A secundum atrial septal defect is located in the central portion of fossa ovalis and is one of the most common congenital heart defects.Reference Hoffman, Kaplan and Liberthson1 Moderate to large atrial septal defect is associated with significant left-to-right shunts and subsequent increase in pulmonary blood flow. This right-side volume overload of the heart may induce symptoms such as failure to thrive, tachypnoea, or recurrent infections.Reference McDaniel2, Reference Lammers, Hager, Eicken, Lange, Hauser and Hess3 Haemodynamically significant atrial septal defects seldom present with symptoms and are preferably closed electively when the child has reached the age of three to 4 years.Reference Feltes, Bacha and Beekman4, Reference Baumgartner, Bonhoeffer and De Groot5 However, symptomatic atrial septal defects, especially combined with other cardiac or premature comorbidities, may need to be closed earlier. Today, there is no consensus concerning the timing of atrial septal defect closure among these children.
Six percent of all children born in Sweden are born preterm.Reference Morken, Kallen, Hagberg and Jacobsson6, Reference Beck, Wojdyla and Say7 Modern perinatal care has led to improved survival.Reference Fellman, Hellstrom-Westas and Norman8, Reference Norman, Hallberg and Abrahamsson9 Children born preterm have altered cardiac morphological structures as well as functional impairment of their heart, withstanding into adulthood, and sometimes along with additional cardiac and pulmonary sequelae.Reference Bensley, Stacy, De Matteo, Harding and Black10–Reference Lewandowski, Augustine and Lamata13 These cardiac alterations and comorbidities may influence these children’s vulnerability to complications when going through procedures later in life.
A shift towards an early atrial septal defect closure in young and small children with symptomatic atrial septal defects have been noted by us and others.Reference Lammers, Hager, Eicken, Lange, Hauser and Hess3, Reference Bishnoi, Everett and Ringel14, Reference Wood, Holzer and Texter15 Early atrial septal defect closure is considered safe and may be beneficial for pulmonary recovery in preterm children.Reference Zaqout, De Baets and Schelstraete16, Reference Du, Koenig, Cao, Waight, Heitschmidt and Hijazi17 However, few studies have assessed the association between the risk of adverse events following an atrial septal defect closure and preterm morbidity.
We hypothesised that preterm children are at risk for adverse events following atrial septal defect closure (surgical and percutaneous device closure) due to the complex comorbidity and additional cardiac remodelling. The main objective of this study was to explore potential neonatal and clinical factors associated with both major and minor events within 1 year after atrial septal defect closure.
Materials and methods
This is a retrospective case–control study including children under the age of 18 years who were born in Sweden and treated with an atrial septal defect closure (surgery or percutaneous device closure) between January 2000 and December 2014 at Skåne University Hospital in Lund and Astrid Lindgren Children’s Hospital at Karolinska University Hospital in Stockholm.
Children born abroad were excluded, as they are not included in the Swedish Medical Birth Registry and thereby missing important demographic data.
All surgeries were performed at Skåne University Hospital in Lund, and the percutaneous device closures were performed at both centres.
Cases were defined as children with one or more adverse events (either major or minor event), and controls were defined as children without adverse events. Factors related to the atrial septal defect, neonatal as well as cardiac morbidity and chronic pulmonary disease were taken into account as confounding factors (Table 1). Preterm children were stratified according to the World Health Organization definition of gestational age at birth, late premature (32 to <37), very premature (28 to <32 weeks), and extreme premature (<28 weeks).
Table 1. Potential clinical risk factors and confounding factors
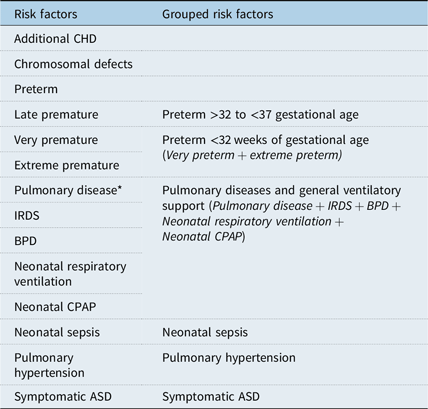
ASD = atrial septal defect; BPD = bronchopulmonary dysplasia; CHD = congenital heart defects; CPAP = continuous positive airway pressure; IRDS = infant respiratory distress syndrome.
