the associations between meteorological variables and HFMD. Meteorological factors such as mean temperature and relative humidity are the most commonly reported environmental agents. However, to date, the effect of temperature variation on childhood HFMD has received limited attention. The temperature variation within one calendar day, i.e., diurnal temperature range (DTR), is an important indicator for climate change. Previous studies have reported that DTR is associated with many infectious diseases (e.g. diarrhoea, pneumonia and respiratory syncytial virus) [Reference Onozuka14–Reference Xu, Hu and Tong16]. But few studies have examined the impact of DTR on children's health, and little is known about the relationship between DTR and childhood HFMD. To the best of our knowledge, there are only three published papers focusing on the relationship between DTR and HFMD [Reference Xu15, Reference Hii, Rocklöv and Ng17, Reference Ma18]. But these findings are inconsistent, and the nature and extent of the relationship remain highly controversial. In addition, no studies have assessed the age-, gender- and pathogen-specific effects of DTR on childhood HFMD. The current study was designed to help address this research gap.
This study aimed to quantify the lag association between DTR and HFMD. Specifically, a multilevel distributed lag non-linear model (DLNM) was adopted to explore the temporal lagged relationship between daily HFMD cases and daily DTR using the data in 18 cities in Sichuan Province, China. Using the more sophisticated model, a better understanding might be achieved for the relationship between DTR and HFMD.
MATERIALS AND METHODS
Study area
Sichuan Province is located in Southwest China between longitude 98·31E to 107·99E and latitude 26·40N to 33·68N. It has a population of approximately 80 million people and encompasses 485 000 km2. There are 18 prefectural-level cities in Sichuan Province.
Data sources
In China, a web-based infectious disease monitor information system (the China Information System for Disease Control and Prevention, CISDCP) has been established in 2004. HFMD was classified as a class “C” notifiable disease by the Ministry of Health of China. Therefore, all HFMD cases should be reported to CISDCP within 24 h after diagnosis using a unified format [19]. The diagnosis of HFMD was made according to the clinical criteria issued by the Ministry of Health of China [19]. The city-specific daily data of reported HFMD cases in 18 prefectural-level cities in Sichuan Province from 2011 to 2015 were obtained from CISDCP. As 99% of cases occurred among children under the age of 15 years according to the preliminary analysis, we focused on the HFMD cases aged 0–14 years in this study.
Daily meteorological data, including maximum temperature, mean temperature, minimum temperature, relative humidity and rainfall of 18 cities in Sichuan Province were obtained from China Meteorological Data Sharing Service System. DTR was calculated as daily maximum temperature minus daily minimum temperature. The city-specific socio-economic data from 2011 to 2015 were collected from the China city statistical year book.
Distributed lag non-linear models
DLNM represent a flexible modelling framework that can flexibly describe a non-linear exposure–response relationship and delayed effects in time series data [Reference Gasparrini, Armstrong and Kenward20]. In this study, a Poisson generalized linear regression combined with DLNM was applied to explore the effect of DTR on HFMD incidence. The model was specified as:
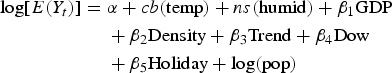 $$\eqalign{ \log [E(Y_t )] = \; &\alpha + cb({\rm temp}) + ns({\rm humid}) + \beta _1 {\rm GDP} \cr &+ \beta _2 {\rm Density} + \beta _3 {\rm Trend} + \beta _4 {\rm Dow} \cr &+ \beta _5 {\rm Holiday} + \log ({\rm pop})}$$
$$\eqalign{ \log [E(Y_t )] = \; &\alpha + cb({\rm temp}) + ns({\rm humid}) + \beta _1 {\rm GDP} \cr &+ \beta _2 {\rm Density} + \beta _3 {\rm Trend} + \beta _4 {\rm Dow} \cr &+ \beta _5 {\rm Holiday} + \log ({\rm pop})}$$
where Y t is the reported daily HFMD case counts on day t; α is the intercept; Pop is the population. cb(temp) indicates the ‘cross-basis’ function, which is obtained by DLNM to model non-linear and distributed lag effects of temperature. ns means a smooth function based on the natural cubic spline. A natural cubic spline DLNM was used to model the nonlinear association between DTR and HFMD. Akaike Information Criterion (AIC) was adopted to choose the degrees of freedom (df) for DTR and lag [Reference Gasparrini, Armstrong and Kenward20]. The final composition of the function was a natural cubic spline of DTR with three df and a natural cubic spline with three df for lag days. Based on previous studies [Reference Zhu12, Reference Xu13], a maximum lag of 14 days was used to explore the potential lag associations. Three df was used to smooth humidity (humid) and rainfall (rain) [Reference Zhu12, Reference Xu13]. GDP (GDP per person) and Density (population density) are socio-economic variables. Trend is a variable of the year and calendar month used to control for seasonality and long-term trend. Dow is a day of the week on day t. Holiday is a binary variable which is ‘1’ if day t was a public holiday. β 1, β 2, β 3, β 4 and β 5 are the regression coefficients. Rainfall was insignificantly associated with HFMD, so the variable was removed from the final model.
