Introduction
Palmer amaranth is a summer annual broadleaf weed belonging to the Amaranthaceae family and is one of dioecious species among pigweeds (Steckel Reference Steckel2007). Human activities in the 20th century such as agricultural development, within- and between-field operations, and seed and equipment transportation have led Palmer amaranth to spread to the northern United States (Culpepper Reference Culpepper2006). Since the first report of Palmer amaranth in Virginia in 1915 beyond its native habitat in the southwest United States, it has become one of the most problematic and troublesome weeds in agronomic cropping systems in the United States (Culpepper et al. Reference Culpepper, Webster, Sosnoskie and York2010). Being dioecious, Palmer amaranth is an obligate outcrossing, wild pollinated species (Sosnoskie et al. Reference Sosnoskie, Webster, Kichler, MacRae, Grey and Culpepper2012), resulting in wide genetic diversity (Jhala et al. Reference Jhala, Norsworthy, Ganie, Sosnoskie, Beckie, Mallory-Smith, Liu, Wei, Wang and Stoltenberg2021; Oliveira et al. Reference Oliveira, Gaines, Patterson, Jhala, Irmak, Keenan and Knezevic2018). High photosynthetic rate along with diaheliotropic movement (i.e., leaves orienting themselves perpendicular to incoming sunlight to intercept radiant energy and light) allow Palmer amaranth to fix carbon at higher rate, resulting in rapid growth (Ehleringer and Forseth Reference Ehleringer and Forseth1980; Ehleringer Reference Ehleringer1985). In a 2-yr field study in Kansas, Horak and Loughin (Reference Horak and Loughin2000) reported that Palmer amaranth had the highest plant dry weight, leaf area, water-use efficiency, and growth rate (0.10 to 0.21 cm per growing degree day) compared to redroot pigweed (Amaranthus retroflexus L.), tumble pigweed (Amaranthus albus L.), and waterhemp [Amaranthus tuberculatus (Moq.) Sauer].
Depending on environmental conditions, Palmer amaranth typically flowers during September and October, although decreasing day length can accelerate the flowering process (Bond and Oliver Reference Bond and Oliver2006). Female Palmer amaranth plants are prolific seed producers, even under competition with agronomic crops (Massinga et al. Reference Massinga, Currie, Horak and Boyer2001). Seeds are usually dispersed by gravity forces; however, dispersal via irrigation, plowing, mowing, harvesting, birds, and mammals has been documented (Costea et al. Reference Costea, Weaver and Tardif2004, Reference Costea, Weaver and Tardif2005). Because Palmer amaranth’s prolific seed production and aggressive growth habit make it difficult to control in agronomic cropping systems (Horak and Loughin Reference Horak and Loughin2000; Ward et al. Reference Ward, Webster and Steckel2013), it is vital to control Palmer amaranth early in the growing season by integrating mechanical, cultural, and chemical practices, including PRE herbicides with multiple sites of action (SOAs; de Sanctis et al. Reference de Sanctis, Barnes, Knezevic, Kumar and Jhala2021; Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012).
Globally, glyphosate is the most widely used agricultural pesticide and is used extensively in glyphosate-resistant (GR) canola (Brassica napus L.), corn (Zea mays L.), cotton (Gossypium hirsutum L.), sugarbeet (Beta vulgaris var. saccharifera), and soybean in the United States (Heap and Duke Reference Heap and Duke2018). Since the commercialization of GR crops, particularly GR corn and soybean in the midwestern United States and GR cotton in the southern United States, continuous use of glyphosate multiple times in a year, along with a decline in the use of residual herbicides (Culpepper Reference Culpepper2006; Young Reference Young2006), has resulted in the evolution of GR weeds (Beckie Reference Beckie2006). As of 2020, 50 weeds have been confirmed resistant to glyphosate worldwide (Heap Reference Heap2021), including six broadleaf weeds such as common ragweed (Ambrosia artemisiifolia L.), giant ragweed (Ambrosia trifida L.), kochia [Bassia scoparia (L.) A. J. Scott], horseweed (Erigeron canadensis L.), waterhemp, and Palmer amaranth in Nebraska (Jhala Reference Jhala2017a).
