Since 1990 organic food sales have been increasing by 20 % per year in Europe and North America(Reference Oberholtz, Dimitri and Greene1). Consumer studies suggest multiple reasons for buying organic fruits and vegetables, for example, they taste better, are healthier and safer(Reference Chassy, Bui and Renaud2). It has been shown that organic fruits and vegetables have a lower content of pesticides than conventionally produced foods. Furthermore, it has been hypothesised that organically grown fruits and vegetables could be healthier due to a higher content of phytochemicals (for example, carotenoids)(Reference Zhao, Carey and Wang3–Reference Mercadante and Rodriguez-Amaya8).
Several studies have been conducted to gain information about the impact of the agricultural system on carotenoids. A higher carotenoid content was found in organically grown sweet peppers, tomatoes, yellow plums, carrots, celery and kale(Reference Perrez-Lopez, Lopez-Nicolas and Nunez-Delicado4–Reference Mercadante and Rodriguez-Amaya8). In contrast a lower content was found in organically produced carrots, tomatoes and grapefruits(Reference Warman and Havard9–Reference Lester and Eischen11). No differences in carrots, cabbage, leek, potatoes, spinach and lettuce were determined in an older study(Reference Schuphan12). These inconsistencies suggest that factors other than the organic production method might affect carotenoid content, for example, the cultivar, the microclimate, the degree of ripeness and the soil conditions. Several of the conducted studies did not take these factors into account(Reference Caris-Veyrat, Amiot and Tyssandier5, Reference Bourn and Prescott13, Reference Haker14). Therefore, the results are inconclusive. Obviously, there is a need for more studies comparing the carotenoid content of fruits and vegetables under well-controlled conditions.
To date, only one human intervention study has been conducted to compare the bioavailability of carotenoids from organically v. conventionally produced tomato purée in human subjects(Reference Caris-Veyrat, Amiot and Tyssandier5). In this study, no differences between the bioavailability of lycopene and β-carotene from organically v. conventionally produced tomato purée were observed. Additionally, there were no significant differences in polyphenol and vitamin C concentrations between the two groups(Reference Caris-Veyrat, Amiot and Tyssandier5).
Further human intervention studies were conducted to investigate the bioavailability of phenolic compounds from a diet based on organic food, red wine and apples(Reference Grinder-Pedersen, Rasmussen and Bugel15–Reference Briviba, Stracke and Rüfer18). A diet based on organically produced food (meat, potato, wheat, rye, fruits, vegetables) resulted in a higher urinary excretion of quercetin and kaempherol, increased protein oxidation and decreased plasma antioxidant capacity in the group that ate organically produced food(Reference Grinder-Pedersen, Rasmussen and Bugel15). The plasma antioxidant capacity in the subjects receiving a diet based on organically produced food was higher than by a diet based on conventionally produced food(Reference Di Renzo, Di Pierro and Bigioni16). In humans the ingestion of conventionally produced red wine led to a decrease in Cu2+-induced formation of thiobarbituric acid-reactive substances. In contrast, the consumption of organically produced red wine increased the catalase activity in erythrocytes(Reference Akcay, Yildirim and Guvenc17). Consumption of organic or conventional apples (1 kg) showed no effect on the antioxidant capacity of LDL (lag-time test), endogenous DNA strand breaks, Fpg protein-sensitive sites or capacity to protect DNA against damage caused by H2O2. However, 24 h after apple consumption in both intervention groups, decreased levels of endonuclease III-sensitive sites and an increased capacity to protect DNA against damage induced by iron chloride were observed(Reference Briviba, Stracke and Rüfer18). Studies have shown that carotenoids positively affect the antioxidant capacity and the immune system in humans(Reference Hughes19).
There are no data on whether the agricultural system affects the bioavailability of carotenoids and physiological effects after the consumption of organically and conventionally produced carrots. It has been shown that the bioavailability of carotenoids may be affected by the food matrix. Organically produced carrots generally have a higher DM content than conventionally produced ones(Reference Fjelkner-Modig, Bengtsson and Stegmark20, Reference Beckmann and Pestemer21); this could result from different food matrices. So, one might speculate that the agricultural system affects the bioavailability of carotenoids from carrots.
Therefore, the aim of the present study was to evaluate the carotenoid content and the antioxidant capacity of carrots grown in Northern Germany under either defined organic or conventional agricultural conditions. Furthermore, we investigated whether the agricultural farming system influences carotenoid bioavailability, antioxidant status and the immune system in healthy young men after the consumption of blanched carrots.
