Excess energy intake is the primary dietary cause of obesity, although other dietary factors need to be taken into account. Nutritional guidelines recommend dietary fibre for health promotion and disease prevention. However, the beneficial effects of dietary fibre in the prevention, or treatment, of obesity and associated metabolic disturbances are not consistent.
Obesity and diabetes are less prevalent in populations consuming large amounts of dietary fibre. Further, excess weight and obesity were substantially lower in vegetarian populations(Reference Newby, Tucker and Wolk1), suggesting that fibre intake could play an important role in the prevention and progression of these metabolic conditions. Cross-sectional studies have shown that, following adjustment for confounding variables, the intakes of total fibre and soluble fibre are inversely related to total fat intake, body-fat content and BMI. Obese subjects typically consume less fibre than normal-weight individuals(Reference Alfieri, Pomerleau, Grace and Anderson2). In a 10-year prospective study in young adults, fibre intake was associated with lower weight increases and a lower risk of developing obesity, after adjustment for fat intake(Reference Ludwig, Pereira, Kroenke, Hilner, Van Horn, Slattery and Jacobs3).
Several mechanisms have been proposed to explain the role of fibre intake on body-weight regulation(Reference Slavin4), apart from displacing energy and nutrients from the diet. Fibre increases the volume of gastric contents, reduces gastric emptying and promotes satiation(Reference Jenkins, Kendall, Axelsen, Augustin and Vuksan5, Reference Schneeman6) while decreasing the absorption of macronutrients(Reference Baer, Rumpler, Miles and Fahey7) and influencing the secretion of gut hormones controlling fat oxidation and storage(Reference Salas-Salvadó, Bulló, Pérez-Heras and Ros8).
Epidemiological data and mechanistic studies support the beneficial effects of fibre in body-weight regulation, but data from randomised controlled clinical trials evaluating the effect of fibre supplementation of the diet on body weight have not been consistent(Reference Pittler and Ernst9–Reference Rossner, Andersson and Ryttig12). In a systematic review, Howarth et al. analysed several clinical trials conducted in small and heterogeneous population samples over relatively short periods of time(Reference Howarth, Saltzman and Roberts13). The findings were that the intake of 12 g fibre/d resulted in a decrease of 10 % in energy intake and a body-weight loss of 1·9 kg over 3·8 months. These authors indicated that this effect on body-weight loss was greater in obese subjects.
However, long-term compliance to fibre supplementation of the diet is poor in adults(Reference Jacobs, Giuliano, Roe, Guillen-Rodriguez, Hess, Alberts and Martinez14). If fibre is to be considered a long-term therapeutic option, an effective and well-tolerated source needs to be found.
The aim of our study was to evaluate the effect of a 16-week consumption of a new fibre mixture on weight loss, tolerance, satiety, the lipid profile, glucose metabolism and high-sensitivity C-reactive protein in overweight (or obese) patients attending primary care centres.
Material and methods
Subjects
Male and female patients between the ages of 18 and 70 years who were overweight or obese (27 < BMI < 35 kg/m2) with a high degree of motivation to achieve weight loss were invited to participate in a parallel, double-blind, randomised placebo-controlled, multi-centred clinical trial using two dose levels of a mixture of fibre. Patients were recruited from eleven primary care centres in Spain. Exclusion criteria for the study were: (1) diabetes mellitus requiring treatment with anti-diabetic drugs or insulin, or other known endocrine disorders; (2) dyslipidaemia requiring pharmacological treatment according to the National Cholesterol Education Program Adult Treatment Panel III (ATP III) guidelines; (3) intake of anti-obesity medication and/or following a low-energy diet over the 8 weeks prior to entry into the present study; (4) previous bariatric surgery; (5) history of eating disorders; (6) intake of dietary fibre supplements in the month prior to inclusion in the present study; (7) acute or chronic infection, inflammatory disease or cancer. The Institutional Review Board of the eleven participating primary care centres approved the study protocol. Written informed consent was obtained from all patients participating in the study.