Glyphosate-resistant Palmer amaranth was first confirmed in Georgia in 2004 (Culpepper et al. Reference Culpepper, Grey, Vencill, Kichler, Webster, Brown, York, Davis and Hanna2006), and since then has been confirmed in 28 states in the United States (Heap Reference Heap2021). Palmer amaranth has evolved resistance to herbicides from at least eight SOA groups: microtubule-, acetolactate synthase (ALS)–, 5-enolpyruvyl-shikimate-3-phosphate synthase (EPSPS)–, photosystem II (PS II)–, hydroxyphenylpyruvate dioxygenase (HPPD)–, very long chain fatty acid (VLCFA)–, protoporphyrinogen oxidase (PPO)–, and synthetic auxin inhibitors (Heap Reference Heap2021). Palmer amaranth resistant to glufosinate was recently confirmed in Arkansas (Barber et al. Reference Barber, Norsworthy and Butts2021). Some populations of Palmer amaranth have also been found to have resistance to multiple herbicides in a few states, including ALS inhibitor/glyphosate resistance in Nebraska (Chahal et al. Reference Chahal, Varanasi, Jugulam and Jhala2017), atrazine/HPPD inhibitor resistance in Nebraska (Jhala et al. Reference Jhala, Sandell, Rana, Kruger and Knezevic2014), and 2,4-D/ALS inhibitor/atrazine/glyphosate/HPPD inhibitor resistance in Kansas (Kumar et al. Reference Kumar, Liu, Boyer and Stahlman2019).
Palmer amaranth resistance to ALS inhibitors was first confirmed in Kansas in 1994 and since then has been confirmed in 14 states (Heap Reference Heap2021; Sprague et al. Reference Sprague, Stoller, Wax and Horak1997). Isoxaflutole, an HPPD-inhibiting PRE corn herbicide, has been available commercially since 1998 (Spaunhorst and Johnson Reference Spaunhorst and Johnson2016). The recently available isoxaflutole/glufosinate/glyphosate-resistant soybean (LibertyLink GT27TM) provides an opportunity to use isoxaflutole applied PRE alone or in mixture with other residual herbicides for early-season weed control. Glufosinate is a contact, POST herbicide for control of emerged broadleaf and grass weeds (Jhala et al. Reference Jhala, Ramirez and Singh2013). It is a nonselective herbicide traditionally used to control weeds in fruit and nut orchards and non-crop areas (Jhala et al. Reference Jhala, Ramirez and Singh2013). Norsworthy et al. (Reference Norsworthy, Griffith, Scott, Smith and Oliver2008) reported 99% control of GR Palmer amaranth with glufosinate. Hoffner et al. (Reference Hoffner, Jordan, Chandi, York, Dunphy and Everman2012) found that glufosinate applied early-POST (EPOST) controlled Palmer amaranth by 73% compared to 76% control with glufosinate applied EPOST followed by a late-POST (LOST). Wiesbrook et al. (Reference Wiesbrook, Johnson, Hart, Bradley and Wax2001) found that glufosinate applied sequentially improved control of broadleaf weeds over a single application. Glufosinate applied EPOST resulted in 71% control, and a sequential LPOST application provided 76% control of GR waterhemp in glufosinate-resistant soybean in Nebraska (Jhala et al. Reference Jhala, Sandell, Sarangi, Kruger and Knezevic2017). An additional option for POST control of GR Palmer amaranth in glufosinate-resistant soybean is glufosinate mixed with residual herbicides such as acetochlor, pyroxasulfone, or S-metolachlor (Aulakh and Jhala Reference Aulakh and Jhala2015). This mixture provides foliar and residual control of Palmer amaranth through overlapping residual activity.