The 18 cities were divided into two groups: group B (Panzhihua city) and group A (the other 17 cities), since small differences in weather conditions (Table 1) were detected within cities except for Panzhihua city. For group A, stratified analyses were performed by gender group (male and female), age group (0–5 and 6–14 years) and pathogen group (EV71 and CV-A16). For group B, stratified analyses were performed by gender group and age group. We did not perform pathogen-specific analysis for group B because there were only 521 laboratory-confirmed cases in Panzhihua city (206 cases were associated with EV71 and 155 cases were associated with CV-A16).
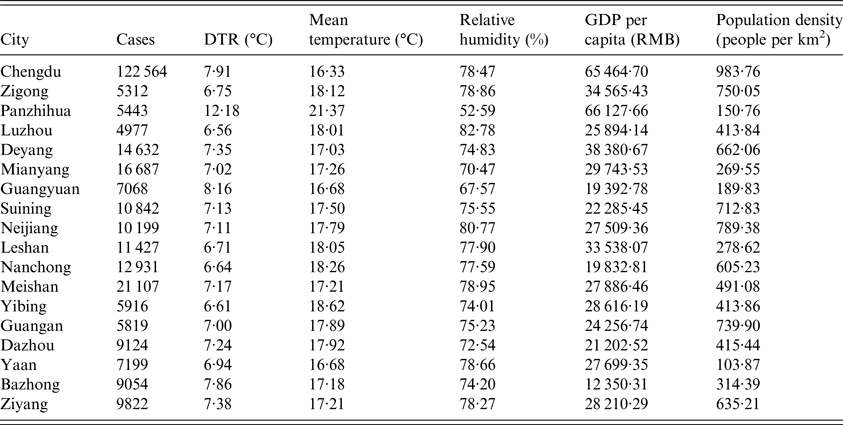
Table 1. Characteristics of the 18 cities in Sichuan Province
Sensitive analyses were performed to test the robustness of our results: varying the df (4–7) for meteorological variables. The ‘dlnm’ and ‘lme4’ packages in the R software (version 3·3·3) were used to create the multilevel DLNM model.
RESULTS
A total of 290 123 HFMD cases aged 0–14 years were reported in the 18 cities in Sichuan Province during 1 January 2011 and 31 December 2015, of which 19 632 (6·77%) were laboratory confirmed. On average, there were 8·83 cases of daily HFMD. Children aged 0–5 years were the majority of the victims, which accounted for 97·25% (282 149 cases) of all reported cases over the study period. Of 290 123 HFMD cases, 174 026 were males and 116 097 were females, with a male-to-female sex ratio 1·50. Among the laboratory confirmed cases, 6166 (31·41%) were associated with EV71 and 4639 (23·63%) were associated with CV-A16. Table 1 presented the descriptive analysis for the 18 cities.
Results of group A
The three-dimensional plot demonstrated the relationship between daily DTR and HFMD cases along 14 lag days (Fig. 1). Overall, the estimated effects of DTR on HFMD incidence were non-linear, with higher relative risk (RR) at large DTR.

Fig. 1. Three-dimensional plot of the relationship between DTR and HFMD over 14 lag days (group A).