Study design and intervention
At the initial visit, a complete medical history and physical examination of the patient was taken. Before randomisation the subjects were stratified by gender and BMI (BMI ≤ 30 kg/m2 or BMI>30 kg/m2). Eligible subjects were assigned to receive fibre (at two fixed doses) or placebo in a 2:1 ratio for each dose level for a 16-week period. A randomisation list was computer generated in a fixed block size of six patients. The details of the randomisation codes were kept in sealed envelopes away from the investigator. Only in cases of the utmost necessity (e.g. serious adverse events) was the assignment of the patient made known to the investigator by the study co-ordinator. Patients, investigators, monitors and statisticians were blinded with respect to the treatment assignment.
All enrolled patients were individually advised to follow a diet that was reduced by 2·5 MJ/d compared to their current diet, as recommended by the National Heart Lung and Blood Institute(15) and the Spanish Consensus on obesity ( < 35, >45 and < 25 % of total energy intake in the form of fat, carbohydrates and protein respectively)(Reference Salas-Salvadó, Rubio, Barbany and Moreno16). No participant received a diet containing < 4·1 MJ/d during the course of the study. Depending on the assigned group, the patients received either a fibre-mix product, or a placebo product twice daily (b.i.d.) or three times daily (t.i.d.), taken with 150 ml water 10 min before lunch and dinner (b.i.d. group) or breakfast, lunch and dinner (t.i.d. group), respectively.
The fibre supplement and the placebo were administered as soluble effervescent powder in sachets (manufactured by Madaus, S.A.) of identical weight and presentation. Each sachet of the fibre supplement contained 3 g Plantago ovata seed husks and 1 g glucomannan. The placebo sachets contained 3 g microcrystalline cellulose.
Measurements
With the patient wearing light clothes, weight was measured with a ± 100 g precision at baseline and every 4 weeks until the study was completed. Weight difference between baseline and final visit was considered the primary efficacy endpoint. Other variables measured at baseline and after 16 weeks of treatment were self-assessment of satiety and adherence to diet and treatment, lipid profile (total cholesterol, VLDL-, LDL- and HDL-cholesterol and TAG), fasting glucose and insulin, oral glucose tolerance test (OGTT), homeostasis model assessment of insulin resistance (HOMA-IR) index, fasting glycosylated Hb and serum high-sensitivity C-reactive protein.
At baseline, and after 8 and 16 weeks of treatment, patients were asked to determine and record in a diary, the level of postprandial satiety on the day of the consultation visit plus 2d prior to the visit, on a 100 mm visual analogue scale. In the same diary, the patient recorded the self-assessment of level of adherence to the diet on a 100 mm visual analogue scale. The investigator monitored treatment compliance by counting the medication sachets returned by the patient at each follow-up visit at weeks 4, 8, 12 and 16.
Fasting blood samples were taken between 08.00 and 09.00 hours at baseline and at the end of the study. Biochemical parameters were assessed by routine methods. Fasting insulin was centrally measured by radioimmunoassay using a commercial kit (Immundiagnostik AG, Bensheim, Germany). The lower limit of detection was 0·1 mg/l and the intra- and inter-assay CV were 0·9 and 1·7 %, respectively. Insulin resistance was assessed by HOMA-IR as follows: fasting plasma insulin (mIU/l) × the fasting plasma glucose (mmol/l) divided by 22·5. Serum high-sensitivity C-reactive protein concentrations were also centrally measured using commercial enzyme-linked immunosorbent assay kits (Alpha Diagnostic International, Texas, USA). Intra- and inter-assay CV were < 10 %. OGTT was performed following a 75 g glucose load in fasting conditions at baseline and after 16 weeks of treatment.
Information on adverse events possibly related to the dietary supplement products was solicited at each visit to the clinic during the study period. At each visit the patients were asked an open-ended question regarding any unusual symptoms, or discomfort, or side effects such as more defaecation, flatulence, or any other events over the previous weeks.
