Introduction
The effect of exogenous growth hormone (GH) on animal growth and meat quality has been studied extensively during the last decades. It has been confirmed that exogenous GH can improve animal daily weight gain, feed efficiency and lean accretion rate (Campbell et al., Reference Campbell, Steele, Caperna, McMurtry, Solomon and Mitchell1989; Brameld et al., Reference Brameld, Atkinson, Saunders, Pell, Buttery and Gilmour1996). The responses of hormones and genes in somatotropic axis, such as GH receptor, IGF-1 and IGF-1 receptor have also been observed closely as the major components in the pathway of GH action (Louveau and Bonneau, Reference Louveau and Bonneau1996; Klindt et al., Reference Klindt, Yen, Buonomo, Roberts and Wise1998; Zhao et al., Reference Zhao, Zhou, Xu, Zhao, Wei, Xia and Chen2003). However, the responses of genes participating in myogenesis and myofibre-type transformation in skeletal muscle have received less attention, despite the fact that skeletal muscle is one of the major targets for GH action and economically the most important part in meat animals.
The skeletal muscle myofibres in postnatal pigs can be classified into four major types, I, IIa, IIx and IIb, according to their metabolic and contractive characteristics. Type I fibres (red; slow-twitch) are the smallest, oxidative fibres with high lipid content and many mitochondria, whereas type IIb fibres (white; fast-twitch) are the largest, glycolytic fibres possessing high glycogen content and few mitochondria. Type IIa and IIx fibres are intermediate oxidative–glycolytic fibres. Generally, IIa fibres are more closely related to type I fibres and IIx fibres are more similar to IIb fibres (Hamalainen and Pette, Reference Hamalainen and Pette1993). The longissimus dorsi (LD) and semitendinosus (ST) muscles differ in myofibre-type composition. LD muscle is composed primarily of glycolytic myofibres, whereas ST muscle has different myofibre-type composition depending on anatomical location. The deep medial portion comprises largely of oxidative myofibres. The myofibre-type composition contributes greatly to both skeletal muscle growth and meat quality (Klont et al., Reference Klont, Brocks and Eikelenboom1998; Eggert et al., Reference Eggert, Depreux, Schinckel, Grant and Gerrand2002; Chang et al., Reference Chang, Da Costa, Blackley, Southwood, Evans, Plastow, Wood and Richardson2003). Compared with its well-defined positive effect on myofibre hypertrophy, the influence of rpGH on myofibre-type composition is still under debate. Many studies reported that exogenous GH had no effect on the skeletal muscle fibre composition (Solomon et al., Reference Solomon, Campbell, Steele, Caperna and McMurtry1988 and Reference Solomon, Campbell and Steele1990; Beermann et al., Reference Beermann, Fishell, Roneker, Boyd, Armbruster and Souza1990; Czerwinski and Martin, Reference Czerwinski and Martin1994), while some experiments demonstrated divergent results (Solomon et al., Reference Solomon, Campbell, Steele and Caperna1991 and Reference Solomon, Caperna, Mroz and Steele1994).
Skeletal muscle growth involves both myofibre-type transformation and myofibre hypertrophy in pigs. Recently, some genes are reported to be involved in these two processes, such as myoD and myogenin (Olson, Reference Olson1990; Li and Olson, Reference Li and Olson1992; Molkentin and Olson, Reference Molkentin and Olson1996) and myostatin (Lee and McPherron, Reference Lee and McPherron1999; Sharma et al., Reference Sharma, Langley, Bass and Kambadur2001; Yang and Zhao, Reference Yang and Zhao2006). Meanwhile, calpain 3, a member of calcium-dependent protease family, acts as a skeletal muscle-specific regulatory protein (Sorimachi et al., Reference Sorimachi, Imajoh-Ohmi, Emori, Kawasaki, Ohno, Minami and Suzuki1989 and Reference Sorimachi, Ohmi, Emori, Kawasaki, Saido, Ohno, Minami and Suzuki1990) and participates in the regulation of myofibre hypertrophy. Peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), a transcriptional coactivator expressed in several tissues including skeletal muscle, was found to activate mitochondrial biogenesis and oxidative metabolism (Knutti and Kralli, Reference Knutti and Kralli2001) and was reported to drive the formation of slow-twitch muscle fibres in rodents (Lin et al., Reference Lin, Wu, Tarr, Zhang, Wu, Boss, Michael, Puigserver, Isotani, Olson, Lowell, Bassel-Duby and Spiegelman2002). However, data describing the responses of these genes to exogenous GH in pigs are scarce (Ji et al., Reference Ji, Losinski, Cornelius, Frank, Willis, Gerrard, Depreux and Spurlock1998a and Reference Ji, Frank, Cornelius, Willis and Spurlock1998b).
