Introduction
Events that occur during the first 1000 d (from conception through to 2 years) in a child's life, including birth outcomes, are significant. They contribute to the commencement of proper development and impact future health(1). Birth outcomes, including birth weight, gestational age, abortion, neonatal mortality and stillbirths, are a measure of babies’ health at birth. Of these, the two most studied indicators are birth weight and gestational age, yet their causes are not fully understood(Reference Wadhwa, Glynn and Hobel2). Worldwide, low birth weight (LBW) is estimated at 15–20 % births(3), and 1⋅1 million babies die as a result of preterm birth complications annually(Reference Risnes, Vatten and Baker4). The WHO estimates that more than 9 million infants were dying within their first birthday or even before birth every year, especially in developing countries(Reference Kim and Saada5). The inequality in birth outcomes between low- and high-income countries is attributed to poor antenatal care (ANC) received by pregnant women in low-income countries(Reference Reinebrant, Leisher and Coory6). Birth outcomes are generally related to social, economic, biological and environmental factors(Reference Brown, Speechley and Macnab7–Reference Maddah, Karandish and Mohammadpour-Ahranjani10).
Maternal nutrition practices, child growth period and the intrauterine environment during pregnancy may also affect birth outcomes(Reference Abu-Saad and Fraser11–Reference Sintayehu and Bedasa14). A study conducted in Ghana on birth weight concluded that mothers with adequate dietary practices were less likely to have babies with LBW(Reference Abubakari and Jahn15). Poor maternal dietary habits have been related to rural living, food insecurity and poverty(Reference Gyimah, Annan and Apprey16). Moreover, unfavourable birth outcomes have been related to low socioeconomic status such as low education level, low income, poor access to health facilities and poor housing conditions(Reference Tanya Nagahawatte and Goldenberg17,Reference Aga Khan University and UNICEF18) . Adverse birth outcomes have also been related to maternal morbidity and insufficient maternal care(Reference Kassebaum, Bertozzi-Villa and Coggeshall19–Reference Yeshialem, Alemnew and Abera21), thus, early identification of complications of pregnancy during antenatal is vital.
ANC is the basic component of maternal care on which the life of mothers and babies depend(Reference Nisar and White22). According to the WHO(23), ANC is part of the three most important care given to pregnant women and a crucial indicator of reducing the global maternal mortality ratio as specified in target 3 of the Sustainable Development Goal (SDG). Accessing ANC services have been directly linked to better birth outcomes and a decrease in infant mortality and malnutrition among low-income and middle-income countries(Reference Kuhnt and Vollmer24). ANC services are also related to socio-demographic or reproductive characteristics, and consequently birth outcomes(Reference Afulani25–28). The Government of Ghana endorsed the Focused Antenatal Care (FANC) strategy of the World Health Organization (WHO) in 2002 to address the significantly higher maternal mortality rate and to increase access, quality and consistency of ANC for pregnant women. ANC is a focal point for pregnant women and their fetuses for receiving a wide variety of preventive and healthcare activities, treatment, monitoring during pregnancy up to delivery(Reference Baffour-Awuah, Mwini-Nyaledzigbor and Richter29). There is late initiation of ANC attendance among pregnant adolescents as they are more likely to hide their pregnancy due to doubt and naivety, lack of finance and stigmatisation(Reference Pell, Meñaca and Were30). Studies among pregnant adolescents have reported a high risk for adverse birth outcomes, such as preterm, fetal death or LBW(Reference Alam31–Reference Weng, Yang and Chiu35). Furthermore, findings have reported an even higher risk for unfavourable birth outcomes in much younger adolescents(Reference Afriyie, Bedu-Addo and Asiamah36,Reference Cooper, Leland and Alexander37) . The WHO(38) reported an estimation of 12⋅8 million births (44 births per 1000 adolescent girls) of teenage girls between 15 and 19 years in 2018, with the highest occurrence in low-income countries (8 times higher), compared with high-income countries.
A maternal health survey conducted in 2017 in Ghana identified 12 % miscarried pregnancies, 2 % stillbirths and 10 % induced abortions among women aged 15–49 years(39). Among these women of reproductive age, the prevalence of adolescent pregnancy was 14⋅2 %, with stillbirths, miscarriages and induced abortion recording 1⋅3, 6⋅8 and 18⋅8 %, respectively. Live births among this group were the least (73⋅1 %) as compared with the adult group (77⋅4 %), who were between 20 and 34 years(39). A study conducted in Kumasi, Ghana, recorded a prevalence of 19 % adverse birth outcomes(Reference Asundep, Carson and Archer40) while an earlier one reported a 44⋅6 % prevalence(Reference Yatich, Yi and Agbenyega41) among pregnant women. Among adolescents in Kumasi, the second-largest city in Ghana, 23 % had LBW(Reference Ayensu, Edusei and Oduro42).