Statistical methods
Based on 80 % power to detect a significant difference of 1·5 (sd 2·66) kg in weight reduction, type 1 error (α) = 0·05 (two-sided), fifty-one valid patients were required for each study group. We randomised a total of 200 patients to compensate for non-measurable patients. Of the 200 randomised patients, 185 were included in the safety analysis, 166 in the intention-to-treat (ITT) analysis, and 126 in the per-protocol analysis. The results are expressed as mean, standard deviation and/or 95 % CI, or as a percentage. The primary and secondary efficacy variables were evaluated by an ANCOVA model using the baseline value and the BMI stratum as covariates. Between-groups comparisons for the rest of the variables were performed using the Fisher exact test or ANOVA, according to the nature of the variables. The last-observation carried-forward approach was applied for the main outcome; the rest of variables were analysed according to the available-data-only approach. The analysis was performed following the ITT principle, using SAS version 9.1.3 software (SAS Institute Inc., Cary, NC, USA), and the level of significance was established at P = 0·05 (two-sided). We show the results of the ITT analysis because no significant differences in the results were found between the ITT and the per-protocol analysis.
Results
Patients
Two hundred adult patients were randomised and assigned to the three treatment groups (t.i.d. group, n 68; b.i.d. group, n 66; placebo group n 66). Of these, thirty-three patients were not included in the ITT analysis: nine due to withdrawal of informed consent, five due to selection criteria violation, and nineteen due to the lack of some measurable variables at baseline or due to other reasons. Subsequent data refers only to the 166 patients of the ITT study population (t.i.d. group, n 58; b.i.d. group, n 53; placebo group, n 55). There were no statistically significant differences among treatment groups with respect to time-on-treatment or causes for discontinuing the study. Table 1 summarises the baseline characteristics of the 166 measurable patients (22 % men and 78 % women). The three groups were well balanced in terms of age, gender, weight and BMI. No significant differences were observed between groups in relation to the plasma lipid profile, fasting glucose, insulin or glycosylated Hb concentrations or glucose tolerance at the beginning of the study. None of the groups showed significant differences in relation to the degree of postprandial satiety.
Table 1 Characteristics of the subjects on entry into the trial* (Values are means with standard deviations)

b.i.d., mixed fibre dose given twice daily; t.i.d., mixed fibre dose given three times daily; OGGT, oral glucose tolerance test; HOMA-IR, homeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein.* There were no significant differences between groups.
Adherence to the treatment (monitored by the investigator by counting the medication sachets returned by the patient at each follow-up visit) and to the diet (evaluated by the patient on the visual analogue scale at each follow-up visit) in all the groups was, in general, very high (>90 % in all the consultation visits). No statistically significant differences were found among groups in relation to treatment compliance or to the self-assessment of the level of adherence to the diet.
Changes produced following the intervention
Weight loss was observed with both fibre doses ( − 4·52 (sd 0·56) kg in the b.i.d. group and − 4·60 (sd 0·55) kg in the t.i.d. group) and with the placebo ( − 3·79 (sd 0·58) kg) at the end of 16 weeks of the study. The weight loss was progressive and constant in the three study groups, without any weight regained at the end of the study. Although the changes tend to be greater in both groups supplemented with dietary fibre, the differences between groups were not statistically significant (P = 0·43) (Fig. 1).