* Pulmonary disease includes: Transient tachypnoea of the new-born, IRDS, BPD, Pneumothorax and Congenital Diaphragmatic Hernia.
Demographic data, potential risk factors, and adverse events were retrieved from medical records, the Swedish Registry of Congenial Heart Disease,18 and Swedish Medical Birth Register19 (Table 1). Adverse events were categorised according to severity and were presented in detail in a previous publication.Reference Tanghoj, Liuba, Sjoberg, Rydberg and Naumburg20
The risk factor “Symptomatic atrial septal defects” was used when stated in the medical journal as diagnoses related to heart failure according to International classification of disease (ICD 10 I50.0-9) and/or if the child was treated with antidiuretic drugs.Reference Masarone, Valente and Rubino21, Reference Kantor, Lougheed and Dancea22
The risk factor “pulmonary hypertension” was used when echocardiographic findings indicated a systolic right ventricular pressure ≥25 mmHg, by measures of the tricuspid regurgitant velocity > 2.6 m/second, or when invasive catheter measurements showed a mean pulmonary artery pressure of ≥25 mmHg pulmonary or a pulmonary vascular resistance of ≥3 WU × m2, all prior to the atrial septal defect closure.Reference Abman, Hansmann and Archer23
Definitions of study group inclusion criteria and cases with conversion from percutaneous device closure to surgery
In 41 children who underwent atrial septal defect catheterisation with intention to treat the atrial septal defect, the procedure was converted to surgery due to unfavourable anatomy or insufficient rims. These children were included in the surgical group, and the conversion was not recorded as an adverse event.
In six children, major adverse events requiring surgery (persistent arrhythmias (n = 4), device embolisation (n = 1), and significant residual shunt (n = 1)) occurred after or during percutaneous device closure. These children required device removal and surgical closure of the defect. They were primarily included in the percutaneous device closure group, and the need of surgery was recorded as an adverse event and then additionally included in the surgical group.
Overall, 419 atrial septal defect closures were identified; 266 closed percutaneously and 153 surgically (Fig 1).
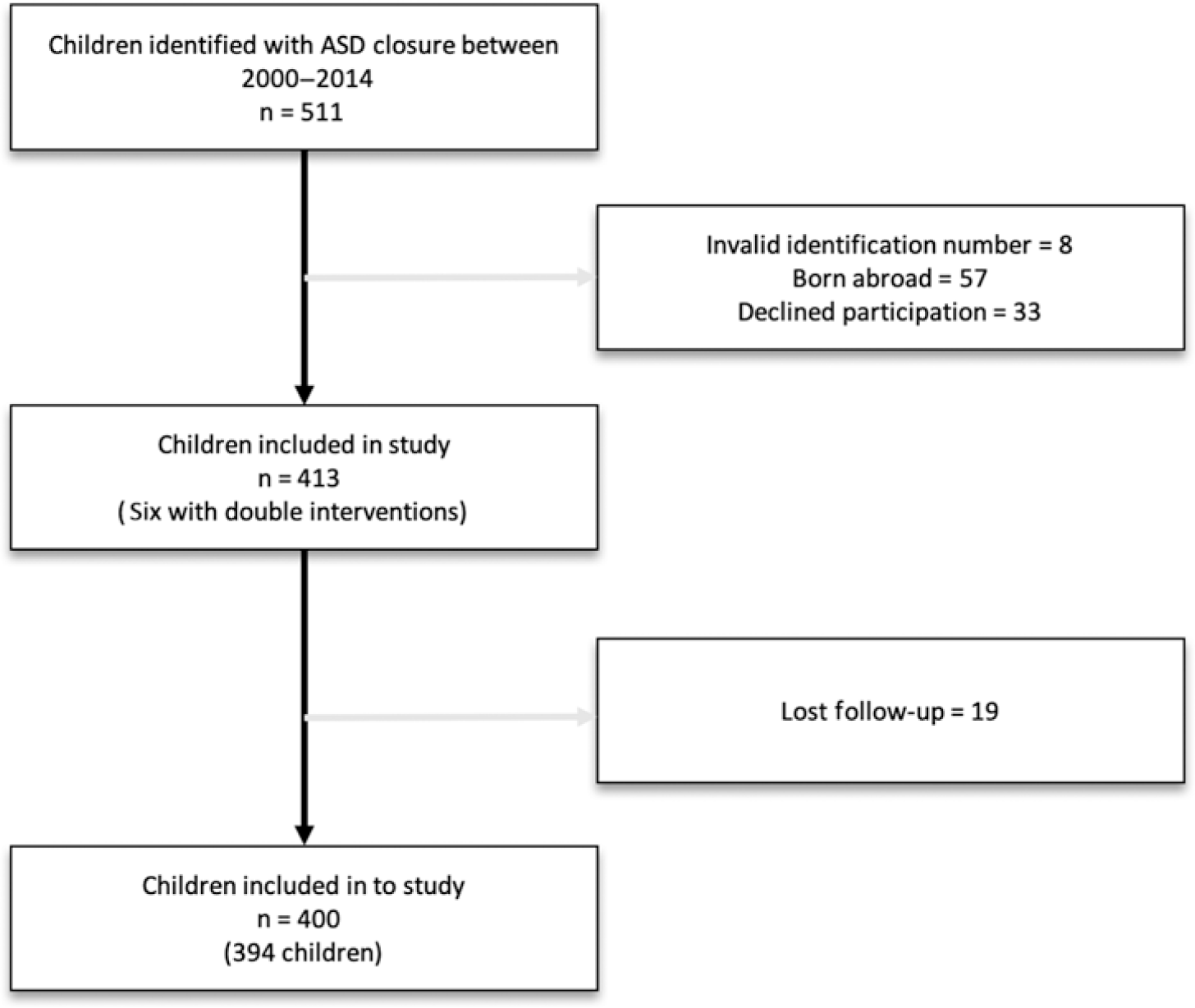