The seventh target of the SDG 3 seeks to ensure universal access to sexual and reproductive healthcare services. To achieve this goal, the Ministry of Gender, Children and Social Protection in Ghana developed a five-year strategic plan (2018–22). The plan seeks to reach adolescents with the correct information and insight and to provide them with training and services to protect them from early and unintended pregnancies(43). Despite this, adolescent pregnancies seem to be on the rise. To prepare them for desirable birth outcomes, a better understanding of the determinants of birth outcomes in this group is required. WHO's global target for 2025 is to reduce LBW by 30 %, that is, a 3 % annually reduction. It is expedient that we identify the factors which lead to these adverse birth outcomes among this age bracket. The present study was, therefore, conducted to explore the predictors of adverse birth outcomes among pregnant adolescents in Ashanti Region, Ghana.
Methods
Study design
In this prospective health centre-based study, 416 pregnant adolescents (aged 13–19 years old) with a maximum of 32 gestational weeks were recruited from May to December 2018 and followed until they gave birth between January and July 2019.
Study area and population
Ashanti Region, the most populated region in Ghana, makes up 19 % of Ghana's total population, which is about 5 792 187 people(44). The prevalence of adolescent pregnancy in the region is 12⋅2 %, according to a recent survey conducted in 2017(39). We recruited participants from health centres serving communities in three urban districts (Kumasi Metropolis, Asante Akim Central Municipal and Ejisu Juaben Municipal) and five rural districts (Bosomtwi, Asante Akim South and North, and Ahafo Ano North and South) in Ashanti Region, Ghana. We recurited participants at selected Community-based Health Planning Services (CHPS) compounds, health centres and hospitals in the selected districts(44).
Sample size
The sample size was statistically calculated using the sample size-based proportion formula by(Reference Charan and Biswas45): n = 2(Z α/2 + Z β )2 p(1 − p)/(P1 − P2)2. Where n is the sample size, Z α/2 = 1⋅96 at type 1 error of 5 %, Z β = 0⋅84 at 80 % power, P1 is the LBW in pregnant adolescents with adequate nutritional status, P2 is the LBW in pregnant adolescents with poor nutritional status, P1 − P2 is the difference in the prevalence of LBW between pregnant adolescents with adequate nutritional status at birth and those with inadequate nutritional status, and P is pooled prevalence = (P1 + P2)/2. Based on a previous pilot study by Ayensu et al. (Reference Ayensu, Edusei and Oduro42), which reported 23 % LBW prevalence, we proposed that LBW in pregnant adolescents with adequate nutritional status would be 11⋅4 %, while those with poor nutritional status would remain 23 %. Using the above descriptives, the sample size n = 2(1⋅96 + 0⋅84)2 × 0⋅1735(1–0⋅233)/(0⋅114–0⋅233)2, n = 2⋅09/0⋅01, n = 209 was calculated, which implied we needed to recruit 209 participants in each arm of the study (half in the poorly nourished group and a half in the well-nourished group) making 418 participants to show a significant association between poor nutrition and LBW. We added 10 % attrition to give 460 participants who were needed.
However, 416 pregnant adolescents were recruited on a first-come, first-served basis for the baseline study. Trained enumerators visited hospitals/health centres selected for the study on antenatal clinic days for pregnant adolescents. All pregnant adolescents within the inclusion criteria who gave written consents were written. In the rural areas, announcements were made at the community information centres to invite participants to the health centres on specific days. These announcements were necessary as some pregnant adolescents refused to visit the health centres for ANC to fear being stigmatised. We trained enumerators in a two-day workshop on each data collection tool used. We obtained birth outcome data from 270 (birth weight) and 303 (gestational age) participants out of the 416 recruited during the baseline study as some participants were lost to follow-up.
Ethics
Ethical approval for the study was obtained from the Committee on Human Research Publication and Ethics (CHRPE) of the Kwame Nkrumah University of Science and Technology, KNUST, (Kumasi, Ghana) (CHPRE/AP/236/18). Study protocols/aims were first explained to all participants in their local language. Written and signed informed consent was obtained from all participants by following CHRPE regulations before recruiting for the study.