Materials and methods
Chemicals
All chemicals were purchased from Carl Roth (Karlsruhe, Germany), Sigma-Aldrich (Taufkirchen, Germany) or Merck (Darmstadt, Germany).
Carrot samples
Carrots (Daucus carota ssp. sativus, variety Narbonne) were harvested in 2005 from two farms in Schleswig-Holstein, County Dithmarschen, Germany. Carrots from organic and conventional farming systems were used. The distance between the organic and conventional fields averaged 3 km. The fertilisation, harvest and distribution of the carrots were monitored by the Institute of Organic Farming of the Johann Heinrich von Thünen-Institute, Federal Research Institute for Rural Areas, Forestry and Fisheries at Westerau. Soil and microclimate conditions were comparable. After harvest the carrot samples were stored at 0·4–4°C and 98 % humidity for 2 weeks. For the human intervention study the carrots were sliced and blanched using a steamer (Konvektomat type E-HD 1011 P; Imperial, Bünde, Germany) for 3 min at 110°C. Thereafter, the carrots were packed in 200 g portions and stored at − 30°C until use.
Subjects
Thirty-six non-smoking men, aged between 19 and 54 years were recruited. The subjects were healthy according to clinical examination and disease history. They refrained from taking vitamin supplements or any medication for 6 months before and during the study. The study was approved by the Landesärztekammer Baden-Württemberg and all participants gave their written consent.
Study design
The study was a randomised, controlled, double-blind trial of 6-week duration. Study participants were allocated to three groups (n 12 per group). Intervention was preceded by a 4-week low-carotenoid period where subjects were asked to refrain from foods rich in carotenoids (for example, carrots, carrot products, lettuce and spinach). Intervention lasted from day 0 to day 14. During the intervention period the volunteers of the intervention groups consumed daily 200 g blanched carrots from organic or conventional agricultural systems for 2 weeks. Subjects were told to consume the carrots with a main meal and with a minimum of 10 g fat. The third group was a control group who maintained a carotenoid-restricted diet throughout the total study period of 6 weeks.
Collection and preparation of blood samples
Fasting blood samples were taken in the morning before carrot intake between 07.00 and 10.00 hours at day 0 as well as on days 2, 7 and 14 of the intervention period. Blood was drawn from an antecubital vein into prechilled tubes containing EDTA (1·6 g/l, Monovette; Sarstedt AG & Co., Nümbrecht, Germany) for carotenoid and antioxidant status and into lithium heparin tubes (Sarstedt) for immune parameters. Plasma was collected after centrifugation at 1500 g for 10 min at 4°C. Tubes without anticoagulant (Serum-Monovette; Sarstedt AG & Co.) were used for serum collections. Blood was allowed to clot at room temperature for 30 min and was centrifuged at 1500 g for 10 min at room temperature. Serum was stored at − 30°C and plasma at − 80°C until analysis.
Methods for analyses in carrots
Carotenoid extraction from blanched carrots
Five grams of one 200 g portion of blanched carrots were homogenised using a laboratory blender (B400; Büchi Labortechnik AG, Flawil, Switzerland). Samples of 0·3 g were further homogenised in 50 μl 100 mm-EDTA and 5 ml acetone containing 0·01 % butylated hydroxytoluene using an Ultra Turrax T25 (IKA, Staufen, Germany). The homogenate was centrifuged at 3000 g for 5 min at 4°C and the organic extracts were collected in a separate tube. Extraction was repeated until the organic extracts were colourless. After washing the combined organic extracts with 5 ml of a saturated NaCl solution the organic solvent was removed using a rotary evaporator (Laborota 4003-digital; Heidolph, Schwabach, Germany). The remaining aqueous phase was extracted three times with 2 ml n-hexane containing 0·01 % butylated hydroxytoluene and the combined organic extracts were evaporated to dryness under a stream of N2 gas. For HPLC analysis the residue was dissolved in 200 μl of acetone–dichloromethane (10:1, v/v, 0·01 % butylated hydroxytoluene) and 50 μl were used for HPLC analysis. In total, samples from five different 200 g portions were analysed.
Analysis of dry matter in carrots
About 5 g of the homogenates were dried for 5 h at 110°C. The analysis was repeated five times.