ALS inhibitor and/or GR Palmer amaranth has been observed in several corn/soybean production fields in south-central and west-central Nebraska, in addition to alfalfa (Medicago sativa L.), corn, and sugarbeet fields in western Nebraska (Vieira et al. Reference Vieira, Samuelson, Alves, Gaines, Werle and Kruger2018). To address the growing need to control GR weeds in cropping systems, multiple herbicide–resistant soybean traits have been developed. For example, isoxaflutole/glufosinate/glyphosate-resistant soybean has been developed to provide an additional herbicide SOA for control of herbicide-resistant weeds, primarily GR weeds; however, herbicide programs need to be developed and tested that provide season-long control of GR Palmer amaranth in this multiple herbicide-resistant soybean. The objectives of this research were to 1) evaluate isoxaflutole- and glufosinate-based herbicide programs for control of ALS inhibitor and GR Palmer amaranth in isoxaflutole/glufosinate/glyphosate-resistant soybean; and 2) evaluate the effect of herbicide programs on Palmer amaranth density and biomass, as well as soybean injury and grain yield.
Materials and Methods
Field Experiments
Field experiments were conducted in 2018 and 2019 in a grower’s field near Carleton, NE (40.30°N, 97.67°W). The field had a GR corn-soybean rotation with reliance on glyphosate for weed control in a no-till production system for the last 10 yr and confirmed to have an ALS inhibitor and GR Palmer amaranth (Chahal et al. Reference Chahal, Varanasi, Jugulam and Jhala2017). Hereafter, we refer to this as multiple herbicide-resistant (MHR) Palmer amaranth. The soil at the experimental site was silt loam (montmorillonitic, mesic, Pachic Argiustolls), pH 6.0; and 19% sand, 63% silt, 18% clay, and 2.6% organic matter content. Winter annual weeds were controlled with glyphosate at 900 g ae ha−1 + 2,4-D ester at 560 g ae ha−1 + liquid ammonium sulfate 3% vol/vol 2 wk prior to establishing an experiment. A soybean cultivar resistant to isoxaflutole/glufosinate/glyphosate was planted in a no-till seedbed at 345,800 seeds ha−1 in rows spaced 76 cm apart. Soybean was planted on May 10, 2018, and May 6, 2019. Individual experimental plot dimensions were 3 m wide by 9 m long. The experimental site was in a rainfed environment with no supplemental irrigation. The precipitation received during both years during crop growing season are listed (Table 1).
Table 1. Monthly mean air temperature and total precipitation during the 2018 and 2019 growing seasons (March to October), along with the 30-yr average at the research site near Carleton, Nebraska. a
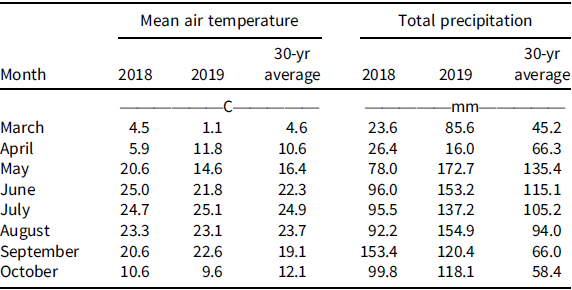
a Data were obtained from National Oceanic and Atmospheric Administration (NOAA 2019).
Treatments were arranged in a randomized complete block design with four replications. Herbicide programs evaluated to control MHR Palmer amaranth consisted of PRE, EPOST, LPOST, and/or PRE fb POST herbicide programs (Table 2). A nontreated control was included for comparison. Herbicides were applied with a handheld CO2-pressurized backpack sprayer equipped with AIXR 110015 flat-fan nozzles (TeeJet® Technologies, Wheaton, IL) calibrated to deliver a 140 L ha−1 flow rate at 276 kPa at a constant speed of 4.8 km h−1. Glufosinate was mixed with liquid ammonium sulfate at 3% vol/vol (Anonymous 2017) and was applied with XR 11005 flat-fan nozzles (TeeJet® Technologies). The PRE herbicides were applied after soybean planting on the same day (i.e., May 10) in 2018, and 4 d after soybean planting (i.e., May 10) in 2019. The EPOST herbicides were applied 31 d after PRE (DAPRE) herbicides were applied. Palmer amaranth was 1 to 8 cm tall depending on herbicide program. Soybean was at the first to second trifoliate (V1 to V2 growth stage). The LPOST herbicides were applied 20 to 22 DAEPOST herbicide application. Palmer amaranth was 8 to 25 cm tall depending on the herbicide program. Palmer amaranth plant height was variable because new plants had emerged and some plants had been partially controlled by the EPOST herbicide.