Data collection tools
A structured questionnaire was used to collect data on the socio-demographic variables, dietary diversity, eating behaviours (pica, food aversion, food craving), food deprivation (household hunger scale – HHS), availability of necessities (lived poverty index – LPI), antenatal interventions compliance (nutrition education, micronutrient supplementation, tetanus injection, malaria tablet intake, use of the ITN, daily intake of micronutrient), maternal factors (maternal morbidity and mode of delivery) and birth outcomes (birth weight and gestational age) of the pregnant adolescents. Data on age and parity were verified from their National Health Insurance Identification cards and maternal health record book. The questionnaire was pretested at selected health centres and validated to ensure appropriate responses from participants. Birth weight and gestational age were the dependent variables.
Assessment of dietary diversity
A previous day's 24-h dietary recall method was used to assess dietary diversity among the participants. The FAO's Minimum Dietary Diversity for Women (MDD-W), was used to determine maternal dietary diversity using ten food groups. The MDD-W score obtained was then used to identify whether maternal dietary diversity was adequate (5–10 food groups) or not (0–4 food groups).
Assessment of eating behaviour
Questions on whether or not the participants were practising food cravings, pica and food aversions were asked. Each question had a yes/no optional response.
Assessment of HHS and LPI
We collected data on food availability and deprivation over the past month using standardised close-ended questionnaires(Reference Ballard, Coates and Swindale46) to assess hunger (HHS) among participants. The responses to these questions were then coded and scored. Scores between <2 meant little or no hunger, 2–3 meant moderate hunger and 4–6 meant severe hunger. LPI was assessed by asking questions on the availability of food, water, cash income, medical care and cooking fuel over the past year. The responses were coded and scored. A score of 0 meant no lived poverty, while the scores of 1–4 meant the absence of one or more necessities(Reference Mattes, Dulani and Gyimah-Boadi47). The averages of the scores were then categorised into low (0–1⋅0), moderate (1⋅01–1⋅5) and high (>1⋅5) LPI.
Assessment of antenatal interventions
A structured questionnaire was administered to participants to identify their compliance with the antenatal interventions given at their various health facilities. Participants were asked if they received nutrition education, tetanus injection, micronutrient supplements, malaria tablet, ITN, consumed daily micronutrient supplement, and whether they used the ITN given.
Assessment of birth outcomes and maternal factors
Follow-up data on several birth outcomes, including birth weight and gestational age at birth were obtained from their maternal health record books and were categorised as normal or adverse. LBW was defined as a birth weight of less than 2⋅5 kg(28). Preterm birth was defined as delivery less than 37 completed weeks of gestation(Reference Lawn, Gravett and Nunes48). Questions on mode of delivery (vaginal delivery, caesarean section) and morbidity during pregnancy were asked.
Data analysis
Microsoft Excel was used to clean the entered data, and Statistical Package for Social Sciences version 25 (SPSS IBM Inc., Chicago, USA) was used to analyse them. We performed χ 2 (Fischer's exact test) and cross-tabulation to compare frequencies of all variables used (antenatal interventions and compliance, maternal factors and birth outcomes) among community types. Frequencies of birth weight and gestational age were compared among socio-demographic variables, dietary diversity, eating behaviours, LPI, HHS, antenatal interventions compliance and maternal factors using Fischer's exact test. We used logistic regression to identify predictors of birth outcomes. We presented the data as frequency (%) and P values < 0⋅05 were considered significant.
Results
Impact of socio-demographic factors on birth outcomes
The distribution of socio-demographic characteristics of pregnant adolescents and among community types used for the present study has been presented in another study(Reference Gyimah, Annan and Apprey16). Effects of socio-demographic status on birth outcomes are presented in Table 1. Among all the factors used, the level of education affected the gestational age at birth significantly (P = 0⋅006). Generally, preterm births (PTBs) decreased with increasing education level (none = 40 %; SHS = 11⋅5 %). PTBs showed slightly different trends by socio-demographic status. Older adolescents (16–19 years) recorded more PTBs (13⋅1 %) than adolescents in the younger bracket (5 %). Similarly, more PTB's were recorded among adolescents who were married (19⋅4 %) and employed (16⋅9 %) than among those who were single (13⋅8 %) and unemployed (10⋅9 %). LBWs were proportionally higher among adolescents who were younger than their older counterparts (22⋅2 % v. 14⋅8 %), married than single (19⋅4 % v. 13⋅8 %), employed than unemployed (16⋅9 % v. 15⋅6 %) and among those with high-income range than those without income (33⋅3 % v. 13⋅6 %).
Table 1. Differences in socio-demographic factors and birth outcomes (birth weight and gestational age)

HHS, Household Hunger Scale; LPI, Lived Poverty Index.
Bold values are significant at P < 0⋅05.
a χ 2.
b Fischer's exact test.