Figure 1. Included children.
Statistical analyses
All data are presented as mean (SD), median (range), or percentage (%) depending on the type and distribution of the data. Continuous data were primarily tested for normality using Shapiro-Wilks test. Student’s t-test (unpaired two-sided) was used for parametrically distributed variables, Mann-Whitney U test for non-parametric distributed variables, and Person’s χ 2 for categorical data, with p < 0.05 considered to be significant.
Conditional logistic regression was performed to evaluate the association between major and minor adverse events and potential risk factors (Table 1). Maximum-likelihood estimates of the OR and 95% CI were obtained, taking into account potential confounding factors.
Univariate logistic regression was performed for both major and minor adverse events and performed for three categories:
1. All atrial septal defect closures
2. Children with percutaneous device closure of atrial septal defect
3. Children with surgical atrial septal defect closure
Conditional logistic regression was performed to evaluate the association between potential risk factors and major as well as minor adverse events following atrial septal defect closure. In this model, additional congenital heart defects and chromosomal defects were excluded as potential risk factors, and all potential synergistic neonatal and clinical factors were grouped according to clinical relation (Table 1). This model was used to reduce the risk of overadjustment and to emphasise clinically linked risk factors.
Results
Overall, 511 children underwent atrial septal defect closure performed at either one of our two centres. A total of 117 children were excluded from the study group due to invalid identification numbers, having been born abroad or declined participation, leaving 394 individual children and 400 individual atrial septal defect closures for analysis (Fig 1).
General characteristics
The median body weight for children with an atrial septal defect closure was 14.5 kg (3.5–110 kg), the median age was 3.0 years (0.1–17.8 years), the median atrial septal defect size 13.0 mm (4.1–37.0 mm), and the median atrial septal defect-to-weight ratio 0.9 mm/kg (0.1–4.3) (Table 2). The female:male ratio was 1.5:1. Further demographic data are presented in Table 3.