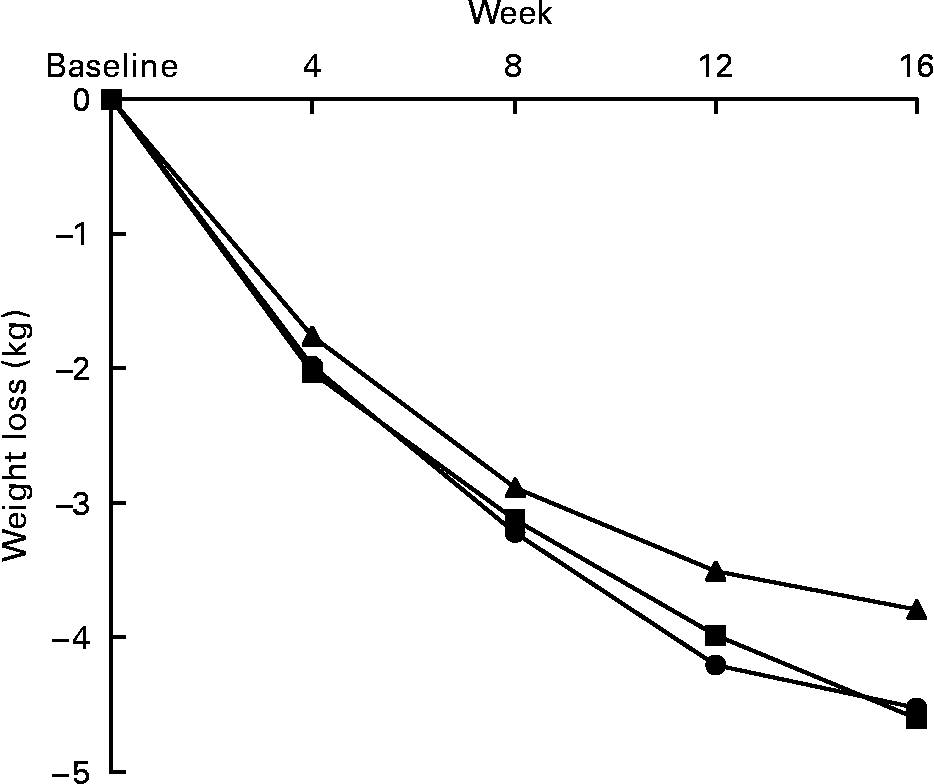
Fig. 1 Effects on body weight of the 16-week fibre supplementation in b.i.d. group (mixed fibre dose given twice daily, –●–) and t.i.d. group (mixed fibre dose given three times daily, –■–) compared with placebo, –▲–. No statistically significant differences in changes were observed between groups.
Table 2 summarises the effect of interventions on the measured plasma biochemical parameters. During the 16 weeks of the trial, glycosylated Hb decreased within all three groups ( − 0·03 (sd 0·06) in the b.i.d. group, − 0·11 (sd 0·06) in the t.i.d. group and − 0·15 (sd 0·06) in the placebo group); the differences between the groups not being statistically significant. Fasting glucose and 2 h post-OGTT decreased within all three groups with the exception of the fasting glucose in the placebo group (fasting glucose changed − 0·12 (sd 0·11), − 0·09 (sd 0·10), 0·03 (sd 0·10) mmol/l and glucose 2 h post OGTT changed − 0·20 (sd 0·29), − 0·17 (sd 0·26) and − 0·24 (sd 0·27) mmol/l in the b.i.d., t.i.d. and placebo groups, respectively). Fasting insulin and 2 h post-OGTT decreased considerably within the b.i.d. group, albeit the changes between groups were not statistically significant. No significant differences in changes on HOMA-IR index were observed among the groups. However, the changes in the lipid profile induced by the interventions were different between groups. The decrease in plasma total cholesterol tended to be greater in the b.i.d. and t.i.d. groups compared to placebo. The differences between groups with respect to the plasma LDL-cholesterol concentrations were statistically significant (P = 0·03), with a greater decrease observed in the two fibre-supplemented groups ( − 0·38 (sd 0·10) and − 0·24 (sd 0·09) mmol/l in the b.i.d. and t.i.d. groups v. − 0·06 (sd 0·09) mmol/l in placebo), although no dose–response pattern was observed for this parameter. The changes in HDL-cholesterol and TAG concentrations did not follow any clear trend within or between the intervention groups. The differences between groups in the plasma total cholesterol:HDL-cholesterol and HDL-cholesterol:LDL-cholesterol ratio were statistically significant (P = 0·03 and P = 0·02, respectively, with a greater change observed in the two fibre-supplemented groups). No differences between groups were observed in relation to serum C-reactive protein concentrations. Table 3 summarises the observed effects of the 16-week fibre supplementation on perceived satiety. The differences between groups in changes in satiety were statistically significant post-lunch (P = 0·04) but not post-dinner. However, no significant differences in satiety were observed between the two fibre-supplemented groups.