Table 2. Herbicides, application timings, and rates used for control of acetolactate synthase inhibitor and glyphosate-resistant Palmer amaranth in isoxaflutole/glufosinate/glyphosate–resistant soybean in field experiments conducted in 2018 and 2019.
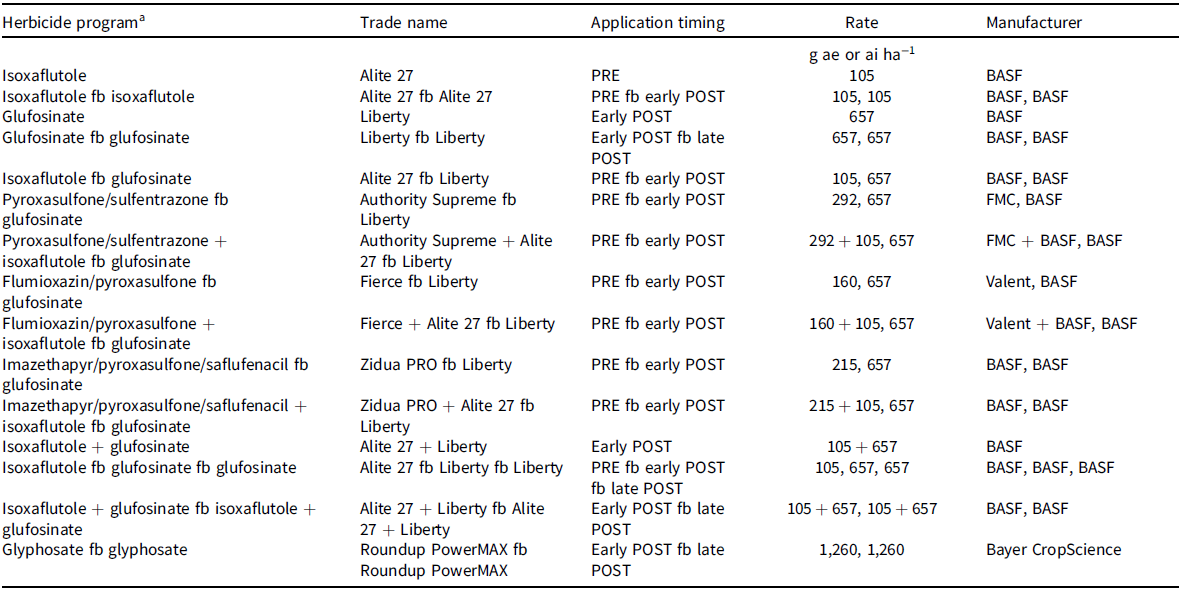
a Glufosinate was mixed with ammonium sulfate (DSM Chemicals North America Inc., Augusta, GA) at 4.2 kg ha−1.