Table 2. Study population
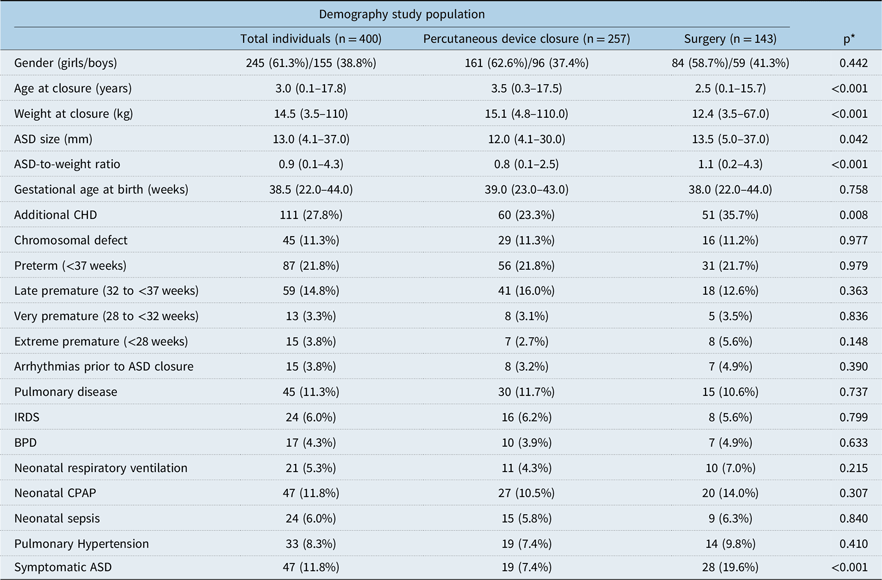
ASD = atrial septal defect; BPD = bronchopulmonary dysplasia; CHD = congenital heart defects; CPAP = continuous positive airway pressure; IRDS = infant respiratory distress syndrome.
* Comparing percutaneous device closure with surgery.
Table 3. Risk factors following all types of ASD closure
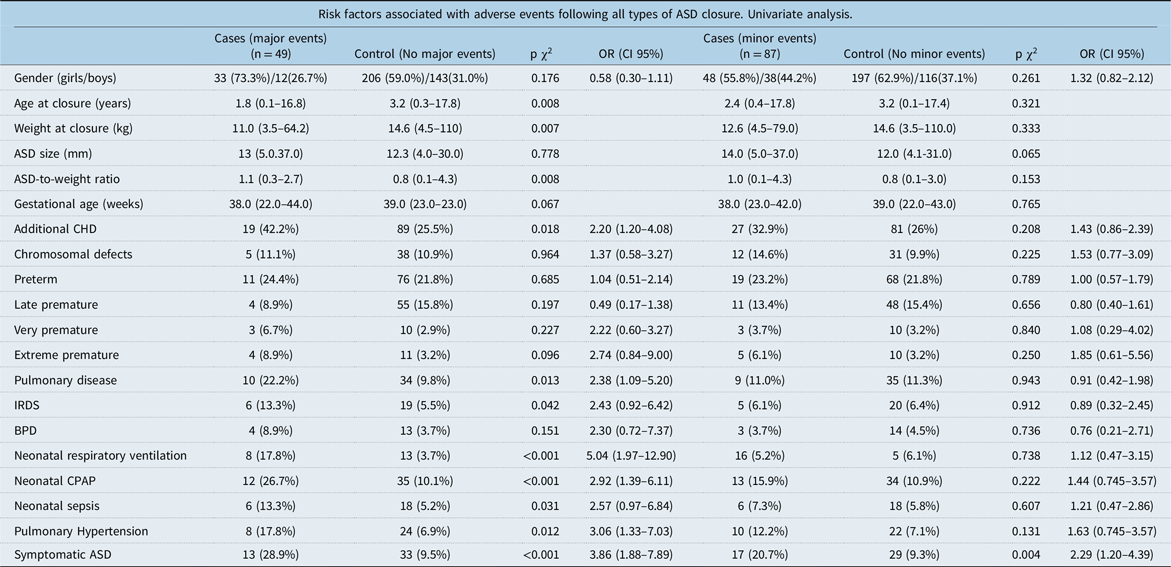
ASD = atrial septal defect; BPD = bronchopulmonary dysplasia; CHD = congenital heart defects; CPAP = continuous positive airway pressure; IRDS = infant respiratory distress syndrome.
Adverse events
Overall, 110 minor adverse events in 87 children and 68 major events in 49 children were recorded. The most common major events were persistent arrhythmias or intraprocedural arrhythmias requiring treatment, accounting for 25 (36.7%) of all major events. Three types of minor events were predominantly common; 34 cases of suspected infection (30.9%), 32 cases with trivial pericardial/pleural effusion (29.1%), and 12 cases of arrhythmias not requiring treatment (10.9%) (Table 4).