ANC service delivery and uptake, maternal factors and birth outcomes
Table 2 presents the frequencies of antenatal interventions, maternal factors and participants’ birth outcomes in the present study. Levels of participants who received nutrition education (P = 0⋅022), tetanus injection (P = 0⋅021), used insecticide-treated nets (P = 0⋅026) and mode of delivery (P = 0⋅009) varied significantly between rural and urban study participants. Generally, about half of pregnant adolescents (49⋅5 %) received nutrition education during antenatal services. Nearly 12 % more of pregnant adolescents from urban areas received nutrition education than their rural counterparts (P = 0⋅022). About 60⋅6 % received micronutrient supplements while only 46 % were taking their supplements daily. Four(Reference Risnes, Vatten and Baker4) out of ten pregnant girls had not received tetanus injection. However, a more significant proportion of urban girls (P = 0⋅021) had received tetanus injection. About four to five out of ten had not received malaria tablets or insecticide-treated net (ITN). About 17 % of pregnant girls who had received the ITN were not using them. The use of ITN among rural girls was significantly higher (P = 0⋅026) than those in urban areas. Though not significant, LBW was higher among deliveries by rural girls (18⋅8 %) than girls (13⋅4 %). Also, preterm births proportion was higher among urban girls (13⋅1 %) than rural (11⋅9 %). About eight in ten pregnant girls delivered their babies using the vaginal method. Significantly, more rural girls (P = 0⋅009) than urban girls delivered through the vaginal method.
Table 2. Antenatal care interventions and uptake, maternal factors and birth outcomes between rural and urban-dwelling pregnant adolescents
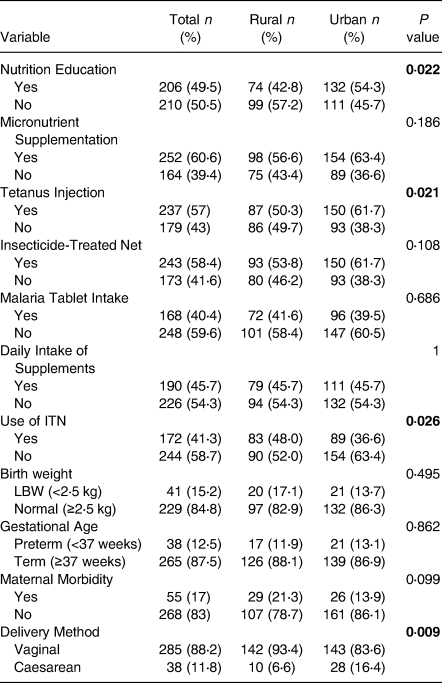
LBW, Low Birth Weight.
Categorical data are presented as frequency (%).
Bold values are significant at P < 0⋅05.
Impact of antenatal service delivery and uptake, and maternal factors and birth outcomes
Table 3 presents the effect of antenatal intervention uptake and maternal factors on birth outcomes. Adolescents who reported ill while pregnant showed a significantly larger proportion of LBWs (27⋅1 %) than those who did not (12⋅6 %; P = 0⋅024). Also, PTBs were over twice more among those reporting ill (24⋅1 %) than those who reported no illness (10 %; P = 0⋅011). LBW was significantly lower among participants who used ITNs (9⋅3 %; P = 0⋅038) as compared with those who did not (19 %). Also, LBW was slightly lower among adolescents who received nutrition education but higher among those who took micronutrient supplements, and malaria tablets, tetanus injection, than their counterparts who did not though these did not reach a statistical significance. Though not significant, participants who delivered through caesarean section (20⋅7 %) gave birth to a greater proportion of babies with LBW as compared with those who gave birth through the vaginal delivery method (15⋅8 %).
Table 3. Comparison of birth outcomes by antenatal care interventions uptake and maternal factors

LBW, Low Birth Weight; NBW, Normal Birth Weight.
Bold values are significant at P < 0⋅05.
Table 4 shows the effect of the HHS, LPI, eating behaviour and MDD on birth outcomes. Among these variables, HHS had a statistically significant effect on birth weights (P = 0⋅006) and PTB (P = 0⋅028), whereby adolescent girls who suffered hunger had a larger proportion of LBW (no hunger = 12 %, moderate = 35⋅5 % and severe = 16⋅1 %; P = 0⋅006) and PTB (no hunger = 10 %, moderate = 24⋅4 % and severe = 13⋅2 %; P = 0⋅028). Likewise, poorer pregnant adolescent had significantly more LBW (P = 0⋅029) and PTB (P = 0⋅006). Although not statistically significant, LBW was lower among those with adequate MDD (11⋅4 %) as compared with those with inadequate MDD (17⋅9 %), while PTB was rather lower among those with inadequate MDD (11⋅6 %) than those whose MDD were adequate (13⋅7 %). Similarly, those who practiced PICA had more LBWs (16⋅2 %) and less PTBs (10⋅7 %) than those who did not (LBW = 14⋅6 %, P = 0⋅057; PTB = 13⋅6 %, P = 0⋅590).