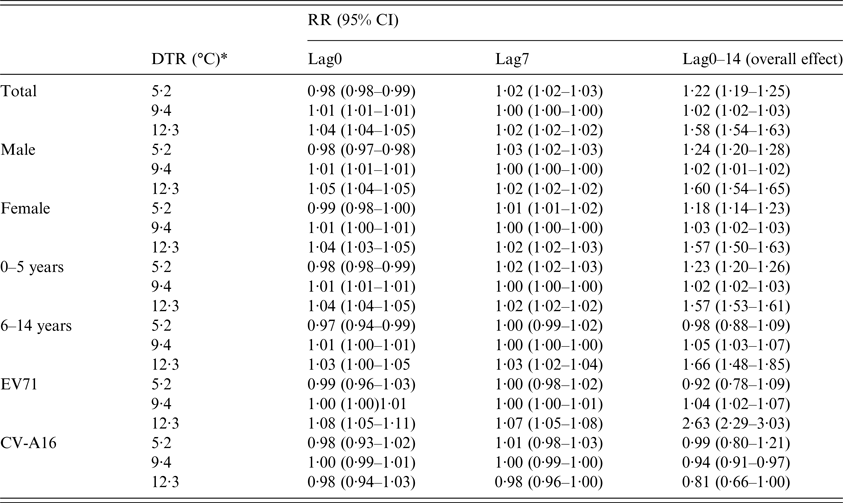
Figure 2 showed the overall effect of temperature, summing up the contributions for the 14 days of lag. It suggested that DTR was significantly associated with childhood HFMD. The RR increased with the increment of DTR and it reached the small peak at 5·2 °C and then the curve became relatively flat, again the RR increased rapidly when DTR was above 8·7 °C.
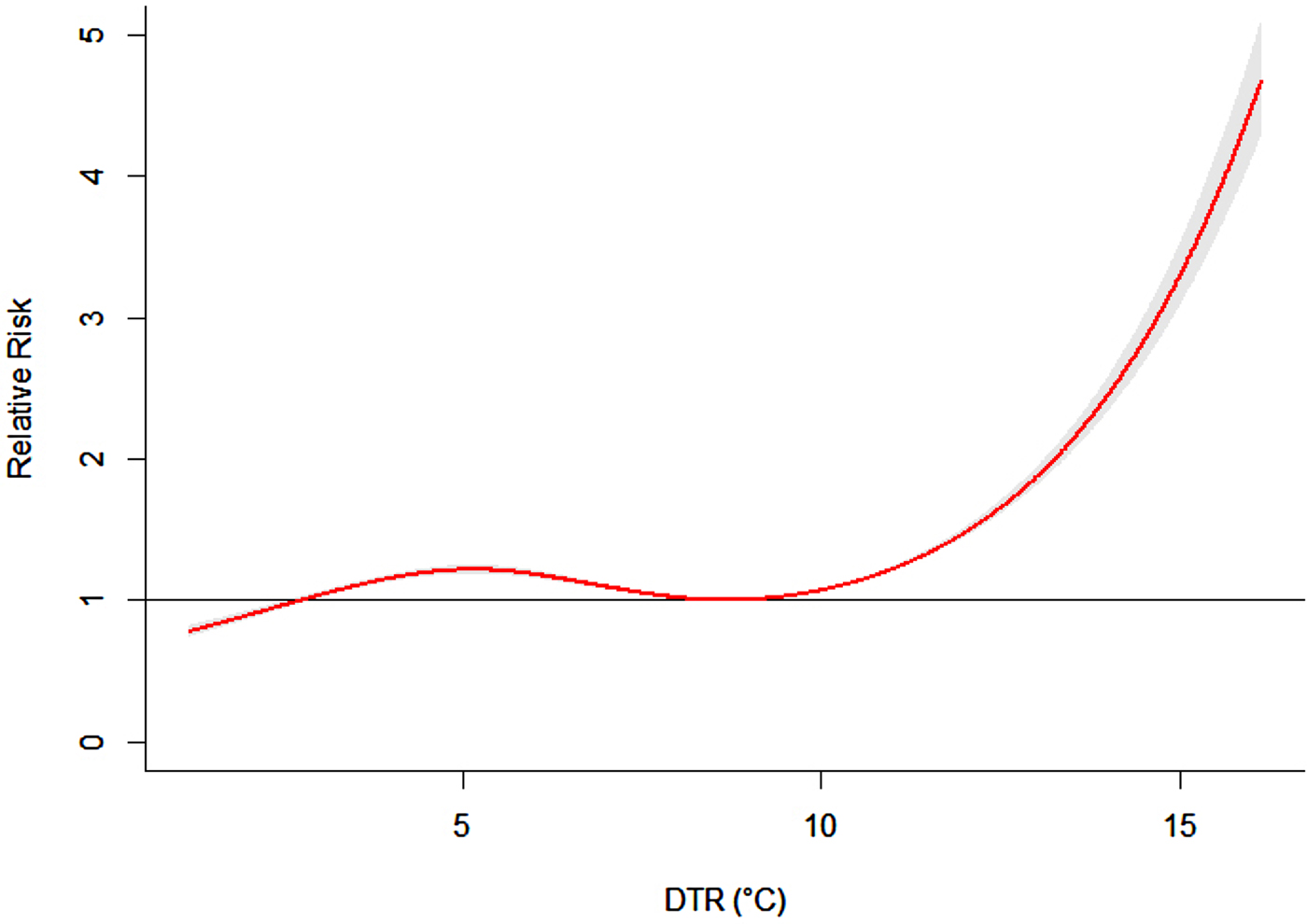
Fig. 2. The overall relative risks of DTR for total HFMD cases over 14 days (group A).