Data Collection
Palmer amaranth control was assessed visually at 21 DAPRE, 14 DAEPOST, and 14 and 28 DALPOST herbicide applications on a scale of 0% to 100% (0% indicating no control of Palmer amaranth and 100% indicating complete control). Palmer amaranth densities were recorded 21 DAPRE, 14 DAEPOST, 14 DALPOST, and 28 DALPOST by counting the number of Palmer amaranth plants in one 0.5-m2 quadrat placed randomly between two center soybean rows in each plot. Soybean injury was assessed visually at 14 DAPRE, 14 DAEPOST, 14 and 28 DALPOST herbicide applications based on a scale of 0% to 100% (0% indicating no soybean injury and 100% indicating complete plant death). Palmer amaranth plants counted during density ratings were clipped at the soil surface, placed into paper bags, then placed in an oven at 65 C until they reached a constant weight. Aboveground biomass was converted into percent biomass reduction and was compared with the nontreated control using the following equation (Wortman Reference Wortman2014):
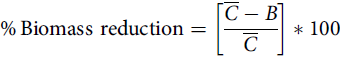 $${\rm{\% }}\,{\rm{Biomass}}\ {\rm{reduction}} = \left[ {{{\overline C - B} \over {\overline C}}} \right]*100$$
$${\rm{\% }}\,{\rm{Biomass}}\ {\rm{reduction}} = \left[ {{{\overline C - B} \over {\overline C}}} \right]*100$$
where
![]() $$\overline C$$
is the biomass of the nontreated control and B is the biomass of an individual treatment plot. Soybean was harvested from the center two rows in each plot using a plot combine. Grain yield was adjusted to 13% moisture content and converted into kilograms per hectare.
$$\overline C$$
is the biomass of the nontreated control and B is the biomass of an individual treatment plot. Soybean was harvested from the center two rows in each plot using a plot combine. Grain yield was adjusted to 13% moisture content and converted into kilograms per hectare.
Statistical Analysis
Data were subjected to ANOVA using the MIXED procedure in SAS version 9.3 (SAS Institute Inc, Cary, NC). Data were tested for normality with the use of UNIVARIATE procedure. Palmer amaranth control, density, and biomass data were arcsine square root–transformed before analysis; however, back-transformed data are presented with the mean separation based on the transformed data. Year and herbicide treatments were considered fixed effects, while replication was considered a random effect in the model. If year-by-treatment was nonsignificant, data from both years were combined. However, if the year-by-treatment interaction was significant, data were analyzed separately by year. Where the ANOVA indicated treatment effects were significant, means were separated at P ≤ 0.05 using Tukey Kramer’s pairwise comparison test.
Results and Discussion
Year-by-treatment interaction for MHR Palmer amaranth control 21 DAPRE was not significant (P > 0.05); therefore, data were combined for both years, while Palmer amaranth control estimates 14 DAEPOST and 28 DALPOST, Palmer amaranth density, and soybean yield were significant (P < 0.05); therefore, data were presented separately for both years. No soybean injury was observed from any herbicide program (data not shown), indicating that the herbicides evaluated in this study are safe to use in isoxaflutole/glufosinate/glyphosate–resistant soybean when applied according to label instructions. Schultz et al. (Reference Schultz, Weber, Allen and Bradley2015) also reported that isoxaflutole is safe to use in isoxaflutole-resistant soybean.
Temperature and Precipitation
The 2018 growing season started off warmer than average, with temperatures of 20.6 and 25.0 C for May and June, respectively, compared with 14.8 C and 21.8 C in 2019 (Table 1). Monthly precipitation varied from the 30-yr average in both years. Below-average precipitation occurred in 2018, with 78 and 96 mm in May and June, respectively, compared with the 30-yr average of 135 and 115 mm, whereas above-average precipitation was observed throughout the 2019 growing season (Table 1).