Table 4. Comparison of birth outcomes by HHS, LPI, eating behaviour and dietary diversity

HHS, Household Hunger Scale; LPI, Lived Poverty Index; LBW, Low Birth Weight; NBW, Normal Birth Weight.
Bold values are significant at P < 0⋅05.
a χ 2.
b Fischer's exact test.
Predictors of adverse birth outcomes
Table 5 shows the findings of logistic regression on predictors of LBW. The use of ITN, LPI, HHS and maternal morbidity were significantly associated with delivering LBW babies. Pregnant adolescents who used ITN presented reduced odds (AOR 0⋅4; P = 0⋅013; 95 % CI 0⋅2, 0⋅9) of giving birth to babies with LBW compared with those who did not use them. Those who were poorer, using LPI presented increased odds (OR 2⋅5; P = 0⋅014; 95 % CI 1⋅1, 4⋅8) of delivering LBW babies than those with low LPI. Also, pregnant adolescents who were moderately hungry had a higher risk of delivering LBW babies (AOR 3⋅0; P = 0⋅028; 95 % CI 1⋅1, 8⋅1) compared with those who were not hungry. Maternal morbidity increased odds (AOR 2⋅7; P = 0⋅020; 95 % CI 1⋅2, 6⋅0) of babies with LBW as compared with those who did not experience any sickness.
Table 5. Predictors of low birth weight
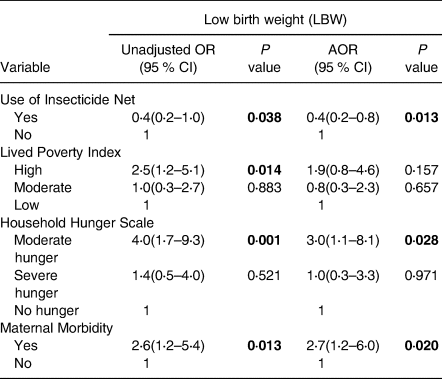
Adjusted for Age and Community type, OR, Odds ratio; AOR, Adjusted odds ratio; CI, Confidence interval, statistically significant p values are bolded.
Table 6 presents a logistic regression analysis of the predictors of PTB. Maternal education status, LPI, HHS and maternal morbidity were significantly associated with delivering PTB. Pregnant adolescents with no formal education presented increased odds (AOR 9⋅0; P = 0⋅004; 95 % CI 2⋅1, 39⋅8) of having PTB compared with those with formal education. Adolescents who suffered hunger had increased odds (OR 2⋅9; P = 0⋅010; 95 % CI 1⋅3, 6⋅5) for PTB, compared with those who were not hungry. Poorer pregnant adolescents presented increased odds (OR 2⋅5; P = 0⋅014; 95 % CI 1⋅2, 5⋅3) of delivering preterm babies than those who were not poor. Experiencing maternal morbidity increased the odds (AOR 3⋅0; P = 0⋅011; 95 % CI 1⋅3, 7⋅0) of having PTB as compared with those who did not experience any form of morbidity.
Table 6. Predictors of preterm birth
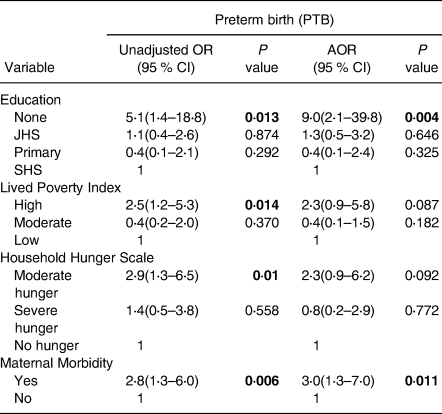
Adjusted for Age and Community type, OR, Odds ratio; AOR, Adjusted odds ratio; CI, Confidence interval, statistically significant p values are bolded.