Figure 3 presented the RR of cumulative exposure to DTR over 14 days for age-, gender- and pathogen-specific HFMD cases. For males and females, the RR followed the similar trends as the total RR, with the first small peak at 5·2 °C for males and 5·0 °C for females. For these two groups, the RR began to increase rapidly when DTR was above 8·7 °C. But For males, the RR increased more rapidly than that of females.
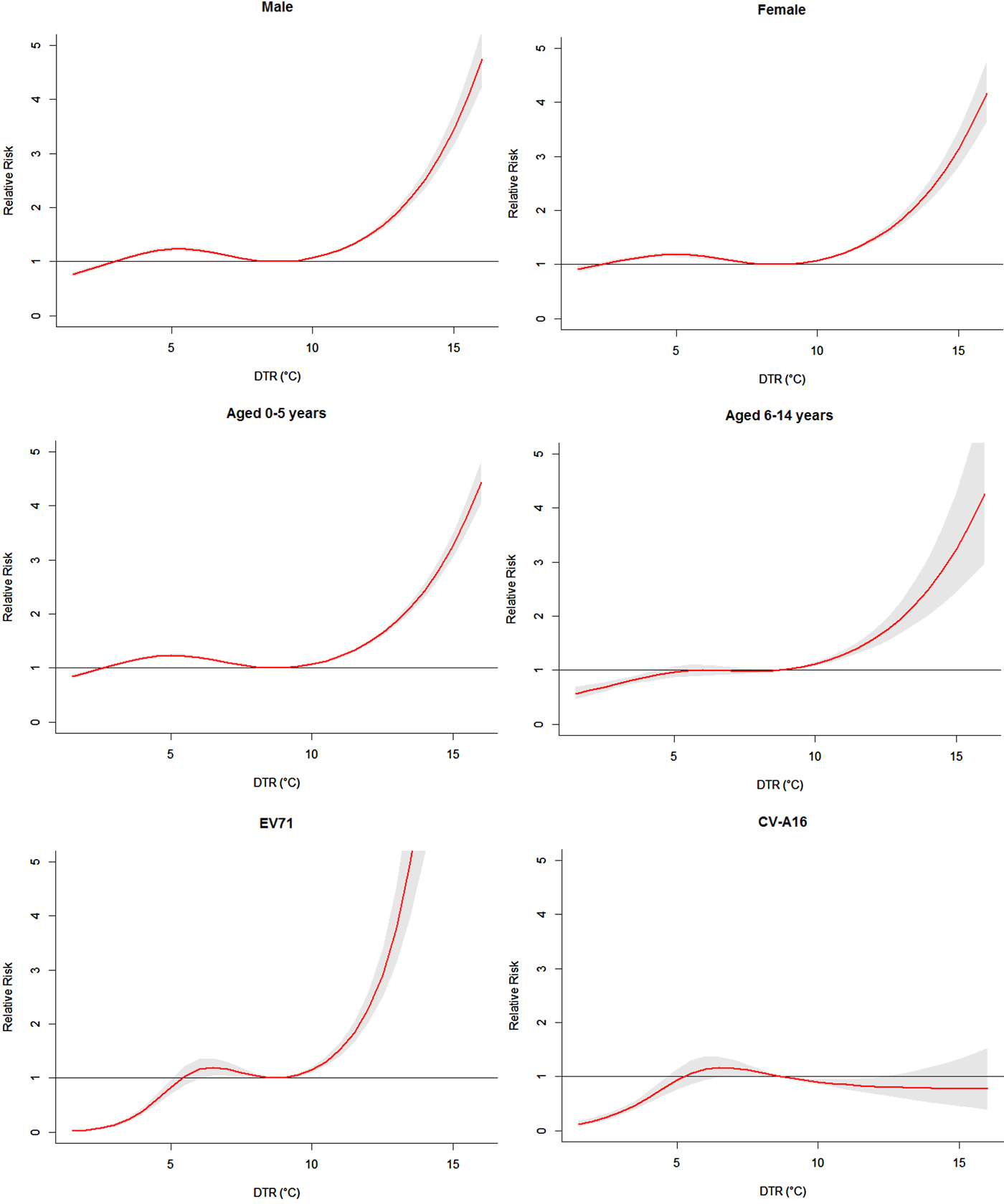
Fig. 3. The overall relative risks of DTR for age-, gender- and pathogen-specific HFMD cases over 14 days (group A).