Table 4. Risk factors following ASD surgical repair
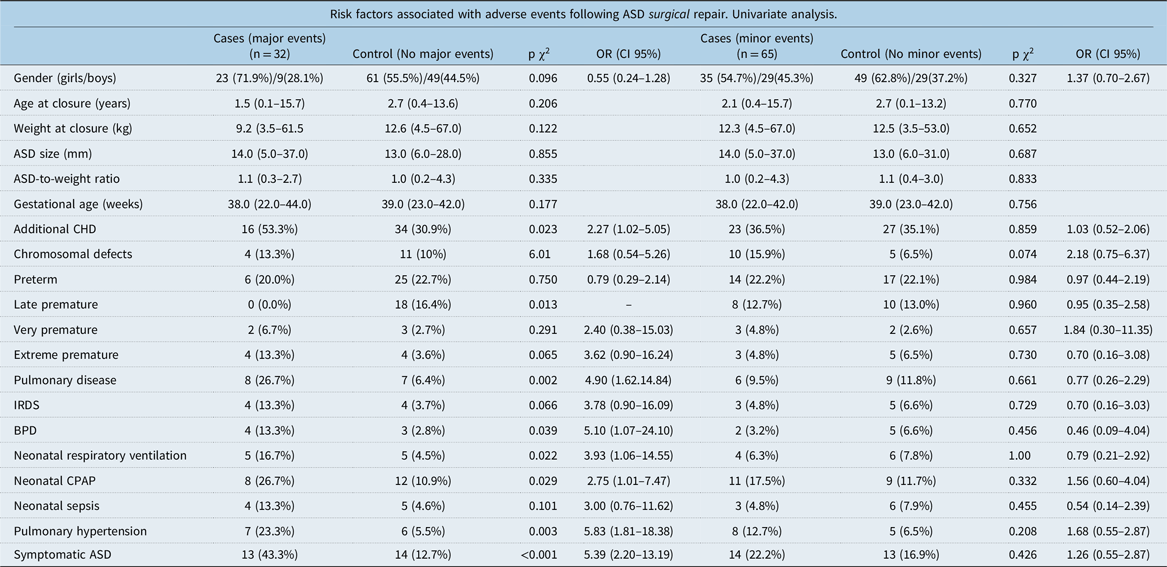
ASD = atrial septal defect; BPD = bronchopulmonary dysplasia; CHD = congenital heart defects; CPAP = continuous positive airway pressure; IRDS = infant respiratory distress syndrome.
Deaths
Four children died during the follow-up period. One 17-year-old child underwent an electrophysiological ablation one day prior to the atrial septal defect closure where a 27 mm Amplatzer septal occlude was used. She died on the 5th day after device placement due to device erosion and pericardial effusion. One child with surgical atrial septal defect closure, at the age of 13 months, died 237 days after surgery due to ventricular arrhythmia. A 12-month-old child with hypertrophic cardiomyopathy died 166 days after surgical atrial septal defect closure due to septic shock. One preterm child, born at a gestational age of 22 weeks, had a surgical atrial septal defect closure at the age of 6 months and died 27 days after surgery due to pulmonary hypertension crisis and multiple organ failure.
Associations between risk factors and adverse events. Univariate regression model and distribution
None of the sub-groups of premature birth were associated with neither minor nor major events, regardless of type of atrial septal defect closure (Table 3).
Some neonatal comorbidities that are common among children born prematurely were more common in cases with major events compared to controls. There were, for example, more cases with pulmonary disease (10 cases (22.2%) versus 34 controls (9.8%), p = 0.013), infant respiratory distress syndrome (IRDS) (6 cases (13.3%) versus 19 controls (5.5%), p = 0.042), need of neonatal respiratory ventilation (8 cases (17.8%) versus 13 controls (3.7%), p < 0.001), neonatal continuous positive airway pressure (CPAP) (12 cases (26.7%) versus 35 controls (10.1%), p < 0.001), and neonatal sepsis (6 cases (13.3%) versus 18 controls (5.2%), p = 0.031)(see Table 3).
Many of these factors also showed increased risks for adverse events in univariate regression analysis (see Table 3–5).