Table 2 Effects of 16-week fibre supplementation on plasma metabolic markers* (Values are means with standard deviations and means with 95 % CI)

b.i.d., mixed fibre dose given twice daily; t.i.d., mixed fibre dose given three times daily; OGGT, oral glucose tolerance test; HOMA-IR, homeostasis model assessment of insulin resistance.* Results from the ANCOVA model. All results are adjusted by stratum and baseline values.
† Changes between start and end of study.
‡ Differences in changes between groups (b.i.d. and t.i.d. v. placebo).
§ Statistical differences in changes between groups.
Table 3 Effects of 16-week fibre supplementation on satiety* (Values are means with standard deviations and means with 95 % CI)
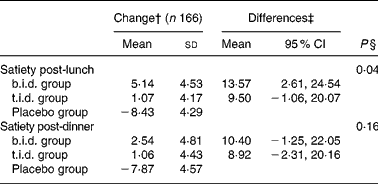
b.i.d., mixed fibre dose given twice daily; t.i.d., mixed fibre dose given three times daily.* Results from the ANCOVA model. All results are adjusted by stratum and baseline values.
† Changes between start and end of the study.
‡ Differences in changes between groups (b.i.d. and t.i.d. v. placebo).
§ Statistical differences in changes between groups.
Safety
No serious adverse event was reported during the study. No significant differences in total adverse events were observed between groups. Gastrointestinal complaints (3·8 % diarrhoea, 32·2 % flatulence, 2·2 % constipation, 2·2 % upper abdominal pain and 0·5 % abdominal distension) were the most frequently reported adverse events, with no significant differences in the proportions of these events reported between the treatment groups. There were no patient withdrawals from the study due to adverse events.
Discussion
The results of our study suggest that fibre supplementation, in the context of a weight-reducing diet, could be beneficial in overweight or obese individuals because the additional fibre induces satiety and a more long-term favourable lipoprotein profile than placebo. However, the present results do not support the hypothesis that fibre supplements in combination with a conventional energy intake restriction can have additional effects on weight reduction and glucose tolerance.
The literature contains several reports of the effect of fibre supplements on body weight(Reference Pittler and Ernst9, Reference Howarth, Saltzman and Roberts13). However, in few of the randomised placebo-controlled clinical trials has the body weight been considered the primary variable in the analysis(Reference Slavin4). In addition, long-term ( ≥ 4 months) trials are rare(Reference Tuomilehto, Voutilainen, Huttunen, Vinni and Homan17–Reference Makkonen, Simpanen, Saarikoski, Uusitupa, Penttila, Silvasti and Korhonen20) and have been conducted in small samples of heterogeneous populations (healthy controls, obese or diabetic patients) while using different types and doses of fibre(Reference Howarth, Saltzman and Roberts13).
To the best of our knowledge, our study is unique in analysing the effect of two doses of soluble-fibre supplementation on body weight and/or other metabolic variables. Because of the great variability expected in the response of body weight following intervention, our study was performed in 200 individuals. Hence, the present study is one of the few large-scale studies analysing the long-term effects of a supplement of fibre on lipid profile, glucose tolerance(Reference Brown, Rosner, Willett and Sacks21), body-weight changes and satiety.
The influence of dietary-fibre supplements on energy regulation remains controversial(Reference Slavin4). One of the best-studied supplements of fibre on body weight is guar gum. A meta-analysis of the randomised placebo-controlled trials identified thirty-four guar gum trials of which only eleven could be analysed(Reference Pittler and Ernst9) and only two were for a period >14 weeks(Reference Tuomilehto, Voutilainen, Huttunen, Vinni and Homan17, Reference Makkonen, Simpanen, Saarikoski, Uusitupa, Penttila, Silvasti and Korhonen20). The meta-analysis concluded that guar gum is not efficacious for reducing body weight and is frequently associated with gastrointestinal complaints such as flatulence, diarrhoea, abdominal pain, and cramps(Reference Pittler and Ernst9).