Discussion
The present study identified predictors of adverse birth outcomes among pregnant adolescents in Ghana. The findings indicated that the use of ITN, maternal education status, hunger (HHS), poverty (LPI) and maternal morbidity were the main determinants of adverse birth outcomes (LBW and PTB) among pregnant adolescents in Ghana. The prevalence of LBW and PTB reported in the present study was 15⋅2 and 12⋅5 %, respectively. Similarly, the LBW prevalence for Western Africa and Ghana reported in 2019 were 15⋅2 and 14⋅2 %, respectively(49). Our population of adolescents is, therefore, similar to the national average with regards to LBW and lower for PTB. Compared with other studies around the world, our findings regarding LBW and PTB were higher(Reference Ganchimeg and Ota32,Reference Bihoun, Zango and Coulibaly50) but lower from other results(Reference Mombo-Ngoma, Mackanga and González51,Reference Usynina, Postoev and Odland52) . Within Ghana, a study in Cape Coast reported a similar LBW prevalence of 14⋅3 % among pregnant adolescents(Reference Afriyie, Bedu-Addo and Asiamah36).
Generally, access to ANC interventions among pregnant adolescents used in the study was relatively low. For example, less than half (49⋅5 %) of the participants reported having received nutrition education at ANC. The uptake of the interventions by adolescents was also low. Four in ten participants did not take their micronutrient supplements, nor used the ITNs, and a few took their malaria tablets (40⋅4 %). The low compliance may negate the benefits of attending ANC, as the mere attendance to ANC sessions is not the fundamental objective. This calls for programmes, including pregnant women's education in general, on the benefits of complying with these interventions. The low compliance may also be due to the fewer number of ANC sessions attended. Pregnant adolescents are likely to delay pregnancy disclosure due to uncertainty and vulnerability, especially during the first trimester, lack of funds and stigmatisation(Reference Pell, Meñaca and Were30). Compared with their adult counterparts, pregnant adolescents are likely to access ANC services later during pregnancy(Reference Blondel, Dutilh and Delour53,Reference Kirbas, Gulerman and Daglar54) . Among all the ANC interventions and compliance assessed, adolescent girls from the urban areas were better off than their rural counterparts. More urban pregnant adolescents received nutrition education (0⋅022), tetanus injection (0⋅021) and had access to ITNs (although more rural girls actually used the ITNs) compared with their rural folks. Tanya and Goldenberg(Reference Tanya Nagahawatte and Goldenberg17) identified the inability to pay for services, lack of transport and prior negative experiences as barriers to healthcare services among rural women. For the present study, we think low socioeconomic status and less access to health care in rural areas are possible reasons for the lower uptake of interventions by rural girls.
The consistent and appropriate use of ITN has been one of the efficient means in preventing malaria infection by limiting the contact and bite of mosquitoes(Reference Yirsaw, Gebremariam and Getnet55). Since 2000, mass and continuous distribution networks have substantially increased access to ITNs in Ghana(Reference Ahorlu, Adongo and Koenker56). Notwithstanding these achievements, a significant gap exists between ITN access and usage. In our study, 58 % of the pregnant adolescents reported having received ITN, while 41 % were using them. Low use of ITN has been reported and associated with discomfort from the smell of chemical used, heat and difficulty installing the net(Reference Manu, Boamah-Kaali and Febir57). Younger women (under 19 years of age) and women with less education or less hurdles to ITN usage include low awareness of ITNs by women, household or cultural constraints such as low social status or economic dependence, age, marital status, education, employment status, knowledge of malaria/ITN and IPTp reception(Reference Hill, Hoyt and van Eijk58). However, our study reported a 10 % less LBW deliveries in ITN non-users than among ITN users (9⋅3 % v. 19 %; P = 0⋅037). This disparity may be related to the possibility that despite these adolescents receiving antenatal interventions, they were at a higher risk of other relating factors such as maternal underweight, hunger, poverty directly associated with LBW compared with those who did not receive antenatal interventions, and these factors may have confounding effects on their birth outcomes rather than the antenatal interventions.
Plasmodium falciparum infection during pregnancy is a prominent cause of LBW. In our findings, the use of ITN was significantly associated with LBW; those who used the ITN presented reduced odds of delivering babies with LBW. Similar to this result, other studies(Reference Eisele, Larsen and Anglewicz59–Reference Appiah, Bukari and Yiri-Erong61) have reported the association of ITN use with birth outcomes. For instance, a significant reduction in the risk of LBW in healthy malaria transmission regions has been associated with the use of antimalarial intermittent pregnancy prevention (IPTp) and ITN during pregnancy(Reference Eisele, Larsen and Anglewicz59). A higher reduction rate of LBW (23 %) and fetal loss (33 %) with the use of ITN has also been reported among women of low gravidity in Africa(Reference Gamble, Ekwaru and Garner60). Kubi Appiah and others also reported increased odds of normal birth weight deliveries (AOR 2⋅17; 95 % CI 0⋅03, 0⋅92) to be associated with women who received a long-lasting ITN from ANC clinics(Reference Appiah, Bukari and Yiri-Erong61).