Therefore, the objectives of the present study were (1) to provide an evidence of the myofibre-type composition responding to rpGH both in LD and ST muscles and (2) to clarify the responses of genes related to skeletal muscle growth at the level of transcription in LD and ST muscles upon exogenous GH administration.
Material and methods
Animals and experimental design
The experiment was undertaken following the guidelines of the regional Animal Ethics Committee. Sixteen castrated male Large White × Landrace pigs with average initial body weight of 50.8 kg (from 48 to 55 kg) were housed individually in floor pens and fed according to the breed standards of USA (National Research Council, 1998). Pigs were randomly allotted to treatment or control group. The treatment group was injected intramuscularly with recombinant porcine growth hormone (rpGH, 4 mg/day) in the extensor area of the neck and the control group with vehicle at 0900 h for 28 days. Animals were slaughtered 4 h after the final injection. LD muscle adjacent to the last rib and the deep medial portion of ST muscle were taken immediately (within 5 to 10 min of death) and rapidly frozen in liquid nitrogen, and then stored at −80°C. rpGH was obtained from Shenzhen Lupeng Agricultural Hi-tech Co. Ltd, People’s Republic of China.
RNA extraction and reverse transcription (RT)
Total RNA was extracted from the tissue samples with a single-step method of RNA extraction by acid guanidinium thiocyanate–phenol–chloroform (Chomczynski and Sacchi, Reference Chomczynski and Sacchi1987). Total RNA concentration was then quantified by measuring the absorbance at 260 nm in a photometer (Eppendorf Biophotometer). Ratios of absorption (260/280 nm) of all preparations were between 1.8 and 2.0. Aliquots of RNA samples were subjected to electrophoresis through a 1.4% agarose–formaldehyde gel to verify their integrity.
Two micrograms of total RNA was reverse transcribed by incubation at 42°C for 1 h in a 25 μl mixture consisting of 10 U avian myeloblastosis virus reverse transcriptase, 10 U RNase inhibitor, 12 μmol/l random primers, 50 mmol/l Tris–HCl (pH 8.3), 10 mmol/l MgCl2, 50 mmol/l KCl, 10 mmol/l DDT, 0.5 mmol/l spermidine and 0.8 mmol/l each dNTP. The reaction was terminated by heating at 95°C for 5 min and quickly cooling on ice.
The expression of myosin heavy chain (MyHC) isoforms
A multiplex PCR procedure reported by Tanabe et al. (Reference Tanabe, Muroya and Chikuni1999) was followed to determine the relative expressions of MyHC isoforms in the muscle of pigs, employing four sense primers specific for MyHC (MyHC I: 5′-AGCCTCTTTCTTCTC CCAGGGACATTC-3′; MyHC 2a: 5′-CACTTGCTAAGAGGGACCTCTGAGTTCA-3′; MyHC 2b: 5′-CATCTGGTAACATAAGAGGTACATCTAG-3′; MyHC 2x: 5′-CTTTCCTCATAAAGCTTCAAGTTCTGCC-3′) and a common anti-sense primer (5′-ATCCAGGCTGCGTAACGCTCTTTGAGGTTGTA-3′) used in that publication. MyHC 1, 2a and 2b were respectively amplified together with MyHC 2x in the same PCR reaction.
The expression of myosatin, myogenin, myoD, calpain 3 and PGC-1α mRNA
Two microlitres of RT reaction mix was used for PCR in a final volume of 25 μl containing 0.5 U Taq DNA polymerase, 5 mmol/l Tris–HCl (pH 9.0), 10 mmol/l NaCl, 0.1 mmol/l DDT, 0.01 mmol/l EDTA, 5% (w/v) glycerol, 0.1% (w/v) Triton X-100, 0.2 mmol/l each dNTP, 1.0 to 2.0 mmol/l MgCl2 and 0.5 μmol/l of each primer specific for each gene. The PCR primers were designed using Primer Premier 5.0 and were synthesised by Haojia Biotech. Ltd., China. The nucleotide sequences of these primers and the PCR conditions are shown in Table 1. The Quantum RNA 18S Internal Standards kit (Catalogue no. 1716, Ambion Inc., Austin, TX, USA) containing primers and competitors was used to normalise variations in pipetting and amplification.