Palmer Amaranth Control
The PRE herbicides evaluated in this study controlled MHR Palmer amaranth by 86% to 99% 21 DAPRE (Table 3). Although statistically similar with other PRE herbicides, pyroxasulfone/sulfentrazone, flumioxazin/pyroxasulfone, and imazethapyr/pyroxasulfone/saflufenacil controlled Palmer amaranth by 97% to 99%. The contribution of the ALS-inhibiting herbicide (i.e., imazethapyr) was minimal; rather, the VLCFA inhibitor (i.e., pyroxasulfone) and PPO-inhibitor (i.e., saflufenacil) primarily contributed to the control. Shyam et al. (Reference Shyam, Chahal, Jhala and Jugulam2021) reported similar findings 14 DAPRE with imazethapyr/pyroxasulfone/saflufenacil, when Palmer amaranth control ranged from 87% to 97% in a 2-yr study in 2,4-D choline/glufosinate/glyphosate-resistant soybean. Sarangi and Jhala (Reference Sarangi and Jhala2019) reported at least 98% Palmer amaranth control 14 and 28 DAPRE with imazethapyr/dimethenamid-P/saflufenacil and flumioxazin/pyroxasulfone. Isoxaflutole applied PRE controlled Palmer amaranth by 86% to 89% 21 DAPRE (Table 3); however, variable control of Palmer amaranth has been reported with isoxaflutole in the literature. Meyer et al. (Reference Meyer, Norsworthy, Young, Steckel, Bradley, Johnson, Loux, Davis, Kruger, Bararpour, Ikley, Spaunhorst and Butts2016) and Johnson et al. (Reference Johnson, Chahal and Regehr2012) reported at least 87% Palmer amaranth control with isoxaflutole 28 DAPRE. In contrast, Spaunhorst and Johnson (Reference Spaunhorst and Johnson2016) reported GR Palmer amaranth control of 57% to 70% 21 DAPRE. The higher control occurred in a higher rainfall year, indicating the importance of moisture for herbicide activation (Spaunhorst and Johnson Reference Spaunhorst and Johnson2016). Isoxaflutole requires 12.7 to 25.4 mm of irrigation or rain to activate, although too much water can cause the herbicide to become diluted and leach, thus reducing its efficacy (Jhala Reference Jhala2017b). If moisture is adequate, isoxaflutole can provide 14 to 21 d of residual activity for Palmer amaranth control (Chahal et al. Reference Chahal, Aulakh, Jugulam and Jhala2015).
Table 3. Effect of herbicide programs on acetolactate synthase inhibitor and glyphosate-resistant Palmer amaranth control in isoxaflutole/glufosinate/glyphosate-resistant soybean 21 d after PRE, 14 d after early-POST, and 28 d after late-POST herbicide application in field experiments conducted in 2018 and 2019. a, b, c

a Year-by-treatment interaction for Palmer amaranth control 14 DAPRE was not significant; therefore, data were combined across years.
b Year-by-treatment interaction for Palmer amaranth control 14 DAEPOST and 28 DALPOST was significant; therefore, data are presented separately for both years.
c Abbreviations: DAEPRE, days after PRE herbicide application, DAEPOST, days after early-POST herbicide application; DALPOST, days after late-POST herbicide application; fb, followed by.
d Means presented within each column with no common letter(s) are significantly different according to Fisher’s protected LSD test at P ≤ 0.05.
e POST herbicides were not applied at the time of evaluation 21 DAPRE.
Palmer amaranth control varied between years with a PRE fb EPOST herbicide programs (Table 3). Glufosinate applied alone controlled MHR Palmer amaranth 95% to 96% in 2018 and 75% in 2019. Glufosinate mixed with isoxaflutole controlled Palmer amaranth 92% to 95% in 2018 and 85% to 94% in 2019 (Table 3). Shyam et al. (Reference Shyam, Chahal, Jhala and Jugulam2021) reported 88% Palmer amaranth control 14 DAEPOST with glufosinate. Conversely, Chahal and Jhala (Reference Chahal and Jhala2015) found that glufosinate in single and sequential applications provided 53% to 76% and 56% to 77% waterhemp control, respectively. Sequential glyphosate applications provided no control of MHR Palmer amaranth in this study, indicating that the population is highly resistant to glyphosate (Table 3). Chahal et al. (Reference Chahal, Varanasi, Jugulam and Jhala2017) reported 37-fold to 40-fold level of glyphosate resistance in MHR Palmer amaranth at this research site; therefore, no control with glyphosate was expected.