Other types of fibre supplements have been studied, also with inconclusive results with respect to weight control(Reference Hylander and Rossner22–Reference Walsh, Yaghoubian and Behforooz24). Among these fibres, Plantago ovata and glucomannan are the most relevant. As with the results of our study, a previous large-scale study performed with ispaghula husk showed no significant short-term (3 weeks) differences in body-weight loss when comparing isphaghula, bran and control groups(Reference Hylander and Rossner22). Also as with the present study, a decrease in hunger ratings was observed at the end of the study period compared to the baseline values in both groups receiving the fibre supplements(Reference Hylander and Rossner22). No significant short-term effects (6 weeks) of 5 g Plantago psyllium on body weight had been observed in type II diabetic patients when evaluating the lipid- and glucose-lowering efficacy of this type of fibre(Reference Rodriguez-Moran, Guerrero-Romero and Lazcano-Burciaga10). In contrast several studies have demonstrated a significant effect of glucomannan supplements on appetite and body weight. However, only some of these studies were randomised placebo-controlled trials, and all were short-term and performed in small study populations(Reference Walsh, Yaghoubian and Behforooz24–Reference Vita, Restelli, Caspani and Klinger27). In our study, the two groups receiving the dietary fibre tended towards greater weight loss than did control individuals. However, the lack of significant differences in body-weight loss observed between groups does not support the hypothesis that this type of fibre has any effect on body weight. Whether significant differences would be observed by increasing the fibre dose is worth considering in future studies.
As with guar gum, isphagula husk (another viscous soluble fibre), has also been associated with gastrointestinal secondary adverse effects because of the rapid fermentation when the fibres arrive intact in the colon(Reference Pittler and Ernst9). In a previous study, we observed that a mixture of Plantago ovata combined with glucomannan slows fermentation in the colon and retards the rate of butyrate and propionate production in vitro (L Fluvià and A Anguera, unpublished results). For this reason, we propose that the intake of this fibre mixture increases subject compliance due to fewer gastrointestinal side effects associated with the delay in gas production, which increases the patient's tolerance of the mixture. It is for this reason that we selected to study the effects of a mixture of these two types of fibre: Plantago ovata husks and glucomannan. In the present study the dietary fibre supplement was well tolerated by the patients. Gastrointestinal symptoms were the most frequent adverse events reported in our study but these were not of any clinical significance. Also, there were no significant differences observed between the groups taking the fibre and the placebo group. As such, our study suggests that long-term administration of Plantago ovata and glucomannan has a high patient-acceptability.
With respect to changes in the lipid profile, our results are in the same direction of a previously-published meta-analysis analysing this effect. We observed a greater decrease in plasma total cholesterol in both groups of patients receiving the fibre. These differences were due, in part, to significant decreases in the plasma LDL-cholesterol concentrations observed in the two groups receiving the fibre compared to the placebo group ( − 0·38 (sd 0·10) and − 0·24 (sd 0·09) mmol/l in the b.i.d. and t.i.d. groups v. − 0·06 (sd 0·09) mmol/l in placebo group). The hypocholesterolaemic effect observed was modest, not dependent on the dose administered, and of the same magnitude as that observed in a meta-analysis where the decrease in total and LDL-cholesterol was estimated to be about 0·05 mmol/l for every gram of soluble fibre added to the diet(Reference Brown, Rosner, Willett and Sacks21, Reference Anderson, Allgood, Lawrence, Altringer, Jerdack, Hengehold and Morel28). As with the conclusions of the meta-analysis cited earlier, our study suggests that the intake of a fibre supplement has no effect on HDL-cholesterol or TAG concentrations. Of note is that obesity is associated with atherogenic dyslipidemia, which has been considered an important CVD risk factor. Hence, fibre supplementation in the context of a weight-reducing diet could decrease the cardiovascular risk in obese people through the mechanism of LDL-cholesterol reduction i.e. 1 % decrease in plasma LDL-cholesterol is estimated to result in a 2 % decrease in coronary disease mortality. Our results highlight significant differences between groups in relation to the changes in the plasma total cholesterol:HDL-cholesterol and HDL-cholesterol:LDL-cholesterol ratios with more favourable changes induced in the two fibre-supplemented groups compared to placebo.