Table 1 Primer sequences of target genes and the condition of PCR

Different controls were set to monitor the possible contaminations of genomic DNA and environment DNA both at the stage of RT and RCR. The pooled samples made by mixing equal quantity of total RNA from all samples were used for optimising the PCR condition and normalising the intra-assay variations. All samples were included in the same run of RT-PCR and repeated at least for three times.
Quantitation of PCR products and statistical analysis
An aliquot of PCR products was analysed by electrophoresis on 2% agarose (for muscle growth-related genes) or 8.0% PAGE (for MyHC isoforms) gels. The gels were stained with ethidium bromide and photographed with digital camera. The net intensities of individual bands were measured using Kodak Digital Science 1D software (Eastman Kodak Company Rochester, NY, USA). Expression of each isoforms of MyHCs was estimated as the relative value of MyHC 1, 2a or 2b to MyHC 2x (Table 2). The mRNA expression of other muscle growth-related genes was normalised to 18S rRNA.
Table 2 Ratios of MyHC isoforms expression in LD and ST muscle (values are presented as means ± s.e.)
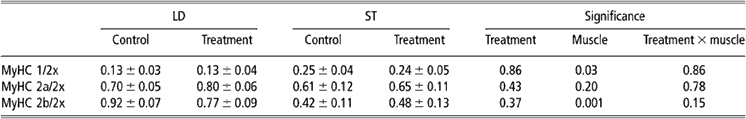
MyHC = myosin heavy chain; LD = longissimus dorsi; ST = semitendinosus; s.e. = standard error.
The results were expressed as mean ± s.e. Data were analysed using the general linear model procedure (Statistical Packages for the Social Sciences, 2001) and the model included the rpGH treatment, muscle and rpGH treatment × muscle interaction. Differences were considered significant when P < 0.05.
Results
Body weight gain and myofibre composition of LD and ST muscles
The initial and final body weights of pigs were 50.5 ± 1.3 kg and 71.5 ± 1.8 kg in the rpGH treatment group and 51.2 ± 1.6 kg and 67.8 ± 1.8 kg in the control group, respectively. Administration of rpGH significantly increased the average daily weight gain by 26.1% (749.6 ± 31.5 g v. 594.6 ± 17.3 g, P < 0.05).
Relative to MyHC 2x, MyHC 2b was expressed more abundantly in LD muscle than in ST muscle, whereas the opposite was true for MyHC 1. In general, rpGH administration had no effect on myofibre-type composition in either LD or ST muscle (Table 2).

Figure 1 The representative electrophoresis photos of myosin heavy chain (MyHC) types: (a) MyHC 1/2x; (b) MyHC 2a/2x; and (c) MyHC 2b/2x; lane 1–4: control group in longissimus dorsi (LD) muscle; lane 5–8: treated group in LD muscle; lane 10: DL2000 marker; lane 11–14: control group in semitendinosus (ST) muscle; lane 15–18: treated group in ST muscle; and lane 9, 19: mixed sample.
mRNA expression of growth-related genes in LD and ST muscle
The abundances of 18S rRNA showed no difference between LD and ST muscles, and were not altered by rpGH treatment (data not shown). No significant rpGH treatment × muscle interaction was found for any of the indices evaluated.
Among all the growth-related genes examined in the present experiment, only calpain 3 exhibited significant muscle effect. LD muscle showed significantly higher abundance of calpain 3 mRNA than in with ST muscle in general (P < 0.001), as shown in Figure 5.
No significant treatment effect was seen in the abundances of myostatin and myogenin mRNA irrespective of muscle types (Figures 2 and 3).

Figure 2 Effect of recombinant porcine growth hormone (rpGH) administration on myostatin mRNA in longissimus dorsi (LD) and semitendinosus (ST) muscles. Upper panel: representative electrophoresis photo. Lane 1: DNA marker PUC19; lane 2: control group in LD muscle; lane 3: treated group in LD muscle; lane 4: control group in ST muscle; lane 5: treated group in ST muscle; and lane 6: mixed sample. Lower panel: results of statistical analysis. No significant rpGH treatment effect, muscle effect or rpGH treatment × muscle interaction for myostatin mRNA expression were observed.