The study predicted that among all socio-demographic factors used, the education status of the mother was significantly associated with PTB. Among pregnant adolescents who had no formal education, 40 % delivered PTB babies. This suggests that maternal education level affects birth outcomes. Access to education can expose an individual to information healthier lifestyles during pregnancy, preventing adverse effects. Similar results of low maternal education level associated with adverse birth outcomes were reported in Quebec, Canada(Reference Luo, Wilkins and Kramer62), and in two meta-analysis(Reference Ruiz, Goldblatt and Morrison63,Reference Silvestrin, Da Silva and Hirakata64) . We understand the correlation between maternal education and maternofetal outcomes by the more likely disposition of women of higher education having a greater understanding of prenatal care, early initiation of prenatal care, more ANC consultations as prescribed and higher socioeconomic status(Reference Silvestrin, Da Silva and Hirakata64,Reference Haider and Bhutta65) . Contrary to our findings, maternal education among Mexican American had little effect on birth outcomes(Reference Gage, Fang and O'Neill66). They observed that the Mexican American presented the least infant mortality rate as compared with the other populations used(Reference Gage, Fang and O'Neill66).
Hunger and poverty presented significant associations with adverse birth outcomes. Hunger led to an increased likelihood of having adverse birth outcomes in the present study. Pregnant adolescents who experienced hunger and were poorer using HHS and LPI, respectively, presented significantly higher odds of giving birth to LBW and preterm babies than those who did not experience any hunger or deprivation. The possible reason for more adverse birth outcomes among pregnant adolescents who experienced poverty and hunger is inadequate maternal dietary intake and low nutritional quality diet, as these have been related to adverse birth outcomes(Reference Abu-Saad and Fraser11). During pregnancy, the intake of adequate dietary diversity diet offers the required micronutrients that are important for the growth and development of the fetus. However, our findings did not present a significant relationship between dietary diversity and adverse birth outcomes though a greater proportion of LBW babies were found among adolescents with inadequate dietary diversity as compared with those with adequate dietary diversity. Contrary to our findings, Quansah and Boateng(Reference Quansah and Boateng67) found inadequate dietary diversity to be associated with higher odds of LBW babies. Maternal nutritional status can have an impact on fetal development. The proper growth and development of the fetus requires the consumption of food items from the various food groups in order to prevent deficiencies of essential micronutrients. Thus, adequate dietary intake is critical in preventing adverse birth outcomes. In developing countries, poor nutritional status is a determining factor of LBW due to low nutrient flow to the developing fetus(Reference Biesalski, Black and Koletzko68). Experiencing hunger during pregnancy can be devastating and can be subsequently linked to poor nutritional status as the pregnancy period requires a significant increase in macronutrients and adequate micronutrients. According to a study on fetal response to satiation and hunger, 74 % of pregnant women experienced an increase in fetal activity when they were hungry. The paper further stated that women with increased fetal activity gave birth to smaller babies(Reference Bradford and Maude69). Studies have related poverty to adverse birth outcomes such as LBW, PTB and fetal loss(Reference Tanya Nagahawatte and Goldenberg17,Reference Aftab, Ainuddin and Kazi70) . Tanya(Reference Tanya Nagahawatte and Goldenberg17) further asserted that the poor might have less access to healthcare systems and maternal care during emergencies. A high risk of malnutrition and subsequent adverse effect on babies leading to nutritional and developmental deficiencies have also been related to poverty(Reference Black, Allen and Bhutta71).
Maternal morbidity was significantly associated with birth outcomes in the present study. The odds of having LBW and PTB among those who got ill during pregnancy were thrice more than those who did not experience any illness. This implies that the health of the mother can be a contributory factor to birth outcomes. According to Tinker(Reference Tinker20), maternal malnourishment and insufficient maternal care are high risks for the development of diseases and death during delivery. This is expected since maternal illness can affect fetal growth and development. Some of the conditions that these pregnant adolescents went through during pregnancy were pregnancy induced hypertension, antepartum haemorrhage and prolonged labour. These findings seem to be consistent with earlier research which reported that participants who experienced morbidity had about five to seven times increased risk of adverse birth outcomes(Reference Yeshialem, Alemnew and Abera21,Reference Chaibva72,Reference Sadiq Abubakar, Poggensee and Nguku73) .