In relation to glucose metabolism, population studies have shown that high-fibre diets may play a protective role in the prevention of type 2 diabetes(Reference Salmeron, Manson, Stampfer, Colditz, Wing and Willett29, Reference Montonen, Knekt, Jarvinen, Aromaa and Reunanen30) and CVD(Reference Rimm, Ascherio, Giovannucci, Spiegelman, Stampfer and Willett31). Diets that are high in complex carbohydrates and fibre were shown to be associated with greater insulin sensitivity and a reduction in plasma insulin levels. Also, high-fibre diets contribute to a better metabolic control in diabetic subjects(Reference Chandalia, Garg, Lutjohann, von Bergmann, Grundy and Brinkley32). However, some clinical studies have shown that only the viscous variety of some soluble fibres play a significant role in reducing postprandial glycaemia(Reference Wursch and Pi-Sunyer33). In our study, the fibre supplements showed no significant effects on insulin resistance, fasting glucose and post-OGTT glucose or insulin concentrations, or glycosylated Hb. This is probably because none of the patients studied had diabetes requiring oral antidiabetic drugs or insulin.
There is a lack of consensus in the literature with regard to the anti-inflammatory effects of fibre. Results from two epidemiological studies indicated that dietary-fibre intake was inversely associated with serum C-reactive protein concentrations(Reference Ma, Griffith, Chasan-Taber, Olendzki, Jackson and Stanek34, Reference Ajani, Ford and Mokdad35), but another large study failed to find any association between whole-grain intake and plasma C-reactive protein, IL-6 or fibrinogen concentrations(Reference Jensen, Koh-Banerjee, Franz, Sampson, Gronbaek and Rimm36). In our study, we were unable to demonstrate any effect of the additional dietary fibre consumption on plasma high-sensitivity C-reactive protein concentrations, although patients receiving the fibre tended to have greater decreases in this parameter.
In conclusion, the present study shows that the administration over a protracted period of 16 weeks of a supplement of a soluble fibre mixture of Plantago ovata and glucomannan to overweight or obese patients induced satiety and a decrease in plasma LDL-cholesterol concentrations, without any effect observed on body weight, TAG, HDL-cholesterol concentrations or other glucose metabolism parameters.
Acknowledgements
This study was supported by MADAUS, S.A. and by the Instituto de Salud Carlos III (Thematic Network G03/140 and RD06/0045, and PI051839), Spain. Dr Salas-Salvadó and Dr Ferrán Torres have received consulting or lecture fees from Madaus Laboratories. Dr Anguera belongs to Madaus Laboratory. The order in which the authors are listed has been agreed on by the investigators. Jordi Salas-Salvadó, Rafel Balanza, Mònica Bulló, Ferrán Torres and Anna Anguera have made the greatest contribution, and wrote the preliminary version. All the authors have contributed equally to the work by discussing and analysing the data and writing the definitive version.
The following investigators and co-investigators participated in the study: Unitat de Nutrició Humana: Jordi Salas-Salvadó, Mònica Bulló, Rafel Balanza, Patricia Casas, Fabiola Márquez. CAP el Remei de Vic: Xavier Farrés, CAP d'Alcover: Xavier Luque, CAP de Centelles: Silvia Narejos, CAP Sarrià, Barcelona: Manel Borrell, CAP Reus I: Josep Basora, CAP Sardenya: Carles Brotons, CAP Sant Gervasi (Parc Sanitari Pere Virgili): Anna Altés, CAP Les Hortes-Poble Sec: Cristina González Fernández, CAP Dr Vicenç Papaceit: Josep Lluis Fernández, CAP Salou: Cristina Ferrández, EAP Reus III: Manuel Prieto, EAP Reus IV: Teresa Basora.