Figure 3 Effect of recombinant porcine growth hormone (rpGH) administration on myogenin mRNA in longissimus dorsi (LD) and semitendinosus (ST) muscles. Upper panel: representative electrophoresis photo. Lane 1: DNA marker PUC19; lane 2: control group in LD muscle; lane 3: treated group in LD muscle; lane 4: control group in ST muscle; lane 5: treated group in ST muscle; and lane 6: mixed sample. Lower panel: results of statistical analysis. No significant rpGH treatment effect, muscle effect or rpGH treatment × muscle interaction for myogenin mRNA expression was observed.
Both MyoD and calpain 3 displayed significant treatment effect irrespective of muscle types. However, the up-regulation of MyoD and calpain 3 mRNA expression responding to rpGH reached the level of statistical significance (P < 0.05) in ST but not in LD muscle when the treatment effects were analysed in each type of muscle, respectively (Figures 4 and 5). In addition, a tendency of down-regulation for the mRNA expression of PGC-1α was seen in ST muscle of rpGH-treated group (P = 0.16, Figure 6).

Figure 4 Effect of recombinant porcine growth hormone (rpGH) administration on myoD mRNA in longissimus dorsi (LD) and semitendinosus (ST) muscles. Upper panel: representative electrophoresis photo. Lane 1: DNA marker PUC19; lane 2: control group in LD muscle; lane 3: treated group in LD muscle; lane 4: control group in ST muscle; lane 5: treated group in ST muscle; and lane 6: mixed sample. Lower panel: results of statistical analysis. No significant muscle effect or rpGH treatment × muscle interaction was seen for myoD mRNA expression. A significant rpGH treatment effect (P < 0.05) was observed irrespective of muscle types. *Indicates significant difference between control and treated group when the differences were compared in LD and ST muscle, respectively (P < 0.05).

Figure 5 Effect of recombinant porcine growth hormone (rpGH) administration on calpain 3 mRNA in longissimus dorsi (LD) and semitendinosus (ST) muscles. Upper panel: representative electrophoresis photo. Lane 1: DNA marker PUC19; lane 2: control group in LD muscle; lane 3: treated group in LD muscle; lane 4: control group in ST muscle; lane 5: treated group in ST muscle; and lane 6: mixed sample. Lower panel: results of statistical analysis. No significant rpGH treatment×muscle interaction was seen for calpain 3 mRNA expression. Significant muscle effect (P < 0.001) and rpGH treatment effect (P < 0.05) were observed. * indicates significant difference between control and treated group when the differences were compared in LD and ST muscle, respectively (P < 0.05).

Figure 6 Effect of recombinant porcine growth hormone (rpGH) administration on PGC-1α mRNA in longissimus dorsi (LD) and semitendinosus (ST) muscles. Upper panel: representative electrophoresis photo. Lane 1: DNA marker PUC19; lane 2: control group in LD muscle; lane 3: treated group in LD muscle; lane 4: control group in ST muscle; lane 5: treated group in ST muscle; and lane 6: mixed sample. Lower panel: results of statistical analysis. No significant rpGH treatment effect, muscle effect or rpGH treatment × muscle interaction for PGC-1α mRNA expression were observed.
Discussion
In the present study, the myofibre-type distribution was not affected by rpGH administration in both LD and ST muscle, as detected with molecular-typing technique developed recently and applied elsewhere (Tanabe et al., Reference Tanabe, Muroya and Chikuni1999; Zhao et al., Reference Zhao, Yang, Wei, Xia and Chen2004). This is in agreement with previous findings obtained by using histochemical staining (Solomon et al., Reference Solomon, Campbell, Steele, Caperna and McMurtry1988 and Reference Solomon, Campbell and Steele1990; Beermann et al., Reference Beermann, Fishell, Roneker, Boyd, Armbruster and Souza1990; Ono et al., Reference Ono, Solomon, Evock-Clover, Steele and Maruyama1995; Sorensen et al., Reference Sorensen, Oksbjerg, Agergaard and Petersen1996) and by directly measuring the mRNA and protein levels of MyHC isoforms (Czerwinski and Martin, Reference Czerwinski and Martin1994). However, there have been some controversial results reported by Solomon et al. (Reference Solomon, Campbell, Steele and Caperna1991) who found that at the body weight of 60 and 90 kg, LD muscle of pigs injected with 100 μg/kg per day pGH from 30 to 60 kg possessed of more oxidative–glycolytic fibres and fewer glycolytic fibres. Later, it is reported that pGH-affected muscle fibre-type composition in a muscle type-specific pattern. ST muscle in pGH-treated pigs (100 μg/kg per day for 42 days) had more oxidative–glycolytic fibres and fewer glycolytic fibres at 30 kg of body weight, whereas this change was not observed in longissimus, semimembranosus and triceps muscle of the same pigs (Solomon et al., Reference Solomon, Caperna, Mroz and Steele1994). The reasons for such discrepancy concerning the effect of rpGH on myofibre types are complex, which involve the age or the body weight when the animals started to receive the rpGH treatment, the length of the treatment, the doses, the routes of administration, the nutritional and physiological status (including genders) of the animals, as well as the muscle types and sensitivity and specificity of the methods used for myofibre typing.