For children aged 0–5 years and 6–14 years, the RR also followed the similar trends as the total RR. For children aged 0–5 years, the RR reached the small peak at 5·1 °C and began to increase rapidly when DTR was above 8·7 °C. For children aged 6–14 years, the curve became relatively flat when DTR was above 5·5 °C. Again the RR increased rapidly when DTR was above 8·7 °C.
Figure 3 also illustrated the cumulative effects of DTR on pathogen-specific HFMD cases. The exposure–response curve of DTR on EV71 was quite different from that of CV-A16. For cases of EV71 infection, the RR reached the small peak at 6·5 °C and then the curve became relatively flat. Again the RR increased very rapidly when DTR was above 8·7 °C. For cases of CV-A16 infection, the RR reached the peak at 6·6 °C and then the curve began to decrease.
The risks of different DTRs for total, gender- , age- and pathogen-specific HFMD cases along the lags were summarized in Table 2. The effects of DTR on childhood HFMD differed between males and females. We found that DTR had the higher risk estimates of HFMD incidence in males than in females. For females, the RR value was 1·57 (95% CI 1·50–1·63) at lag0–14 when DTR was 12·3 °C. While for males, the RR value was 1·60 (95% CI 1·54–1·65) at lag0–14. For age-specific RRs, DTR had the higher risk estimates of HFMD incidence in children aged 6–14 years. For children aged 0–5 years, the RR value was 1·57 (95% CI 1·53–1·61) at lag0–14 when DTR was 12·3 °C. For children aged 6–14 years, the RR value was 1·66 (95% CI 1·48–1·85) at lag0–14.
Table 2. The relative risks of different diurnal temperature ranges for age-, gender- and pathogen-specific HFMD cases (group A)
* 9·4 °C and 12·3 °C represented the 75th percentile and 95th percentile of DTR, respectively; 5·2 °C represented the first small peak value of DTR for total children.
For pathogen-specific RRs, large DTR had the higher risk estimates of HFMD incidence in cases of EV71 infection, while small DTR had the higher risk estimates of HFMD incidence in cases of CV-A16 infection. For cases of EV71 infection, the RR value was 2·63 (95% CI 2·29–3·03) at lag0–14 when DTR was 12·3 °C. For cases of CV-A16 infection, the RR value was 0·81 (95% CI 0·66–1·00) at lag0–14.
In the sensitivity analyses, when the df for climate variables were varied between 4 and 7, similar results were obtained.
Results of group B
The three-dimensional graph presented the relationship between daily DTR and HFMD cases along 14 lag days (Fig. 4). Overall, the estimated effects of DTR on HFMD incidence were non-linear, with higher RR at large DTR.

Fig. 4. Three-dimensional plot of the relationship between DTR and HFMD over 14 lag days (group B).
Figure 5 showed the overall effect of temperature. It suggested that DTR was significantly associated with childhood HFMD. The exposure–response curve was an approximately inverted V-shape. The RR increased with the increment of DTR and it reached the peak at 13·7 °C and then began to decrease.
