Fe is an essential trace element, and it is required for the function of numerous critical enzymes in many biological processes for humans and animals( Reference Strube, Beard and Ross 1 , Reference Wang, Di and D’Agostino 2 ). Therefore, it is crucial to provide an optimal Fe level in the diet to prevent clinical deficiencies or ensure that the animals reach their optimal growth. The Fe requirement of broilers from 1 to 21 d of age was recommended to be 80 mg/kg by the National Research Council (NRC)( 3 ), and only estimated values were given for broilers after 3 weeks of age. Moreover, it was based on the limited information of few early studies using purified or semi-purified diets, and the parameters selected were growth performance, Hb concentration or haematocrit, which might be not the most sensitive indices to reflect the metabolic and molecular basis for the requirements of Fe in broiler chicks( Reference Davis, Norris and Kratzer 4 – Reference Southern and Baker 6 ). In addition, the results from early studies might not be applicable to practical diets because of the absence of antinutritional factors like phytate and fibre, which could inhibit Fe absorption by binding Fe in the intestinal tract and, thus, decrease Fe bioavailability( Reference Widdowson and Mccance 7 – Reference Reinhold, Garcia and Garzon 9 ). Therefore, it is necessary to look for more sensitive criteria to evaluate Fe requirements of broiler chicks fed a practical maize–soyabean-meal diet.
It seems that most physiological consequences of Fe deficiency are attributed to anaemia, which can be identified by Hb concentration or haematocrit. However, many patients could also suffer from definite symptoms of Fe deficiency without showing anaemia( Reference Waldenström 10 , Reference Waldenström 11 ). Thus, it is difficult to characterise other manifestations that are not related to anaemia but that may be attributed to compromised metabolic functions, in which Fe serves either as a cofactor or as an integral part of a protein or enzyme molecule( Reference Dallman, Beutler and Finch 12 , Reference Rao and Jagadeesan 13 ). Fe is required for the function of numerous Fe-containing enzymes, such as succinate dehydrogenase (SDH), cytochrome c oxidase (COX) and catalase (CAT), which are intimately associated with energy generation or elimination of reactive oxygen species of the mitochondria in various tissues( Reference Siimes, Refino and Dallman 14 , Reference Hagler, Askew and Neville 15 ). Further, it has been reported that the activity of SDH, COX and CAT could be affected by the dietary Fe levels in rats and pigs( Reference de Deungria, Rao and Wobken 16 , Reference Feng, Ma and Xu 17 ). More recently, in our laboratory, Ma et al.( Reference Ma, Liao and Lu 18 ) reported that the expression of liver SDH, COX and CAT and heart SDH activity as well as liver Sdh and Cox and heart Cox mRNA levels were new and sensitive criteria to evaluate the dietary Fe requirements of broilers from 1 to 21 d of age, and 97–136 mg Fe/kg was required to support their full expression in various tissues. However, dietary Fe requirements for full expression of the above Fe-containing enzymes in various tissues of broiler chicks from 22 to 42 d of age has not been investigated. Therefore, the objective of this study was to investigate the effect of dietary Fe level on growth performance, blood parameters, Fe concentrations as well as on the activity and mRNA levels of Fe-containing enzymes in various tissues, so as to find sensitive indices and evaluate the Fe requirements to support the full expression of Fe-containing enzymes in broilers fed a practical maize–soyabean-meal diet from 22 to 42 d of age.