Generally, the effect of geographical location, environmental conditions, socio-cultural practices, eating behaviour and maternal factors on birth outcomes have been mixed. For instance, in the present study, LBW was more prevalent among rural girls (17 %) than the urban participants (13⋅7 %), similar to a study conducted in Malaysia(Reference Kaur, Ng and Badon74), but contrary to other studies in Ghana(Reference Afriyie, Bedu-Addo and Asiamah36,Reference Abubakari, Kynast-Wolf and Jahn75,Reference Tampah-Naah, Anzagra and Yendaw76) , which reported a higher prevalence of LBW among urban than rural participants. A higher prevalence of LBW is expected in rural areas due to poorer socioeconomic conditions, food insecurity, low nutrition knowledge and limited access to health care(Reference Payghan, Kadam and Reddy77–Reference Tran, Nguyen and Nguyen79). In the present study, the prevalence of caesarean delivery, 11⋅8 %, was similar to studies conducted in Ghana(Reference Tampah-Naah, Anzagra and Yendaw76) but lower than two studies conducted in Bangladesh(Reference Hasan, Alam and Hossain80) and Pakistan(Reference Aftab, Ainuddin and Kazi70), which recorded 23⋅9 and 26⋅4 %, respectively. A greater proportion of caesarean deliveries in this study (16⋅4 %) were from the urban centres. This may seem to be due to urbanisation(Reference de Loenzien, Schantz and Luu81) and access to qualified personnel to carry out the section. Hasan(Reference Hasan, Alam and Hossain80) also presented similar findings where the rate of caesarean deliveries was relatively high in urban women than in rural women. Caesarean sections are less accessible among poor or low-income countries(Reference Ronsmans, Holtz and Stanton82). Generally, about 21 % of those who delivered through caesarean section gave birth to LBW babies. Literature suggests that caesarean sections are usually performed as emergencies without adequate preparations and can lead to a challenge in the newborn's proper initial development(83).
The overall nutritional status such as weight gain, health, parity, previous birth outcomes and biochemical status of the mother during pregnancy may affect birth outcomes. Some studies have presented an increased odds of obstetric, placental and medical complications with increasing parity(Reference Aliyu, Jolly and Ehiri84–Reference Eskenazi, Fenster and Jama86). Furthermore, poor socioeconomic status and its consequences, such as less access to health facilities and low levels of education have been associated with adverse birth outcomes(Reference Nordin, Fen and Isa87,Reference Ozumba and Igwegbe88) . Moreover low birth weight have been associated with pregnant women with chronic anaemia, diabetes and hypertension(Reference Aliyu, Jolly and Ehiri84,Reference Shah89) .
In summary, this study explored the predictors of adverse birth outcomes among pregnant adolescents, and among many factors found that hunger, poverty and lack of formal education predicted preterm births. Pregnant girls with no formal education had nine times increased odds for PTB compared with those with at least secondary education. Girls with high LPI had two and a half increased odds for PTB compared with those with no poverty, and those who experienced moderate hunger were about two and a half times increased odds for PTB compared with those with no hunger. Finally, those who were ill during pregnancy were thrice increased odds for PTB compared those who were not ill. For LBW, the significant predictors were similarly hunger, poverty and maternal morbity. The levels of the odds recorded for the predictors of LBW were simlar to that of the PTB. On the other hand, the use of ITN reduced the odds for LBW deliveries by about 60%.
Limitation
The study had a significant dropout as less than 75 % of baseline participants were followed up for the birth outcomes study. Future studies should be designed to reduce this, either by increasing the sample size or improve measures for tracking participants.
Conclusion
The risk of adverse birth outcomes observed among the pregnant adolescents were associated with ANC compliance and maternal socioeconomic factors. Strengthening antenatal services, promoting girl education, livelihood support for pregnant girls, and interventions to improve socio-economic status and to address hunger and poverty are broad and targeted interventions to both reduce the incidence of adolescent pregnancies, and reduce the risk for adverse birth outcomes among those who still become pregnant. Focused care to encourage attendance to ANC and improve compliance to ANC interventions by pregnant adolescents is strongly recommended. Food security and hunger should be evaluated during antenatal services to identify and address the vulnerable.
Acknowledgements
The authors thank the Health Directors at the health centres where the research was done, and most of all the pregnant adolescents who participated in the study.
The Nestle Foundation provided funding for this research. The funder had no role in the design, analysis or writing of this article.
Formulating the research questions: R. A. A., C. A., L. A. G., H. E. L., W. A. and A. K. E.; designing the study: R. A. A., C. A., H. E. L., L. N., W. A. and A. K. E.; carrying it out: R. A. A., C. A., L. A. G., L. N., H. E. L., W. A. and O. A.-B.; analysing the data: R. A. A., L. A. G., O. A.-B. and C. A.; writing the article: R. A. A., L. A. G., C. A., H. E. L., W. A. and A. K. E.
The authors declare that they have no conflicts of interest.