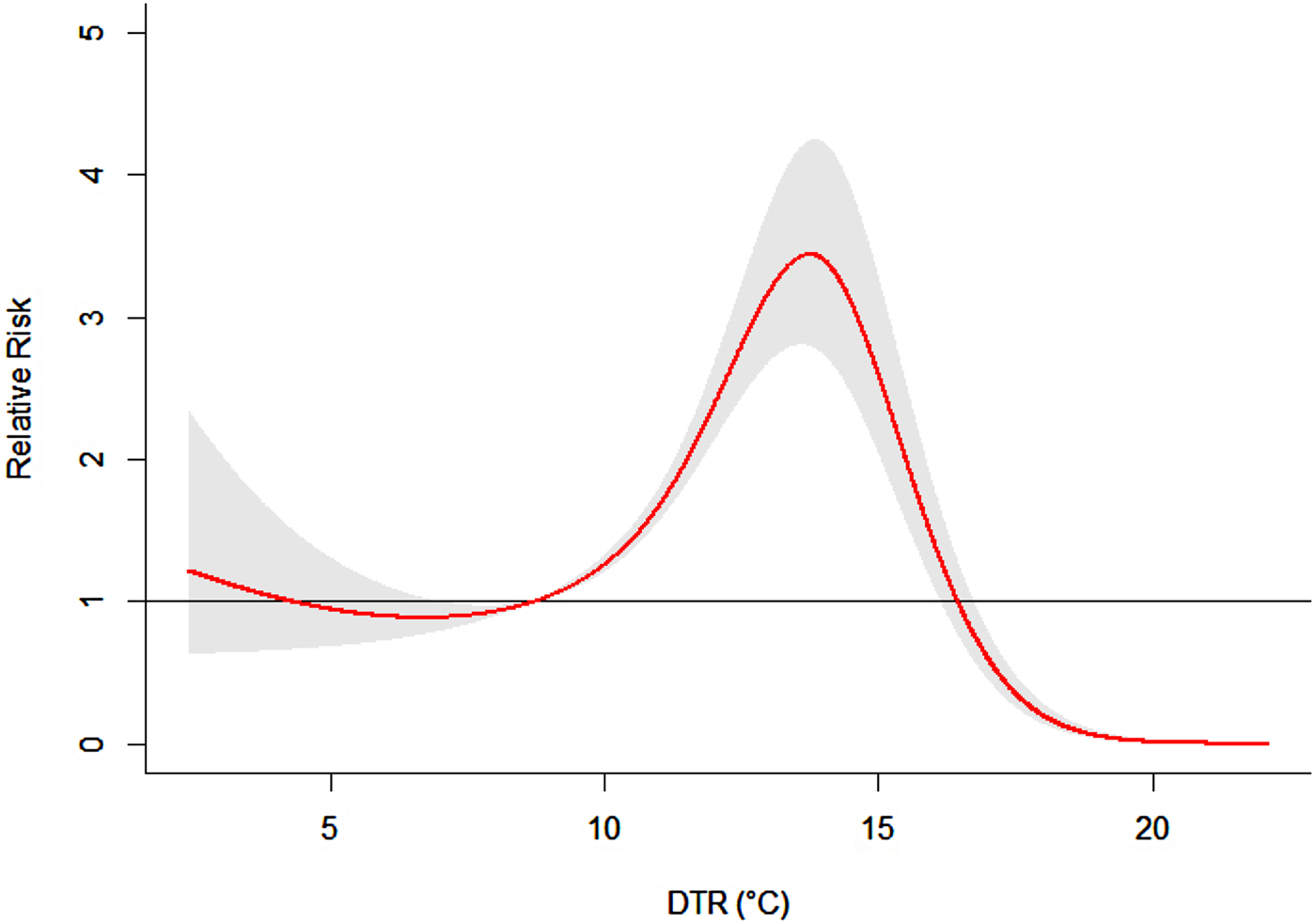
Fig. 5. The overall relative risks of DTR for total HFMD cases over 14 days (group B).
Figure 6 demonstrated the RR of cumulative exposure to humidex over 14 days for age- and gender-specific HFMD cases. For males and females, the RRs followed the similar trends as the total RR, with the peak at 13·6 °C for males and 13·8 °C for females. For children aged 0–5 years and 6–14 years, the exposure–response curves also followed the similar trends as the total RR, with the peak at 13·7 °C for children aged 0–5 years and 14·1 °C for children aged 6–14 years.