Methods
Animals, diets and experimental design
All experimental procedures were approved by the Office of the Beijing Veterinarians. Arbor Acres male broilers (Huadu Broiler Breeding Corporation) were maintained in accordance with the broiler management guidelines for lighting and feeding and allowed ad libitum access to tap water containing no detectable Fe( Reference Yang and Diao 19 ). Birds were housed in an electrically heated, thermostatically controlled room with fibreglass feeders, waterers and stainless-steel cages coated with plastics. From days 1 to 21 post-hatching, all birds were fed the same maize–soyabean-meal diet supplemented with 70 mg Fe/kg as FeSO4.7H2O (Table 1, containing 127 mg Fe/kg diet by analysis); the dietary Fe level was based on the result of our recent study in broilers from 1 to 21 d of age( Reference Ma, Liao and Lu 18 ). At 22 d of age, 288 birds were assigned randomly to one of six dietary treatments of six replicate cages with eight birds per cage in a completely randomised design, and fed an Fe-unsupplemented basal maize–soyabean-meal diet (Table 1, 47·0 mg Fe/kg) or the basal diet supplemented with 20, 40, 60, 80 or 100 mg Fe/kg in the form of reagent-grade FeSO4.7H2O. The added Fe for each treatment was pre-mixed with maize starch to the same weight and mixed with each aliquot of the basal diet. The diets were formulated to meet or exceed the NRC( 3 ) requirements for broilers for all nutrients except for Fe. The dietary Fe level by analysis on an as-fed basis were 47·0, 66·5, 84·5, 104, 122 and 142 mg/kg, respectively. Chicken weight and feed intake per cage were measured weekly to calculate the daily body weight gain, daily feed intake, and feed:gain ratio during days 22 to 42.
Table 1 Ingredients and chemical composition of the basal diets for broilers on an as-fed basis
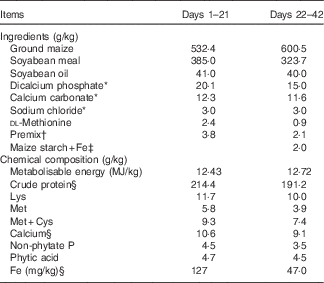
* Reagent grade.
† Provided per kg of diet during days 1–21: retinyl acetate, 4·3 mg; cholecalciferol, 0·094 mg; dl-α-tocopheryl acetate, 20 mg; menadione, 2·5 mg; thiamin, 2·5 mg; riboflavin, 8 mg; pyridoxine, 2·5 mg; vitamin B12, 0·015 mg; pantothenic acid, 12·5 mg; niacin, 32·5 mg; folic acid, 1·25 mg; biotin, 0·125 mg; choline, 700 mg; Cu, 8 mg; Zn, 60 mg; Mn, 110 mg; Fe, 70 mg; iodine, 0·35 mg; Se, 0·15 mg. Provided per kg of diet during days 22–42: retinyl acetate, 3·4 mg; cholecalciferol, 0·085 mg; dl-α-tocopheryl acetate, 12·8 mg; menadione, 1·6 mg; thiamin, 0·8 mg; riboflavin, 6·8 mg; pyridoxine, 1·6 mg; vitamin B12, 0·008 mg; pantothenic acid, 8 mg; niacin, 26 mg; folic acid, 0·8 mg; biotin, 0·126 mg; choline, 500 mg; Cu, 8 mg; Zn, 60 mg; Mn, 80 mg; iodine, 0·35 mg; Se, 0·15 mg.
‡ Fe supplements added in place of equivalent weights of maize starch.
§ These values were determined by analysis based on triplicate determinations; other values in the table are as formulated.
Sample collections and preparations
At 42 d of age, chicks in each cage were individually weighed to calculate the cage average body weight for each of six treatments after fasting for 12 h, and then four birds close to the cage average body weight were selected from each cage. Birds were killed by cervical dislocation, and blood samples were promptly obtained using cardiac puncture with a heparinised syringe equipped with stainless-steel needles. A part of the blood samples was stored at 4°C for the analysis of Hb concentration and haematocrit and the rest was centrifuged to yield plasma; subsequently, plasma samples were frozen (−20°C) for analysis of plasma Fe (PI) and total Fe-binding capacity (TIBC). After blood collection, liver, heart and kidney samples were collected. A set of subsample was stored at −20°C for determinations of SDH, CAT and COX activity and Fe concentrations, and another set of subsample was snap-frozen in liquid N2 and then stored at −80°C for analysis of Sdh, Cox and Cat mRNA levels. The right-femur marrow samples were collected immediately and also snap-frozen in liquid N2 and subsequently stored at −80°C for analysis of Hb mRNA level. The pancreas, spleen, breast muscle and left tibia were also collected and stored at –20°C for analysis of Fe concentration. All samples from four broilers in each cage were pooled into one sample in equal ratios before analysis.