Table 5. Risk factors following all types of percutaneous device ASD closure
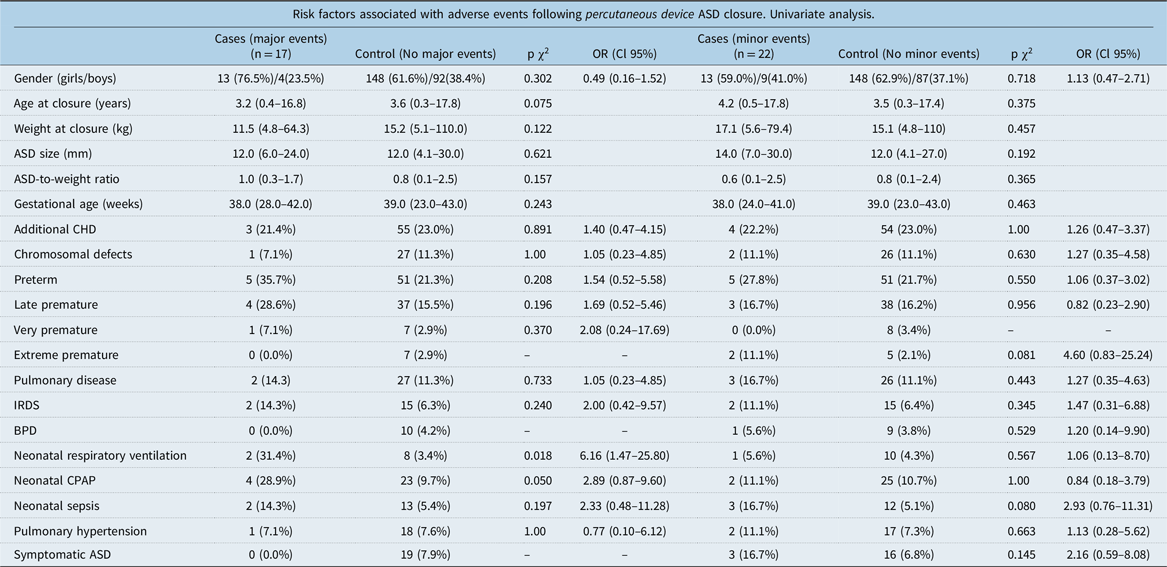
ASD = atrial septal defect; BPD = bronchopulmonary dysplasia; CHD = congenital heart defects; CPAP = continuous positive airway pressure; IRDS = infant respiratory distress syndrome.
Especially among children with surgically closed atrial septal defect, risk factors such as neonatal pulmonary disease (OR = 4.90 CI 95% 1.62–14.84), bronchopulmonary dysplasia (OR = 5.10 CI 95% 1.07–24.10), need of neonatal respiratory ventilation (OR = 3.93 CI 95% 1.06–14.55), neonatal CPAP (OR = 2.75 CI 95% 1.01–7.47), symptomatic atrial septal defects (OR = 5.39 CI 95% 2.20–13.19), pulmonary hypertension (OR = 5.83 CI 95% 1.81–18.38), and additional congenital heart defects (OR = 2.27 CI 95% 1.02–5.05) were all associated with major adverse events in the univariate conditional regression analyses (Table 4).
Among children with percutaneously closed atrial septal defect, neonatal respiratory ventilation (OR = 6.16 Cl 95% 1.47–25.80) was associated with major events in univariate conditional regression analyses (Table 5).
Associations between risk factors and adverse events. Multivariate adjusted regression model
Neither premature birth, as a group or by gestational sub-groups, pulmonary diseases, general ventilatory support nor neonatal sepsis were associated with major events following atrial septal defect closure after adjusting for potential confounding factors (Table 6). Symptomatic atrial septal defects were associated with both major adverse events (OR = 2.80 CI 95% 1.23–6.37) and minor adverse events (OR = 2.18 CI 95% 1.05–8.06) following all types of atrial septal defect closure and adjusted for confounding factors. Among children with surgical atrial septal defect closure, symptomatic atrial septal defects were associated with major adverse events (OR = 4.50 CI 95% 1.47–13.80), taking into account potential confounding factors (Table 6).