Compared with the inconsistent effect of rpGH on myofibre types, the effect of rpGH on myofibre hypertrophy was observed in nearly all the published studies. Our previous finding of increased expression of GHR and IGF-1 mRNA in ST muscle upon GH administration would explain partly the mechanism of rpGH on myofibre hypertrophy (Zhao et al., Reference Zhao, Zhou, Xu, Zhao, Wei, Xia and Chen2003). However, the responses of candidate downstream genes, such as myostatin, myogenin, myoD, etc. following the activation of GHR and IGF-1, have not been clarified.
Myostatin knockout mice deposit two- to three-fold greater muscle mass than their wild-type littermates due to increase in both muscle fibre number (hyperplasia) and the fibre cross-sectional area (hypotrophy) (McPherron et al., Reference McPherron, Lawler and Lee1997) during embryonic and early postnatal development (Wehling et al., Reference Wehling, Cai and Tidball2000; Whittemore et al., Reference Whittemore, Song, Li, Aghajanian, Davies, Girgenrath, Hill, Jalenak, Kelley, Knight, Maylor, O’Hara, Pearson, Quazi, Ryerson, Tan, Tomkinson, Veldman, Widom, Wright, Wudyka, Zhao and Wolfman2003). A disruption of myostatin function by transgenic expression of its propetide (the 5′ region, 866 nucleotides) was reported to result in significant muscle growth (Yang et al., Reference Yang, Ratovitski, Brady, Solomon, Wells and Wall2001). Nevertheless, the expression of myostatin mRNA did not seem to be influenced by rpGH administration in growing pigs (Ji et al., Reference Ji, Losinski, Cornelius, Frank, Willis, Gerrard, Depreux and Spurlock1998), which is supported by our result that shows no significant difference in myostatin mRNA abundance between control and rpGH-treated pigs. These results hint that myostatin may not be involved in the pathway that mediates the effect of rpGH on muscle growth in pigs.
Myogenin and myoD both belong to the MRF family, but with different functions. MyoD is required for commitment of myoblasts during early myogenic process, whereas myogenin plays a pivotal role in the terminal differentiation of myofibres. Myogenin knockout mice can survive foetal development but die immediately after birth with a severe reduction of all skeletal muscles (Hasty et al., Reference Hasty, Bradley, Morris, Edmondson, Venuti, Olson and Klein1993). During myogenesis, the stimulation of differentiation by IGF-I is (at least in part) a result of substantially increased levels of the mRNA for myogenin (Florini et al., Reference Florini, Ewton and Coolican1996). However, no information is available whether the expression of myoD and myogenin is influenced in rpGH-treated pigs. Our finding is that myogenin expression was unaffected while myoD mRNA expression was up-regulated especially in the ST muscle after rpGH administration. This may indicate that myogenin plays a specific role in early myogenesis or is not involved in the action of rpGH in pigs. Hughes et al. (Reference Hughes, Taylor, Tapscott, Gurley, Carter and Peterson1993 and Reference Hughes, Koishi, Rudnicki and Maggs1997) reported a dramatic increase in myoD expression in the soleus muscle of rats after thyroid hormone treatment. Hence, myoD is not only responsible for myoblast commitment during embryonic myogenesis, but also participates in the satellite cell recruitment during fast growth induced by rpGH in postnatal life of pigs.
The muscle type-specific expression of myoD and myogenin has been addressed previously in some publications. Hughes et al. (Reference Hughes, Taylor, Tapscott, Gurley, Carter and Peterson1993) demonstrated that myoD selectively accumulative in the white muscle while myogenin is selectively accumulative in the red muscle in adult rats. But in cattle, Muroya et al. (Reference Muroya, Nakajima and Chikuni2002) reported no significant difference in myogenin mRNA abundance among slow and fast muscles. Te Pas et al. (Reference Te Pas, Verburg, Gerritsen and de Greef2000) noted that myogenin and myoD expression in different muscles were inconsistent in selection for leanness and selection for porcine growth rate. In our study, these two genes did not show selective expression patterns between the two types of muscle, which partly agrees with Te Pas et al. (Reference Te Pas, Verburg, Gerritsen and de Greef2000) concerning the fast-growing pigs.