Measurements of iron concentrations, blood parameters and enzyme activity
The Fe concentrations in diets, water and tissues were determined using an inductively coupled plasma emission spectroscope (model IRIS Intrepid II; Thermal Jarrell Ash) after wet digestions with HNO3 and HCIO4 as described by Huang et al.( Reference Huang, Lu and Luo 20 ). The lowest limit of Fe detection is 0·05 mg/kg. Validation of the Fe analysis was conducted concurrently using bovine liver powder (GBW (E) 080193; National Institute of Standards and Technology) as a standard reference material (SRM). The actual Fe-recovery rates for the bovine liver SRM were determined to be about 99 % in the present study. Hb concentration and haematocrit were measured using an automatic haematology analyser (URIT Group Company Ltd) according to the manufacturer’s instruction. PI and TIBC were measured by the colorimetric method using commercial assay kits (catalogue no. A039 and A040; Nanjing Jiancheng Bioengineering Institute). Ferric Fe in plasma is bound to transferrin; therefore, SDS was used to release Fe from the complex to obtain ferric Fe, which could be reduced to the ferrous state by hydroxylamine. Subsequently, the divalent Fe reacted with bathophrenanthroline to form a coloured complex, which was measured at 520 nm. Transferrin in the plasma was saturated with Fe ions, and the unbound Fe was removed using analytical reagent-grade magnesium carbonate adsorption, and subsequent centrifugation was performed to collect the supernatants that were used for TIBC assays at 520 nm. Finally, transferrin saturation (TS) was calculated as (PI/TIBC)×100( Reference Huebers, Eng and Josephson 21 , 22 ). The liver, heart and kidney were homogenised in 10 % (w/v) ice-cold physiological saline, and then sonicated with an ultrasonic-wave cell grinder (JY92-11; Ningbo Xinzhi Bio-technology Co., Ltd) for 1 min (1 s with 2 s interval). The homogenates were centrifuged at 1500 g for 15 min at 4°C and supernatants were collected to determine total protein contents as well as SDH, CAT and COX activities. Total protein contents were determined using a BCA protein assay kit (catalogue no. 23225; Pierce). The activities of SDH and CAT in the supernatants were determined spectrophotometrically at 600 and 405 nm using commercial assay kits (catalogue no. A022 and A007-1; Nanjing Jiancheng Bioengineering Institute) and expressed as U/mg protein, respectively; the COX activity was measured spectrophotometrically at 550 nm by means of a reagent set (GMS10014.3.2; Genmed Scientifics Inc.) and expressed as mU/mg protein( Reference Nulton-Persson and Szweda 23 – Reference Hou, Li and Zhao 25 ). All of the above procedures were carried out according to the manufacturers’ instructions.
RNA extraction, reverse transcription and real-time PCR
Total RNA was isolated from the liver, heart, kidney and femur marrow using Trizol reagent (Invitrogen) according to the manufacture’s instruction. The concentration of each isolated RNA sample was determined using a NanoDrop Spectrophotometer (ND-2000; Gene Company Ltd), and the integrity of the RNA was checked using denatured RNA electrophoresis. A total of 1 μg of RNA was used to obtain complementary DNA by reverse transcription using the Super Script First-Strand Synthesis System (Invitrogen). Real-time PCR reactions were performed on an ABI 7500 real-time PCR system using SYBR-Green PCR Master Mix (Applied Biosystems). The primer sequences for Sdh, Cat, Cox, Hb, β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are given in Table 2. The protocol for PCR was as follows: denaturation at 95°C for 10 min followed by forty cycles of 94°C for 15 s and 60°C for 1 min. The
![]() $$2^{{{\minus}\Delta \Delta C_{T} }} $$
was used to calculate the mRNA level of each target gene(
Reference Livak and Schmittgen
26
). The geometric mean of internal reference genes, β-actin and GAPDH, was used to normalise the expression level of the target gene. The run was performed in triplicate.
$$2^{{{\minus}\Delta \Delta C_{T} }} $$
was used to calculate the mRNA level of each target gene(
Reference Livak and Schmittgen
26
). The geometric mean of internal reference genes, β-actin and GAPDH, was used to normalise the expression level of the target gene. The run was performed in triplicate.