Table 6. Adjusted risk factors
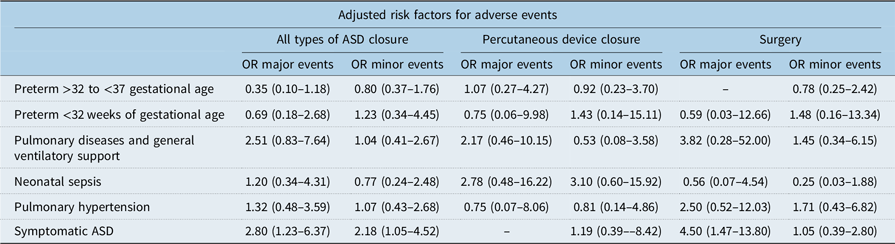
ASD = atrial septal defect.
Discussion
This is one of the first studies assessing neonatal risk factors’ association to adverse events after atrial septal defect closure. Some risk factors such as neonatal respiratory support as well as presence of pulmonary hypertension, additional congenital heart defects, pulmonary disease, and symptomatic atrial septal defects were associated with major adverse events following atrial septal defect closure in the univariate analyses. However, in the multivariate analysis, adjusted for potential confounding risk factors, this association was only present among children with symptomatic atrial septal defects. Hence, few or none of the neonatal factors seem to be associated with adverse events after atrial septal defect closure. Though preterm children are a heterogeneous group with multifactorial morbidity, our results are encouraging and an early indicated necessitous atrial septal defect closure can be regarded as safe in these children.
Few studies have previously described adverse events in small children after atrial septal defect closure.Reference Sadiq, Kazmi, Rehman, Latif, Hyder and Qureshi24, Reference Du, Hijazi, Kleinman, Silverman and Larntz25 These studies have assessed several risk factors but have not included neonatal factors and comorbidities associated with preterm birth.Reference Nykanen, Forbes and Du26–Reference Wyss, Quandt and Weber28 The combination of cardiac alterations and neonatal morbidity may have an aggravating synergistic effect and potentially contribute to an increased risk of adverse events after atrial septal defect closure.Reference Broadhouse, Finnemore and Price11, Reference Schubert, Muller, Abdul-Khaliq and Norman12
It is well known that there are technical difficulties inherent in percutaneous device closure in smaller children, and thus surgical closure might have been the choice of preference in smaller children in our study. Further, a larger atrial septal defect-to-weight ratio is a known risk factor associated with adverse events in surgical closure.Reference Du, Hijazi, Kleinman, Silverman and Larntz25, Reference Butera, Carminati and Chessa29 Our study supports these findings, as children with major events following all atrial septal defect closure were lighter, younger, and had a larger atrial septal defect-to-weight ratio compared to controls.
Preterm birth, regardless of gestational age group, was not associated with an increased risk of major adverse events following atrial septal defect closure.
Additional congenital heart defects, neonatal respiratory support, pulmonary comorbidity, and neonatal sepsis often have to be taken into consideration in the care of the preterm child. These factors were more common among cases with major events following atrial septal defect closure, but did not reach significance as risk factors in the adjusted model. Furthermore, the prematurely born children may suffer from reduced right ventricular function, altered pulmonary capillary bed maturation, and sub-clinical pulmonary hypertension.Reference Bensley, Stacy, De Matteo, Harding and Black10, Reference Schubert, Muller, Abdul-Khaliq and Norman12, Reference Saleemi, El-Khuffash, Franklin and Corcoran30, Reference Levy, Patel, Choudhry, Hamvas and Singh31 All this combined may contribute to an increased risk of symptoms of atrial septal defect as well as adverse events following closure. None of the above risk factors, except for symptomatic atrial septal defects, were independently associated with adverse events after atrial septal defect closure when adjusted for potential confounding factors. This indicates that the altered morphological, global structural differences and functional impairment of the preterm child’s heart does not influence the risk of adverse events after atrial septal defect closure.Reference Bensley, Stacy, De Matteo, Harding and Black10–Reference Lewandowski, Augustine and Lamata13 Premature children with severe pulmonary comorbidity may however benefit from early atrial septal defect closure as has been suggested previously.Reference Bishnoi, Everett and Ringel14, Reference Diab, Cao, Bacha and Hijazi32 Further studies identifying benefits of early atrial septal defect closure in this group of new survivors are needed.