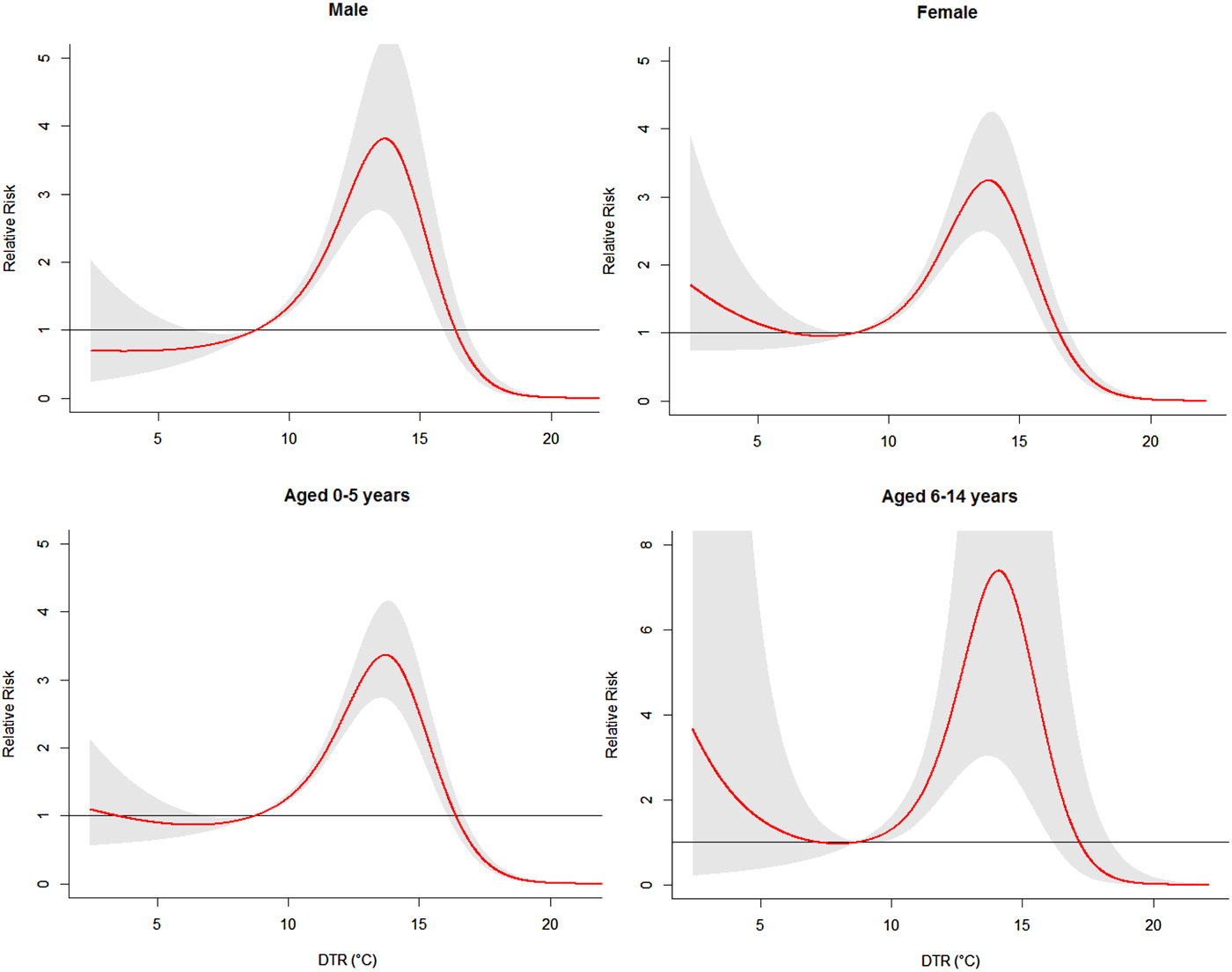
Fig. 6. The overall relative risks of DTR for age- and gender-specific HFMD cases over 14 days (group B).
The risks of different DTR for total, gender- and age-specific HFMD cases along the lags were summarized in Table 3. The effects of DTR on childhood HFMD differed between males and females. We found that DTR had the higher risk estimates of HFMD incidence in males than in females. For males, the highest RR value was 3·80 (95% CI 2·70–5·34) at lag0–14. While for females, the highest RR value was 3·24 (95% CI 2·48–4·24) at lag0–14. For age-specific RRs, DTR had the higher risk estimates of HFMD incidence in children aged 6–14 years. For children aged 0–5 years, the highest RR value was 3·37 (95% CI 2·73–4·15) at lag0–14. While for children aged 6–14 years, the highest RR value was 7·24 (95% CI 3·02–17·35) at lag0–14.