Table 2 Primers used for the target and reference genes

Cat, catalase; Cox, cytochrome c oxidase; Sdh, succinate dehydrogenase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
The effect of dietary Fe treatment was analysed by one-way ANOVA using the general linear model procedure of SAS (version 9.2; SAS Institute Inc.). Differences among means were tested using the least-significant difference method. The replicate cage of eight chicks for growth performance indices or four chicks for other indices served as the experimental unit. Orthogonal comparisons were applied for linear and quadratic responses of dependent variables to independent variables. Regression analysis of broken-line, quadratic and asymptotic models were performed, respectively, and the quadratic models were shown to have the best fits between responsive criteria and dietary Fe levels; therefore, these quadratic models were chosen to estimate the optimal dietary Fe levels (the maximum responses from quadratic models) for broiler chicks( Reference Ma, Liao and Lu 18 , Reference Corzo, Dozier and Kidd 27 – Reference Lu, Chang and Liao 29 ). The level of statistical significance was set at P<0·10( Reference Li, Luo and Liu 30 – Reference Liao, Li and Lu 32 ).
Results
Growth performance
Dietary Fe level did not affect (P>0·25) daily body weight gain, daily feed intake and feed:gain ratio of broilers during days 22–42 (data not shown).
Blood parameters
Dietary Fe level did not affect (P>0·35) PI, TIBC, TS, Hb concentration and haematocrit of broilers at 42 d of age (Table 3).
Table 3 Effect of dietary iron level on blood iron status variables of broilers at 42 d of age (Mean values with their pooled standard errors, n 6)

PI, plasma Fe; TIBC, total Fe-binding capacity; TS, transferrin saturation.
Iron concentrations
The Fe concentrations in kidney, pancreas, spleen and tibia bone ash of broilers at 42 d of age were not affected (P>0·29) by dietary Fe level (Table 4). However, Fe concentrations in liver, heart and breast muscle of broilers at 42 d of age were affected (P<0·05) by the dietary Fe level, which increased linearly (P<0·007) as dietary Fe level increased; therefore, none of these three tissues was suitable for assaying Fe requirement.
Table 4 Effect of dietary iron level on tissue iron concentrations of broilers at 42 d of age (Mean values with their pooled standard errors, n 6)

a,b,c Mean values within a column with unlike superscript letters were significantly different (P<0·07).
* Fresh-weight basis.
mRNA levels
Dietary Fe level did not affect (P>0·27) Sdh and Cat mRNA levels in liver and heart, Cat and Cox mRNA levels in kidney and Hb mRNA level in femur marrow, but affected (P<0·08) Cox mRNA level in liver and heart as well as Sdh mRNA level in kidney of broilers at 42 d of age (Table 5). The heart Cox mRNA level increased quadratically (P<0·03) as dietary Fe level increased, and reached a plateau at a supplementation of approximately 60–80 mg Fe/kg.
Table 5 Effect of dietary iron level on mRNA levels of tissue iron-containing enzymes of broilers at 42 d of age (Mean values with their pooled standard errors, n 6)

RQ, relative quantities; Sdh, succinate dehydrogenase; Cat, catalase; Cox, cytochrome c oxidase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P<0·09).
* The mRNA levels were calculated as the RQ of the target gene mRNA to the geometric mean of β-actin mRNA and GAPDH, RQ=
![]() $$2^{{{\minus}\Delta \Delta C_{T} }} $$
(C
T
, threshold cycle).
$$2^{{{\minus}\Delta \Delta C_{T} }} $$
(C
T
, threshold cycle).
Enzyme activity
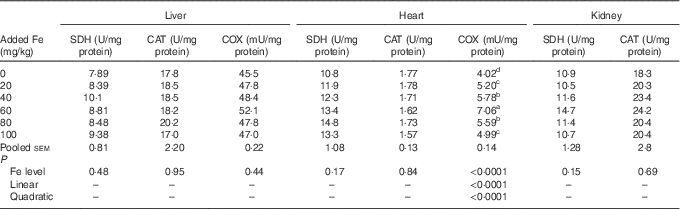
Dietary Fe level did not affect (P>0·15) the SDH, CAT and COX activity in liver, SDH and CAT activity in heart as well as SDH and CAT activity in kidney; however, COX activity in heart of broilers at 42 d of age was affected (P<0·0001) (Table 6). The COX activity in the heart increased quadratically (P<0·0001) as dietary Fe level increased, and reached the highest point at a supplementation of 60 mg Fe/kg.
