INTRODUCTION
On 8 April 2010, the manager of a residential aged care facility in a south-western suburb of Sydney, Australia notified the Sydney South West Public Health Unit (SSW PHU) of an outbreak of gastroenteritis. Under the New South Wales Public Health Act (1991), aged care facilities are required to notify the local PHU of gastroenteritis in two or more people of any age who have an onset within 24 h of each other [1]. Initially, the manager reported gastroenteritis in 6/109 residents, with the first onset on 6 April. In the ensuing days, 26 further residents and three staff members developed gastrointestinal symptoms, and stool specimens isolated Salmonella enterica serotype Infantis as the causative organism.
In Australia, an estimated 17·2 million cases of infectious gastroenteritis occur annually, of which 32% are considered foodborne [Reference Hall2]. Although only 7% of all foodborne gastroenteritis outbreaks in Australia occur in aged care facilities [3], they are cause for concern due to the greater number of hospitalizations and deaths seen in this vulnerable population compared to the general population [Reference Kirk, Roberts and Horvath4, Reference Kirk5]. Most outbreaks of infectious gastroenteritis in aged care facilities in Australia are caused by norovirus infections, and Salmonella spp. is an unusual agent in these settings [Reference Kirk5].
Salmonella spp. are bacteria transmitted by eating contaminated food, drinking contaminated water, contact with infected animals, or contact with an infected person who has diarrhoea. It is the most common pathogen isolated in foodborne disease outbreaks, accounting for one third of outbreaks in Australia [3]. Disease caused by Salmonella spp. typically presents as acute gastroenteritis, and the organism has an incubation period of 6–72 h, sometimes longer [Reference Behravesh, Lynch, Schlundt and Heymann6]. Salmonella Infantis has previously been found in pigs, poultry, chicken eggs, cattle and animal feed, and although it is not common, has been implicated in an increasing number of human infections in New South Wales since the beginning of December 2009 [Reference Kardamanidis7].
Here we report on our investigation of the outbreak of salmonellosis that identified a novel vehicle of transmission.
METHODS
Facility
The aged care facility was licensed to care for a maximum of 112 residents. It was divided into three wards on a single level, separated by locked doors. Two of these were for residents requiring high-level care: High-care North (HCN) which consisted of 28 beds and catered to those with dementia, and a single split ward with 44 beds, the two sections being known as High-care East (HCE) and High-care West (HCW). In these wards the residents were accommodated in single or double rooms. The third ward catered to residents requiring low-level care (LLC), and consisted of 40 single rooms. At the time of the outbreak, there were 109 residents of the facility and 127 staff members. Seventy residents lived in the high-level care areas, including the dementia ward. Of these, 57 were women and 13 were men, with ages ranging from 63 to 100 years. There were 22 residents in HCE, 21 in HCW, and 27 in HCN. There were 39 residents in the LLC area.
Epidemiological investigation
We maintained daily contact with the aged care facility throughout the outbreak and were updated on new symptomatic cases through updated line listings of residents. Nursing staff at the facility reported cases of diarrhoea, took stool specimens from affected residents and reported symptoms other than diarrhoea. We undertook a retrospective cohort study of residents; because all cases with positive cultures were confined to the high-level care areas, we restricted our study to these areas. We defined a case as any resident in a high-care area with a stool culture positive for S. Infantis between 6 and 30 April 2010, regardless of symptoms.
We did not interview residents of the high-level care areas because many were cognitively impaired. We interviewed the facility manager about possible exposures and methods of food preparation and distribution, and obtained the set resident menus for the period from 29 March to 11 April. Accurate individual foods consumed could not be assigned as residents had the option of having alternative meals to those on the set menus, and documentation of their choices had been discarded by staff, as was routine at this facility. Facility management staff provided information regarding all residents, including basic demographic data, room and bed number, symptom history, diet type (full diet, soft diet, puréed diet or enteral feeds), whether they were on thickened fluids, whether patients had been reviewed and whether they had stool specimens collected. The set resident menu was compared with onset of illness for cases to determine any correlation between meals, diet type, and high-risk foods. Reports of ill staff were obtained from the facility manager. All symptomatic staff members were asked by the facility manager to submit stool specimens.