At 28 DALPOST, isoxaflutole applied PRE or in sequential applications (PRE fb EPOST) controlled MHR Palmer amaranth by 10% and 53% in 2018, respectively, while providing no control in 2019 (Table 3). This indicates that isoxaflutole applied alone at 105 g ai ha−1 will not provide effective control later in the growing season and that mixture with other herbicide(s) is needed to achieve economically acceptable control. In this study isoxaflutole was applied at 105 g ai ha−1; however, it can be applied in a range of 140 to 210 g ai ha−1 in a single application with a season maximum of 210 g ai ha−1 (Anonymous 2020). Relatively lower use rate in this study is because the study was conducted before isoxaflutole label approved in 2020. In addition, isoxaflutole is primarily a residual herbicide with limited foliar activity; therefore, effective control of emerged Palmer amaranth at the time of application should not be expected. Janak and Grichar (Reference Janak and Grichar2016) reported similar findings of 51% Palmer amaranth control with a single application of isoxaflutole 101 DAPRE. When mixed with metribuzin, isoxaflutole has been shown to provide 97% to 98% control of redroot pigweed and Powell amaranth (Amaranthus powellii S. Watson; Smith et al. Reference Smith, Soltani, Kaastra, Hooker, Robinson and Sikkema2019). With the exception of isoxaflutole, PRE fb POST herbicide programs provided 80% to 99% MHR Palmer amaranth control in 2018 and 78% to 99% control in 2019 at 28 DALPOST (Table 3). Whitaker et al. (Reference Whitaker, York, Jordan and Culpepper2010) reported greater than 80% late-season control of GR Palmer amaranth with flumioxazin/S-metolachlor applied PRE fb fomesafen, although less than 30% late-season control was achieved with flumioxazin/S-metolachlor without fomesafen applied POST. A single herbicide application is less likely to provide a season-long control of Palmer amaranth and a PRE followed by a POST herbicide program is required for effective control and reducing Palmer amaranth seedbank (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012).
Palmer Amaranth Density and Biomass
Palmer amaranth density and biomass were affected by herbicide programs (Table 4). At 14 DAEPOST isoxaflutole reduced MHR Palmer amaranth density 0% and 48% in 2018 and 2019, respectively, whereas isoxaflutole applied PRE fb EPOST reduced density 49% and 53% in 2018 and 2019, respectively. Similarly, Meyer et al. (Reference Meyer, Norsworthy, Young, Steckel, Bradley, Johnson, Loux, Davis, Kruger, Bararpour, Ikley, Spaunhorst and Butts2016) reported 62% Palmer amaranth density reduction with isoxaflutole applied PRE. Meyer et al. (Reference Meyer, Norsworthy, Young, Steckel, Bradley, Johnson, Loux, Davis, Kruger, Bararpour, Ikley, Spaunhorst and Butts2015) reported 78% to 93% Palmer amaranth density reduction with flumioxazin/pyroxasulfone in soybean in a multiyear, multistate study, while Sarangi et al. (Reference Sarangi, Sandell, Kruger, Knezevic, Irmak and Jhala2017) reported 91% and 98% density reduction of GR waterhemp with flumioxazin/pyroxasulfone and imazethapyr/dimethenamid-P/saflufenacil, respectively.
Table 4. Effect of herbicide programs on glyphosate-resistant Palmer amaranth density reduction and biomass reduction and isoxaflutole/glufosinate/glyphosate-resistant soybean yield in field experiments conducted in 2018 and 2019. a

a Abbreviations: DAEPOST, days after early-POST herbicide application; DALPOST, days after late-POST herbicide application; fb, followed by.
b Year-by-treatment interaction for glyphosate-resistant Palmer amaranth density and soybean yield were significant; therefore, data were not combined across the two years.
c Biomass reduction data is only available for 2019.
d Means presented within each column with no common letter(s) are significantly different according to Fisher’s protected least significant difference test at P ≤ 0.05.
Herbicides applied PRE fb glufosinate reduced MHR Palmer amaranth density by at least 87% in 2018 and 2019. Similar findings were reported by Shyam et al. (Reference Shyam, Chahal, Jhala and Jugulam2021) and Norsworthy et al. (Reference Norsworthy, Korres, Walsh and Powles2016). Glufosinate applied alone reduced MHR Palmer amaranth density 89% and 58% in 2018 and 2019, respectively, whereas glufosinate mixed with isoxaflutole reduced density by 63% to 100% in 2018, and 85% to 94% in 2019 (Table 4). Chahal and Jhala (Reference Chahal and Jhala2015) reported 50% waterhemp density reduction with glufosinate applied EPOST; and 83% density reduction with glufosinate applied EPOST fb LPOST 45 DALPOST in glufosinate-resistant soybean in Nebraska.