Table 6 Effect of dietary iron level on tissue iron-containing enzyme activity of broilers at 42 d of age (Mean values with their pooled standard errors, n 6)
SDH, succinate dehydrogenase; CAT, catalase; COX, cytochrome c oxidase.
a,b,c,d Mean values within a column with unlike superscript letters were significantly different (P<0·07).
Dietary iron requirements
Results of the dietary Fe requirements of broilers as estimated by the non-linear regression analysis are presented in Table 7. Based on fitted quadratic-curve models (P<0·008) of COX mRNA level and activity in the heart, optimal dietary Fe levels were estimated to be 104–110 mg/kg for broiler chicks fed a maize–soyabean-meal diet from 22 to 42 d of age.
Table 7 Dietary iron requirements of broilers from 22 to 42 d of age as estimated based on fitted quadratic-curve models

* Y is the dependent variable and X is the analysed Fe concentration in the basal diet plus supplemental Fe level (mg/kg).
Discussion
It is difficult to characterise other manifestations that are not related to anaemia but that may be attributed to metabolic defects( Reference Hagler, Askew and Neville 15 ). Therefore, it is critically important to find sensitive indices to evaluate Fe nutritional status of animals. The present study demonstrates that heart COX mRNA level and activity are new and more sensitive criteria for evaluating Fe nutritional status of broilers fed a maize–soyabean-meal diet from 22 to 42 d of age than growth, Hb concentration or haematocrit.
To our knowledge, very limited information concerning Fe requirement of broilers during days–42 is available so far, and only estimated value of Fe requirement for broilers after 3 weeks of age was given by the NRC( 3 ). Moreover, in these earlier studies, growth performance, Hb concentration and haematocrit were often used to assess Fe requirements in chicks fed purified or semi-purified diets, and the Fe requirements for broilers during days 1–21 (or days 1–29) were defined as 45–85 mg/kg( Reference Davis, Norris and Kratzer 4 , Reference McNaughton and Day 5 , Reference Southern and Baker 6 , Reference Waddell and Sell 33 – Reference Bao, Choct and Iji 35 ). On the other hand, Vahl et al.( Reference Vahl and van ‘T Klooster 36 ) and Ma et al.( Reference Ma, Liao and Lu 18 ) reported that the Fe requirements in broilers fed a practical maize–soyabean-meal diet were 100 and 118 mg/kg for the optimum growth from 1 to 21 or 1 to 39 d of age, respectively. The Fe requirements in broilers fed the practical diets are higher than requirements for those fed purified or semi-purified diets, which might be due to the adverse effect of phytate and fibre on Fe absorption( Reference Widdowson and Mccance 7 – Reference Reinhold, Garcia and Garzon 9 ). Nevertheless, the growth performance is always influenced by the type of diets or growth phase and, thus, it might not be a good index for assessing Fe requirements for broilers. Besides, many studies have demonstrated that haematological indices, such as Hb concentration or haematocrit, could reflect the Fe nutritional status or be a sensitive criteria for estimating the Fe requirement of broilers fed purified or semi-purified diets( Reference Davis, Norris and Kratzer 4 , Reference Vahl and van ‘T Klooster 36 ). Ma et al.( Reference Ma, Liu and Lu 37 ) found that blood Hb concentration and haematocrit of chicks fed a casein–dextrose diet increased linearly as dietary Fe level increased; Davis et al.( Reference Davis, Norris and Kratzer 4 ) reported that the Fe requirements of chicks fed a soyabean protein–casein–gelatin diet were 78 and 77 mg/kg for Hb concentration and haematocrit, respectively. However, the present study showed that dietary Fe concentration had no effect on growth performance and blood Fe status parameters of broilers from 22 to 42 d of age, suggesting that Hb concentration and haematocrit were not sensitive criteria for estimating Fe requirement of broilers when a conventional maize–soyabean-meal diet was used.