Statistical methods
Data were entered into a Microsoft® Excel spreadsheet and analysed using Stata® version 10.0 (Stata Corporation, USA). Exposures were expressed as dichotomous variables, and crude relative risks (RR) with 95% confidence intervals (CI) were calculated. A logistic regression model was fitted to the study data with an outcome variable of confirmed cases, and adjusted odds ratios were calculated with 95% confidence intervals. Exposure variables were included in the model if they showed an independent statistical association (P < 0·05) with the dependent variable during univariate analysis. Stratified analysis was also conducted to further explore possible confounding.
Environmental investigation
Inspectors from the NSW Food Authority, the State government food regulation agency, commenced an onsite investigation of the facility on 13 April. Facility staff were interviewed to assess food-handling practices and hygiene. Preparation of meals and dietary supplements were reviewed to identify opportunities for cross-contamination. Food samples and environmental swabs were collected for Salmonella testing. Food samples consisted of raw meats (chicken mince, marinated chicken breast, marinated pork, beef mince) from the facility's cold store, whole eggs, and leftover chocolate confectionery that was served to residents prior to onset of illness. Other than chocolate, there were no residual foods or ingredients available for testing that would have been consumed by residents of the aged care facility during the incubation periods. Environmental swabs were collected from food blending equipment, chopping boards and a meat slicer. Food samples were handled aseptically and placed into sterile 500 ml containers. Environmental swabs were obtained using sterile sponge swabs moistened with neutralizing buffer solution. All food and swab samples were sealed and placed under refrigeration immediately after sampling in the facility's cold store. Samples were kept under refrigeration during transport to the laboratory using a car fridge.
Public health personnel visited the facility on 16 April and conducted an inspection of the premises. They examined the facility's general sanitary conditions and interviewed nursing and kitchen staff about infection control practices. All symptomatic staff members were asked by the facility manager to submit stool specimens. Asymptomatic kitchen staff were also requested to submit stool specimens for culture.
On 20 April the Food Authority revisited and obtained residual thickened fluids powder and environmental swabs of equipment used to prepare and dispense this product for laboratory analysis.
Laboratory investigation
Stool samples were sent for microscopy and culture to private laboratories used by medical practitioners caring for patients at the aged care facility. Stool samples were also tested for norovirus. Salmonella isolates were forwarded to the NSW Enteric Reference Laboratory where they were serotyped by standard methods [Reference Grimont and Weill8]. Food and environmental samples were sent to the Division of Analytic Laboratories for bacterial culture and serotyping. A subset of four clinical Salmonella isolates and one food Salmonella isolate were sent to the Microbiological Diagnositics Unit at Melbourne University for pulsed-field gel electrophoresis (PFGE). PFGE was conducted using standardized methods proposed by the PulseNet collaboration [Reference Ribot9]. Electrophoresis was performed at 6 V/cm and 14 °C. The run time was 22 h with pulse time ramping 5–40 seconds. The PFGE patterns obtained were visually assessed and interpreted according to guidelines proposed by Tenover et al. [Reference Tenover10].
RESULTS
Epidemiological investigation
In total, 32 (29%) residents of the facility were identified by nursing staff as having symptoms of diarrhoea and/or vomiting; 29 of those with symptoms were from high-care areas. Twenty-two residents (all from high-care areas) had stool cultures positive for S. Infantis and were classified as confirmed cases. Dates of symptom onset ranged from 6 to 17 April, the majority of these being reported in the first week (Fig. 1). Cases in the first 3 days were largely reported from the dementia ward. Of the seven symptomatic residents in high care without positive stool cultures, two were reported to have had diarrhoea and a fever and five were reported to have had an episode of diarrhoea only. Among the 22 confirmed cases identified, 21 were reported to have had diarrhoea, two vomiting, and one was reported to have had a temperature >38 °C. Two confirmed cases died on 10 April including one of the five cases requiring hospitalization. Further analysis was restricted to those 70 residents in the high-level care areas.

Fig. 1. Cases of S. Infantis by date of symptom onset and ward.
Residents were either on full, soft, puréed, or enteral diets, depending on their swallowing ability. Those on a full diet were able to eat food of all textures, while those on a puréed diet required all their food to be puréed in a food blender; any meats these two groups consumed were prepared separately and came from different batches of meat. Those on a soft diet generally consumed the same meats as the puréed diet with the remainder of their meal coming from the full diet. These three diets were composed of the same breakfast and dessert items. Those on an enteral diet were entirely unable to swallow and were fed via a feeding tube; their feeds were medically prescribed and did not include the foods in the other diets.