At 14 DALPOST in 2019, PRE herbicide fb glufosinate applied EPOST reduced MHR Palmer amaranth biomass by 49% to 97% compared to 95% biomass reduction with glufosinate applied LPOST (Table 4). Aulakh and Jhala (Reference Aulakh and Jhala2015) reported 79% to 88% weed biomass reduction with dimethenamid-P/saflufenacil, or imazethapyr/sulfentrazone fb glufosinate. Shyam et al. (Reference Shyam, Chahal, Jhala and Jugulam2021) reported 100% Palmer amaranth biomass reduction with imazethapyr/pyroxasulfone/saflufenacil fb glufosinate and 99% biomass with glufosinate applied EPOST followed by LPOST in 2,4-D choline/glufosinate/glyphosate–resistant soybean. Single or sequential applications of isoxaflutole resulted in no biomass reduction due to poor Palmer amaranth control (Table 4). Chahal and Jhala (Reference Chahal and Jhala2015) reported 80% to 91% and 92% to 95% biomass reduction with glufosinate applied in single and sequential applications, respectively, in glufosinate-resistant soybean. Thus, a PRE herbicide with multiple SOAs fb glufosinate has consistently provided >90% control of Palmer amaranth and >90% density and biomass reduction in most studies.
Soybean Yield
Year-by-treatment interaction was significant (P < 0.05); therefore, yield data are presented separately for both years (Table 4). Soybean yield in 2019 was higher compared to 2018 due to higher precipitation in 2019 that provided sufficient moisture for soybean growth and development (Table 1). Isoxaflutole mixed with pyroxasulfone/sulfentrazone applied PRE fb glufosinate had soybean grain yield of 2,290 kg ha−1 in 2018, and it was comparable with several herbicide programs (Table 4). In 2019, several herbicide programs resulted in similar soybean yield in the range of 3,140 to 4,282 kg ha−1 (Table 4). Shyam et al. (Reference Shyam, Chahal, Jhala and Jugulam2021) reported soybean yields with similar PRE herbicides used in combination with glufosinate.
Practical Implications
A new soybean trait resistant to isoxaflutole/glufosinate/glyphosate has been available commercially since the 2019 growing season in the United States. Results of this study suggest that herbicide programs are available for effective control of MHR Palmer amaranth in isoxaflutole/glufosinate/glyphosate–resistant soybean. No soybean injury was observed with any of the herbicide programs evaluated in this study, including isoxaflutole applied in sequential applications. Isoxaflutole (Alite™ 27) was registered in 2020 for application in isoxaflutole-resistant soybean; however, use of this herbicide is limited to certain counties in a few states. For example, isoxaflutole (Alite™ 27) is labeled for application in only four southwest counties (Chase, Dundy, Hitchcock, and Red Willow) in Nebraska (Anonymous 2020). In addition, isoxaflutole cannot be applied on coarse-textured soils (e.g., sandy, sandy loam, loamy sand) with less than 1.5% organic matter content, limiting the use of this herbicide. The majority of soybean in Nebraska is grown in eastern Nebraska, so although growers can plant isoxaflutole/glufosinate/glyphosate–resistant soybean in this region, they cannot use isoxaflutole (Alite™ 27) due to label restriction (Anonymous 2020). Therefore, adoption of soybean resistant to isoxaflutole/glufosinate/glyphosate in Nebraska will likely be very limited.
Acknowledgments
We thank Irvin Schleufer, Will Neels, Jose H. Sanctis, and Adam Leise for their assistance with the project and Ian Rogers for editing the manuscript. This project was partially supported by the Nebraska Agricultural Experiment Station with funding from the Hatch Act through the U.S. Department of Agriculture–National Institute of Food and Agriculture Project # NEB-22-396. This project was also supported by the Nebraska Soybean Board. No conflicts of interest have been declared.