Table 3. The relative risks of different diurnal temperature ranges for age- and gender-specific HFMD cases (group B)

* 14·8 °C and 17·2 °C represented the 75th percentile and 95th percentile of DTR, respectively.
† The peak values of humidex for total children, gender-specific and age-specific BD cases, respectively.
In the sensitivity analyses, when the df for climate variables were varied between 4 and 7, similar results were obtained.
DISCUSSION
Our study investigated the association of DTR with age-, gender- and pathogen-specific HFMD among children. A DLNM was adopted to explore the temporal lagged association of daily DTR with HFMD. This is, to our knowledge, the first study to assess the pathogen-specific effects of DTR on HFMD.
In this study, DLNM was adopted to explore the non-linear and lagged relationship between DTR and HFMD. The results of this study indicated that there was a significant association between DTR and childhood HFMD. Although the exact mechanism remains largely unknown, it is possible that DTR can affect the survival and transmission of the HFMD virus in the environment, as well as children's behavior, thereby influencing the transmission dynamics of HFMD.
The two groups in this study demonstrated different DTR impact patterns. The effect of DTR on HFMD incidence in group A generally showed a positive relationship. While the exposure–response curve in group B shown as an approximately inverted V-shape. The mean DTR in group A was 7·09 (ranged from 1 to 16), while the mean DTR in group B was 12·18 (ranged from 2 to 22). However, in this study, there were only 5443 cases in group B. More studies are needed in large DTR regions.
EV71 and CV-A16 are the predominant pathogens responsible for HFMD outbreaks worldwide [Reference Mao21, Reference Yip22]. But few studies have examined pathogen-specific effects of meteorological factors on HFMD. Dong et al. provided a clue that mean temperature had a stronger relationship to EV71 than CV-A16 [Reference Dong23]. But there was no convincing evidence to conclude that significant differences exist between the influences of the mean temperature on EV71 and on CV-A16 because of the small sample size of their study. In this study, we explored the potential difference of the influence of DTR on EV71 and CV-A16. The pathogen-specific results of this study suggested that the exposure–response curve of DTR on EV71 was quite different from that of CV-A16. Large DTR had the higher risk estimates of HFMD incidence in cases of EV71 infection, while small DTR had the higher risk estimates of HFMD incidence in cases of CV-A16 infection.
The age-specific results suggested that children aged 6–14 years appeared to be more vulnerable to DTR effects. This might be partly because children aged 6–14 years play outdoors more often than younger children, which makes them have more chances of being exposed to the virus and are more easily infected by HFMD. The gender-specific results showed a greater association of DTR with HFMD for males than for females. These results may suggest gender differences in susceptibility at the host genetic level [Reference Chen24].
This study examined the effect of DTR on HFMD at a daily scale since the current study focused on the short-term effect of DTR on HFMD. The study based on daily data may be more suitable for the prevention and control of HFMD since it could provide more timely information and the local health department could have enough time to prepare for the potential epidemic. However, the relationship between DTR and HFMD may vary over different time scales. Further studies are needed to evaluate the effect of DTR on HFMD at different time scales such as a monthly and yearly scale.
A few limitations of this study should be acknowledged: (1) This study was ecological in nature, which did not allow us to explore individual-level association and limited our capacity for casual inference. (2) This study was based on surveillance data. Surveillance data for HFMD did not capture all cases. Therefore, the potential bias caused by under-reporting may exist.
In conclusion, our study provided a comprehensive picture of the non-linear relationship between daily DTR and HFMD in a large province of Southwest China. Children aged 6–14 years and male children were more vulnerable to the temperature changes. Large DTR had the higher risk estimates in cases of EV71 infection, while small DTR had the higher risk estimates in cases of CV-A16 infection. Our study provides information to better understand the impact of DTR on HFMD and might have potential importance for disease prevention and control. It is important for both health practitioners and parents to be aware of the effect of DTR on childhood HFMD, and take protective strategies to reduce the associated risks. In addition, different DTR–HFMD associations among different sub-groups could be considered to optimize public health prevention strategies.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (grant no. 81402766) (FY) and the National Natural Science Foundation of China (grant no. 81602935) (TZ).
AUTHOR CONTRIBUTIONS
FY and YM conceived of the project concept; QL and YL cleared the data. FY, XZ, YM and TZ performed the data analysis, model development and interpretation. FY and XL drafted the manuscript. All of the authors have read and approved the final manuscript.
DECLARATION OF INTERESTS
None.