According to the set menus, in the 48-h period prior to the onset of symptoms in the first case residents on a full diet received seafood pie with vegetables and chicken paprika and rice as their two main meals, while those on a puréed diet received puréed fish with vegetables and minced chicken with vegetables. The minced chicken arrived at the facility already minced and was derived from an unrelated batch of chicken to that used for the chicken paprika. However, approximately five of the residents may have consumed the available alternate meals of lamb paprika and ham pie; exact numbers were unavailable.
In addition, 18 residents required their fluids to be thickened to promote safe swallowing; the thickening agent used was a powder composed primarily of guar gum. This was added to 1 litre of flavoured milk, coffee or cordial in quantities to form three consistencies of fluid: honey-thick (one scoop), nectar-thick (two scoops), and pudding-thick (three scoops). All but one of those 18 residents on thickened fluids required oral medications and received these crushed into a spoonful of the pudding-thick mixture. An additional 16 residents who did not require thickened fluids as a prescribed part of their diet chose to receive their medications crushed in the pudding-thick mixture.
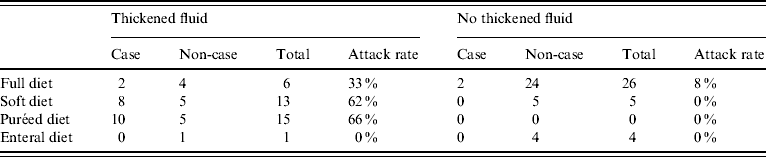
Of the 22 cases, most (82%) were on a soft or puréed diet, and most (91%) received thickened fluids either as a prescribed part of their diet or only with medications. In univariate analysis, there was no significant association between illness and age, gender, or geographical location within the high-level care areas (Table 1). However soft (RR 3·6, 95% CI 1·2–10·2) and puréed (RR 5·3, 95% CI 2·0–14·3) diets were significantly associated with illness compared to those on a full diet (Table 2). Thickened fluids were also associated with illness; this association was significant for those on honey-thick fluid (RR 8·8, 95% CI 1·3–60·4), nectar-thick fluid (RR 13·6, 95% CI 3·4–54·7) and pudding-thick fluid (RR 17·5, 95% CI 4·6–67·2) and also those taking thickened fluid with tablets alone (RR 5·1, 95% CI 1·1–23·9). There was an increasing relative risk of illness with increasing concentration of thickened fluid.
Table 1. Demographics of the 70 high-care residents in the aged care facility, by case status
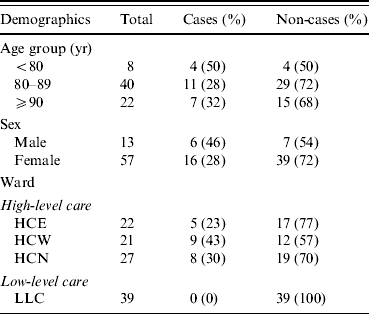
Table 2. Exposure history of the 70 high-care residents in the aged care facility, by case status

OR, Odds ratio; CI, confidence interval; n.s., not significant.
* Microbiologically confirmed (n = 22).
† Results from logistic regression (note summary result for any thickened fluid is from a separate model. controlling for diet type).
‡ Either with medications (tablets) or as a prescribed part of diet.
Simple stratified analysis showed that consuming any thickened fluid was associated with illness, regardless of diet type (Table 3). Diet type and thickened fluid (each variable over several levels) were included in a logistic regression model; diet type ceased to be a significant predictor of case status in this model and the association with thickened fluids was strengthened as was the apparent dose–response relationship (Table 2).
Table 3. Stratified analysis of attack rate by diet type by whether thickened fluid consumed
Three nurses were identified as having symptoms of diarrhoea and/or vomiting and had stool specimens taken; one of these cultured S. Infantis. This nurse was a night manager who worked on 10 and 11 April, consuming only coffee and milk while on duty. While on duty, she crushed medications into thickened fluids for some residents. In addition, on 11 April she assisted in the direct transfer of a confirmed case returning from hospital from the ambulance to their bed. She developed symptoms on 13 April, and did not work again until 18 April. All 12 kitchen staff tested were asymptomatic and had negative stool specimens.