Symptomatic atrial septal defects or clinically manifested heart failure has been reported to be present in 11.6–20% of children with atrial septal defect and even more common in children with large atrial septal defects.Reference Azhari, Shihata and Al-Fatani33, Reference Shaddy, George and Jaecklin34 This is in line with our study, as 48 (12%) of the children had symptomatic atrial septal defects. This rules out the risk of selection bias due to overtreatment in our study. Children with symptomatic atrial septal defects were almost three times as common among children with major events (28.9 vs. 9.5%) and twice as common among children with minor events (20.7 vs. 9.8%) compared to controls in our study. Even after adjusting for potential confounding factors, the risk of major adverse events was almost three times as high and minor events twice as high in children with symptomatic atrial septal defects. This indicates that children with symptomatic atrial septal defects may carry a more severe morbidity compared to children with no symptoms. The bias for selection to surgical repair might be in favour for children with severe atrial septal defect morbidity, which in turn may increase the risk of major events in the surgical cases even after adjusting for all other factors.Reference Du, Hijazi, Kleinman, Silverman and Larntz25, Reference Butera, Carminati and Chessa29 For complex and large atrial septal defect, surgical repair is still considered the most favourable method with long-term favourable outcomes.Reference Cuypers, Opic and Menting35
Significant arrhythmias occurred in 11 (22.9%) of all children in our study. This was more common among children with symptomatic atrial septal defects. Symptomatic atrial septal defects, often with an enlarged right ventricle caused by volume overload, may induce the same trigger to arrhythmias in cases of atrial septal defect closure as in other congenital heart defects such as tetralogy of Fallot.Reference Redington36 Thus, to decrease the risk of arrhythmias, these children might benefit from an early atrial septal defect closure.
This study is limited by the retrospective design, which may increase the risk of selection and recall bias. However, we believe that the risk of missing data due to incomplete registration in medical records and national registers is limited as we used both sources to retrieve demographic data, potential risk and confounding factors, and information on adverse events. Recent validation studies of the two registries in our study have found good coherence between the medical records and registries.Reference Cnattingius, Ericson, Gunnarskog and Kallen37, Reference Bodell, Björkhem, Thilén and Naumburg38 Further, the number of included cases and controls in our study is substantial, which often increases the power in the conditional logistic analyses. However, since our study had cases with few adverse events, a risk of non-robust models was present.Reference Sperandei39 Thus, to reduce this risk, we grouped the similar potential synergistic factors in a second model of analysis (Table 1). The adjusted risk of adverse events within one year after atrial septal defect closure did not alter in the second model.
In our study, we obtained data from two of the Swedish paediatric cardiac centres, performing two-thirds of all percutaneous device closure and half of all paediatric heart surgeries, according to the 2017 annual rapport from the Swedish Registry of Congenital Heart Disease.Reference Ulf Thilén, Jeremiasen, Björkhem and Bergman40 One of the two interventional centres refers patients for surgery to the other centre. By this approach, a harmonisation concerning indications and choice of interventional method (surgery or percutaneous) is made and reduces the risk of selection biased due to method in this study.
Conclusion
Neonatal comorbidities were not associated with increased risk of major and minor adverse events after atrial septal defect closure. Children with neonatal pulmonary morbidity may be a selected group suffering from right-ventricular function impairment and reduced pulmonary capillary bed maturation, and a careful assessment and risk stratification can be advised in this group.
Children with symptomatic atrial septal defects have a two to four times increased risk of major events, predominantly peri-interventional arrhythmias, during or following an atrial septal defect closure, suggesting that a post-interventional follow-up with regard to arrhythmias should be considered in this group.
Acknowledgements
We would like to thank the Swedish Registry of Congenial Heart Disease register as well as Swedish Medical Birth Register and their steering committees for sharing data, and we acknowledge all the paediatric cardiology doctors and nurses in Sweden. Further, we also thank Annika Maxedius, Skåne University Lunds Hospital for her support.
Financial Support
This study was funded by the Unit of Research, Education and Development, Östersund Hospital, Region Jämtland Härjedalen.
Conflict of Interests
None.
Ethical Standards
The authors assure that all procedures contributing to this work is in adherence with the ethical standards of the Helsinki Declaration of 1975, as revised in 2008.
This study was approved by the Ethics Committee for Human Research at Umeå University (D-nr 2015-10-31M allteration 2015-88-32M), and informed consent was optained by everyone in the study population or each guardian of the included children.