Calpain 3, as a skeletal muscle-specific protease, participates in the regulation of myogenesis in cultured C2C12 cell lines via its action on certain proteins belonging to the myogenic regulator factor (Dargelos et al., Reference Dargelos, Moyen, Dedieu, Veschambre, Poussard, Vuillier-Devillers, Brustis and Cottin2002). Mutations in the calpain 3 have been shown to be responsible for limb–girdle muscular dystrophy type 2A (Sorimachi et al., Reference Sorimachi, Kinbara, Kimura, Takahashi, Ishiura, Sasagawa, Sorimachi, Shimada, Tagawa and Maruyama1995). Jones et al. (Reference Jones, Parr, Sensky, Scothern, Bardsley and Buttery1999) demonstrated that calpain 3 was present in all fibres of pigs but at a significantly lower level in slow type I compared with fast type IIA/IIB fibres, as observed in the present study. In the stopped growth caused by feeding a protein-free diet, van den Hemel-Grooten et al. (Reference Van den Hemel-Grooten, te Pas, van den Bosch, Garssen, Schreurs and Verstegen1997) demonstrated that the calpain 3 mRNA expression in LD muscle of pigs was significantly decreased. In the present study, calpain 3 expression increased upon rpGH administration. Moreover, the up-regulation of calpain 3 expression was more remarkable in ST than in LD, demonstrating the muscle type-specific responses of this gene to rpGH treatment. However, Ji et al. (Reference Ji, Frank, Cornelius, Willis and Spurlock1998b) stated that calpain 3 showed no response to 3 mg/day pGH injection in the longssimus muscle of barrow with the initial body weight of 64.2 to 67.4 kg. This difference may be due to the methods used to detect the calpain 3 mRNA expression; the RT-PCR used in the present study is more sensitive than the ribonuclease protection assay used in Ji’s study.
To our knowledge, this is the first report describing the effect of exogenous GH on the expression of PGC-1α in domestic animals. Lin et al. (Reference Lin, Wu, Tarr, Zhang, Wu, Boss, Michael, Puigserver, Isotani, Olson, Lowell, Bassel-Duby and Spiegelman2002) demonstrated that PGC-1α was expressed preferentially in muscle enriched in type I fibres and drove the formation of the oxidative muscle fibre type (slow twitch) in transgenic mice. After prolonged low-intensity physical exercises, PGC-1α expression was increased both in rats (Goto et al., Reference Goto, Terada, Kato, Katoh, Yokozeki, Tabata and Shimokawa2000; Baar et al., Reference Baar, Wende, Jones, Marison, Nolte, Chen, Kelly and Holloszy2002; Terada and Tabata, Reference Terada and Tabata2004; Taylor et al., Reference Taylor, Lamb, Hurst, Chesser, Ellingson, Greenwood, Porter, Herway and Winder2005) and in humans (Russell et al., Reference Russell, Feilchenfeldt, Schreiber, Praz, Crettenand, Gobelet, Meier, Bell, Kralli, Giacobino and Deriaz2003), accompanied with the increased percentage of oxidative fibres. We found in our previous study that PGC-1α mRNA abundance was significantly higher in LD muscle of Erhualian pigs (a Chinese indigenous breed) which possess more oxidative fibres (MyHC 1 and MyHC 2a) compared with Large White pigs (Zhao et al., Reference Zhao, Yang, Wei, Xia and Chen2004). In this context, the tendency of down-regulated PGC-1α expression in ST muscle of treated pigs would indicate more anaerobic metabolisms although no significant alterations were detected for myofibre types. The small proportion of slow myofibre in LD muscle may be attributed to the irresponsiveness of PGC-1α mRNA expression to rpGH treatment, as observed in the present experiment.
In conclusion, myoD, calpain 3 and probably PGC-1α may be involved in the pathway that mediates the action of rpGH skeletal muscle growth. rpGH up-regulates mRNA expression of myoD and calpain 3 in a muscle type-specific manner, being more remarkable in oxidative fibre-enriched muscles.
Acknowledgements
This work was supported by National Basic Research Program of China (2004CB117505) and National Natural Science Foundation of China (project no. 39830290).