Environmental investigation
This was a well-kept, accredited facility, with the current building only 5 years old. A single kitchen serviced the facility. All meals were prepared and plated in the kitchen, which appeared to be clean. The facility operated according to a food safety programme and had previously passed an audit by the NSW Food Authority on 17 August 2009.
It was reported by cleaning staff that food contact surfaces including equipment such as blenders, were regularly cleaned and sanitized with a quaternary ammonium compound at a concentration of 200 ppm. A designated sink was used for washing and sanitizing of all fruit and vegetables with a chlorine-based sanitizer at 100 ppm. The kitchen area designated for preparation of fresh foods and sandwiches was in close proximity to a sink and bench top used for preparation of raw meats and chicken. Preparation of thickened fluids was undertaken by kitchen staff close to a sink used for washing of pots.
Thickened fluid was usually mixed using a standard blender reserved for this purpose, but on occasions a stick blender was used. This stick blender was also used for preparing raw eggs for scrambled eggs and similar dishes and to purée cooked foods. Blenders were reported to be cleaned and sanitized appropriately between use. Kitchen staff reported that washing of pots never occurred when thickened fluids were being prepared and the area was thoroughly cleaned and sanitized prior to use.
The thickened fluid powder was decanted by kitchen staff every 2–3 weeks from a larger bulk storage container in the dry foods pantry into a smaller container in the kitchen for ease of use. A plastic scoop and knife used to dispense the thickened fluid powder were stored in the smaller container. New batches of thickened fluid were made up daily by kitchen staff, placed into jugs, stored in the kitchen fridge and distributed to fridges on wards. Kitchen staff reported that no direct hand contact occurred with the thickened fluid powder. However, the handles of the plastic scoop and knife were observed to be in contact with the powder when stored in the smaller container and these utensils were not replaced after each use of the powder. The smaller container was refilled at some time during the period 29 March to 5 April, was refilled again from the bulk storage container on 15 April and was removed from use on 16 April when the facility was visited by public health staff. The plastic scoop and knife used in the small container up to 16 April were thoroughly washed and were not swabbed.
Policy and audit documents were produced by the facility management as evidence of an ongoing infection control programme. On inspection most infection control and environmental practices on the wards were satisfactory. The facility was well maintained, visibly clean and tidy. Some minor discrepancies were noted including a carer distributing clean bed linen directly after changing a dirty bed, a shower trolley contaminated with a small amount of faecal matter, and an unrestricted communal access snack and drink refrigerator in the low-care patient dining room allowing some potential for cross-contamination of food and drink. Ward practices associated with handling of thickened fluid were reviewed. Nurses doing medication rounds in the high-care unit would pour a single tumbler of thickened fluid to combine with crushed medications to help make medications easier to swallow. Oral medications were observed to be broken and partly crushed in a nurse's hand directly and then mixed with a multi-use spoon in a small drug pot to be dispensed to individual patients at the bedside. Partially used tumblers of thickened fluid were stored in a drug refrigerator draped by a paper hand towel dated for re-use on the next drug round. Nurses' hands, crushing utensils or paper towel were potential sources of cross-contamination of thickened fluid; storage may have facilitated growth.
Laboratory investigation
In total, 22 residents and one staff member cultured Salmonella spp. from stool specimens; these 23 isolates were typed as Salmonella enterica serovar Infantis. One resident was found to be co-infected with norovirus. However, there was no other evidence to suggest a concurrent outbreak of norovirus in the facility. The symptoms of the cases were mostly diarrhoea and 15/16 stool specimens tested for norovirus were negative. Four random isolates underwent PFGE analysis at the Microbiological Diagnostics Unit, Melbourne, were indistinguishable, and were designated as having a ‘C’ pattern [Reference Tenover10] when compared with an unrelated S. Infantis reference strain received earlier in the year.