Many studies showed that the Fe concentration in tissues increased as the dietary Fe level increased, and the change in Fe concentration in tissues could reflect the Fe status of animals: especially, the Fe concentration in liver( Reference Ma, Liao and Lu 18 , Reference Wensing, Abdelrahim and Schotman 38 , Reference Furugouri 39 ). In the present study, it was also found that Fe concentrations in the liver and heart increased as dietary Fe level increased, which was similar to findings from early studies( Reference Ma, Liao and Lu 18 ). However, Fe contents in these tissues did not reach a plateau with increasing dietary Fe levels, and a better linear response was observed, suggesting that these indices would be better used to evaluate the bioavailability of different Fe sources in broilers than to assess the threshold of adequacy.
The gene expression and activity of Fe-containing enzymes might be another type of new and more sensitive biomarkers for assessing Fe nutritional status of animals as, like all other essential trace elements, Fe is a component or a cofactor of numerous Fe-containing enzymes, and functions in the body mainly through Fe-containing enzymes, such as SDH, COX and CAT( Reference Rao and Jagadeesan 13 , Reference de Deungria, Rao and Wobken 16 ). It was found that SDH and CAT activity in the liver of pigs( Reference Feng, Ma and Xu 17 ) and broilers( Reference Ma, Liao and Lu 18 ) first increased, and then decreased when the added Fe levels increased from 0 to 120 mg/kg in the maize–soyabean basal diet, suggesting that liver SDH and CAT activity could sensitively reflect the Fe nutritional status of animals. However, the results from our current study showed that dietary Fe level did not affect the SDH and CAT activity in the heart, liver and kidney of broilers, which might be due to the different types of animals and growth phases studied or due to previous Fe accumulation in these tissues (the diet containing 127 mg Fe/kg during days 1–21). In the present study, it was found that the COX mRNA level and activity in heart increased quadratically as dietary Fe level increased, and they were sensitive biomarkers to assess Fe status and Fe requirements for broilers fed a maize–soyabean-meal diet from 22 to 42 d of age. Our previous study also found that Cox mRNA level in the heart was a new and sensitive criterion for assessing the Fe requirements of broilers fed the maize–soyabean-meal diet from 1 to 21 d of age( Reference Ma, Liao and Lu 18 ). Moreover, a similar phenomenon was also reported in other studies. Siimes et al.( Reference Siimes, Refino and Dallman 14 ) reported that even the mildest degree of Fe-deficiency anaemia was also accompanied by depletion of cytochrome c; de Deungria et al.( Reference de Deungria, Rao and Wobken 16 ) found that supplemental Fe increased the COX activity in the brain of rats. The above-mentioned results, together with those of the present study, demonstrated that COX expression was a sensitive criterion for estimating Fe nutritional status of animals. Therefore, in order to meet all metabolic Fe needs, the dietary Fe levels of 104–110 mg/kg would be recommended as the dietary Fe requirements for broilers. However, considering the effect of sex on dietary Fe requirements of broilers, a combined study (male and female) or separate study focusing on female broilers needs to be conducted in the future to justify the requirement of Fe during 22 to 42 d of age.
In conclusion, the results from the present study indicate that COX mRNA level and activity in the heart are new and sensitive criteria to evaluate the dietary Fe requirements of broilers, and the dietary Fe requirements are 104–110 mg/kg to support full expression of COX in the heart of broilers fed the maize–soyabean-meal diet from 22 to 42 d of age, which are higher than the dietary Fe requirement (80 mg/kg) of broiler chicks from 1 to 21 d or 22 to 42 d of age as recommended by the NRC( 3 ).
Acknowledgements
The authors would like to thank the personnel of these teams for their kind assistance.
The present study was supported by the Agricultural Science and Technology Innovation Program (ASTIP-IAS08), the China Agriculture Research System (project no. CARS-42), the Program of the National Natural Science Foundation of China (project no. 31672440), the Research Program of the Key Laboratory of Animal Nutrition (project no. 2004DA125184G1606) and the Program of Student Community of Professor Yang Sheng (project no. 2016A20010).
The authors’ contributions are as follows: X. Luo and X. Liao designed the experiment; X. Liao drafted the article; X. Luo and L. L. participated in writing and editing the article; C. M. conducted most of the experiments and analysed the data; L. Z. performed the Fe analysis; X. Luo had primary responsibility for the final content. All authors read and approved the final version of the article.
The authors have no financial or personal conflicts of interest to declare.