The raw chicken mince sample obtained on 13 April was also positive for S. Infantis. This sample was from a bag of chicken mince from the usual supplier but would not have been from the same bag used in preparation of meals just prior to onset of the initial cases in the outbreak. All other food and environmental samples collected on this and subsequent occasions from the aged care facility were negative. Isolates from clinical stool samples and chicken mince together with various other representative S. Infantis isolates were rerun on the same gel. The gel image (Fig. 2) indicates that the two clinical isolates (lanes 5 and 6) were indistinguishable. Isolates from the chicken mince (taken from the facility, lane 4), chicken feed (lane 3), and chicken meat (lane 2) were respectively 2, 4 and 6 bands different – i.e. closely related but not identical to either each other or the clinical isolates.

Fig. 2. Pulsed-field gel electrophoresis of clinical samples and selected food-source samples of S. Infantis. M, Marker lanes; lane 1, epidemiologically unrelated isolate; lane 2, chicken meat isolate (surveillance isolate); lane 3, chicken feed isolate (surveillance isolate); lane 4, chicken mince isolate (from aged care facility); lane 5, patient isolate; lane 6, staff isolate; lane 7, epidemiologically unrelated isolate.
DISCUSSION
We identified an outbreak of S. Infantis among aged care facility residents and a staff member. Symptom onset dates of cases were spread over 11 days suggesting prolonged exposure to a source or vehicle. We found a strong epidemiological association between consumption of thickened fluid and illness and a dose–response relationship. However, we did not isolate S. Infantis from thickened fluid powder. Identification of the same serotype of Salmonella in the raw chicken mince suggests cross-contamination of a batch of thickened fluid powder as a mechanism. Infection directly from contaminated chicken was unlikely as not all residents had consumed a meal with chicken mince, symptom onset dates of cases were spread over 11 days, and the meal with chicken mince had been cooked thoroughly and served immediately. Once thickened fluid was identified as a possible vehicle, the facility acted on advice to improve storage and preparation of the product. This included dispensing with a dedicated knife and spatula that were cleaned and sanitized between uses, and not stored in contact with the powder.
The timing of the outbreak strongly suggested a possible role for thickened fluid powder. The same batch of powder, as emptied into the smaller container from bulk storage, was used from some time between 29 March and 5 April until 16 April, and symptom onset dates ranged from 6 to 17 April, corresponding with the incubation period of 6–72 h for Salmonella [Reference Behravesh, Lynch, Schlundt and Heymann6]. There are also some theoretical reasons for the plausibility of thickened fluid powder as a vehicle. Salmonella can survive for extended periods of time in low-moisture foods, such as dry milk powder and flour [Reference Podolak11]. Guar gum, the primary component of the thickened fluid powder, has been successfully used as a substitute for agar in microbial culture media [Reference Jain, Anjaiah and Babbar12], so plausibly, Salmonella may have survived in the container of powder, with the prepared thickened fluid mixture providing an appropriate environment for Salmonella to grow. Although Salmonella was not isolated from the residual thickened fluid powder in the small container, it should be noted that this sample was taken at a later visit by which stage the plastic knife and scoop in the container had been thoroughly washed.
That chicken mince was the source food introduced to the kitchen was supported by the close similarity of the PFGE pattern for S. Infantis isolated from the stools of clinical cases and the PFGE pattern for S. Infantis isolated from chicken mince: there were only four band differences between these isolates. Guidelines suggest this indicates a possible link in the context of an outbreak [Reference Tenover10]. A number of theories for the method of cross-contamination of thickened fluid powder were postulated. As the plastic scoop and knife used to dispense the thickened fluid powder were also stored in the container, and wearing of gloves was not required for this process, there was potential for contamination of the powder by staff that may have handled the chicken mince and not thoroughly cleaned and dried their hands before preparing thickened fluids. Alternatively, the container of thickened fluid powder and the equipment used to prepare mixtures of thickened fluid stored near the pot-washing sink may have been splashed from the washing of pots used to prepare meals that involved chicken mince. A further possibility is that either the stick blender or standard blender used to prepare thickened fluids may have acted as vehicles for cross-contamination. However, these blenders were not reported by kitchen staff to have been used in the preparation of chicken mince.
There are a number of limitations to our study. Case finding and obtaining symptom and meal histories from residents was not possible as many of them had some cognitive impairment. Obtaining accurate meal histories was also made difficult as documentation of meal choices had been discarded prior to our investigation. Our investigation consequently lacks an analytical investigation of cases and non-cases by foods consumed. There remains a reasonable possibility that the implicated raw food source was introduced directly through a meal and subsequent cases were by person-to-person transmission. However, the only meal containing chicken mince consistent with symptom onset in the outbreak was a meal served to those on a puréed diet. Two-thirds of those on a puréed diet became ill but the illness onsets of those on a full diet, for example, were 8, 10, 12 and 17 April, i.e. included dates quite early in the outbreak. Given the range of symptom onset dates over 11 days, a single meal was unlikely to have caused the outbreak.
Our finding of a dose–response relationship between presumed amount of thickened fluid powder consumed and confirmed illness (attack rate) is also limited by imprecision or possible misclassification. Dose depends on both volume and concentration. It was not possible to measure how much thickened fluid residents consumed as a beverage or dietary supplement. Assuming that those on honey-, nectar- and pudding-thick fluid consumed similar amounts of fluid this would be equivalent to a threefold increase in dose from honey- to pudding-thick fluid. Those residents that had thickened fluids solely with their medications consumed pudding-thick fluid but may have consumed as little as a spoonful (15 ml) per day. Even if this is diluted threefold to the dilution of honey-thick fluid this is only 45 ml in volume and far less than the 1·5–2 litres of fluid that may have been ingested by a person whose sole fluid intake was thickened fluid. So although there is uncertainty about total dose of thickened fluid powder, the ordering of the categories is probably sound and the dose–response relationship provides supporting evidence.
Two of the cases among the residents and the single confirmed staff member did not consume thickened fluid as far as we are aware. Possibly these cases may be explained by person-to-person transmission. The ill staff member was known to have handled a symptomatic, confirmed case 2 days before her own symptom onset; the mechanism of potential transmission is less clear for the two resident cases who did not consume thickened fluids.
Detecting outbreaks in aged care facilities due to Salmonella and other bacterial pathogens is important because morbidity and mortality may be high [Reference Kirk5]. Person-to-person and environmental transmission of salmonellae in aged care facilities – usually in the context of deficient infection control standards – is not commonly described [Reference Olsen13, Reference Kay14] but certainly occurs. Dementia may be a factor that increases the risk of Salmonella infection because of inability to maintain personal hygiene [Reference Arshad15]. Transmission of Salmonella via powdered thickeners used to prepare thickened fluids appears to be highly unusual: a search of articles indexed by Medline from 1966 did not reveal any reports of transmission of Salmonella with this vehicle. However, there are reports of Salmonella contamination of powdered infant formula [Reference Brouard16], another powdered dietary supplement requiring preparation. Although it appears that contamination of powdered infant formula – a non-sterile product – typically occurs at source [Reference Cahill17] rather than at time of preparation. Pre-prepared enteral feeds have also been reported to have caused Salmonella outbreaks in an institutional settings either from cross-contamination in the kitchen/preparation area [Reference Gill and Gill18] or linked to albumin from eggs [Reference Matsuoka19]. The outbreak we report appears to be an unusual example of cross-contamination in a kitchen preparation area from a high-risk food to a powdered dietary supplement/swallowing aid which is probably not thought of as a likely vehicle for a bacterial pathogen. Fluids thickened by starches or gums are frequently recommended to elderly in aged care facilities because of real or perceived swallowing problems to prevent aspiration, although the prevalence of this practice and the evidence supporting it may be poorly described [Reference Hine20]. Thickened fluids are very likely prepared in the kitchen alongside other foods. Our results highlight the potential role of this ubiquitous dietary supplement in the transmission of Salmonella and other foodborne pathogens. There is a need for education of staff – both kitchen and nursing – about the risks associated with the handling of all foods and beverages including thickeners and the potential for cross-contamination. Storage of a knife and scoop in a container of thickened fluid powder is a kitchen practice that should be avoided.
ACKNOWLEDGEMENTS
We acknowledge Brett Campbell and Marianne Tegel of the NSW Food Authority who contributed to the environmental investigation, and Jeremy McAnulty, Director of Health Protection at NSW Health, who provided epidemiological advice and general support. We thank Peter Howard and the NSW Enteric Reference Laboratory for serotyping and assistance with methods and also gratefully acknowledge the work of Hua Yi Li who performed the pulsed-field gel electrophoresis. We are also grateful to an anonymous referee who suggested a simple modification to the analysis of the dose–response relationship that we reported.
DECLARATION OF INTEREST
None.