High-performance liquid chromatography analysis
HPLC analysis was performed on a low-pressure gradient system from Shimadzu (Duisburg, Germany) equipped with an autoinjector, column oven and photodiode array detector. The autoinjector was set to 10°C and the column oven to 27°C.
Separation was carried out on a 250 × 4·6 mm internal diameter, 5 μm, YMC ‘Carotenoid’ S5 reversed-phase C30 column with a corresponding 10 × 4·0 mm internal diameter guard column (YMC Europe GmbH, Dinslaken, Germany). Solvent A consisted of tert-butyl methyl ether, solvent B of methanol and solvent C of water. A linear gradient was used starting with 20 % A, 60 % B and 20 % C going to 80 % A, 20 % B and 0 % C within 50 min. The flow rate was 1 ml/min and the detection wavelength was set to λ = 450 nm. Quantification was performed by external calibration using reference compounds. Calibration curves of different carotenoids were performed in the range of 0·025–25 mmol/l, in which the linearity of the response was given. The recovery for all carotenoids was greater than 95 %. The CV of the method was below 5 % (intra-assay).
Extraction of lipophilic antioxidants from carrots
Samples of 0·2 g of the homogenised carrots (see above) were further homogenised in 10 ml acetone for 10 min in an ice-cold ultrasonic bath. The homogenate was centrifuged at 3000 g for 5 min at 4°C and the supernatant fraction was collected. Extraction was repeated until the pellets were colourless. After washing the combined organic extracts twice with 5 ml n-hexane and 1 ml saturated NaCl solution, the organic extracts were evaporated to dryness under a stream of N2 gas. The extracts were dissolved in 3 ml acetone for analysis.
Antioxidant activity in lipophilic carrot extracts
To determine the lipophilic antioxidant activity in carrots the Trolox equivalent antioxidative capacity (TEAC) assay as described by Re et al. was used(Reference Re, Pellegrini and Proteggente22). The reaction was carried out in ethanol; the final reaction mixture volume was 300 μl. Trolox at 10, 20, 30 and 50 μmol/l (used as standards), 100 μl of carrot samples (in acetone) (see below) or acetone alone (blank) were added in ninety-six-well plates (Greiner, Frickenhausen, Germany). The 2,2′-azino-bis(3-ethxylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical cation (ABTS∙+) was formed by the reaction of a 7 mm-ABTS stock solution with 2·45 mm-potassium persulfate (final concentrations) in the dark at room temperature for 12–16 h before use. The ABTS∙+ solution was diluted with ethanol to an absorbance of 0·70 (sd 0·02) at 734 nm. The reaction mixture was started by the addition of 200 μl prewarmed (30°C) ABTS∙+ solution per well. The absorbance was measured after 2·5 min incubation at 30°C in a microplate reader (Tecan safire2; Tecan, Crailsheim, Germany). The microplate was shaken before each reading. The carrot extracts were diluted appropriately in acetone. All analyses were performed fourfold. The Trolox equivalent concentration of the carrots was calculated by subtracting the blank reading from samples and standards. A linear calibration curve for each assay was constructed. Intra- and inter-assay variations were always less than 5 %.
Methods for analyses in blood samples
Serum parameters
Glucose, TAG, cholesterol and uric acid were determined using enzymic kits (Boehringer Mannheim, Germany).
Carotenoids, vitamin E and vitamin C in plasma
Concentrations of carotenoids in plasma (α- and β-carotene, lycopene, lutein, zeaxanthin and β-cryptoxanthin) and vitamin E (α-tocopherol) were determined according to the method described previously(Reference Briviba, Schnäbele and Rechkemmer23). The same HPLC system as for the determination of carotenoids in carrots was used. The vitamin C concentration was determined as described earlier(Reference Watzl, Kulling and Möseneder24).
Antioxidant activity in plasma
Total antioxidant status in plasma was analysed using the ferric-reducing ability of plasma (FRAP)(Reference Benzie and Strain25), the oxygen radical absorbance capacity (ORAC)(Reference Cao and Prior26) and the TEAC(Reference Re, Pellegrini and Proteggente22) assays.
Low-density lipoprotein oxidation
An ultracentrifugation method was used to isolate LDL from plasma(Reference Kleinveld, Hak-Lemmers and Stalenhoef27). The ex vivo oxidation of LDL was performed using a modification of the procedure described by Esterbauer et al. (Reference Esterbauer, Striegl and Puhl28). The LDL concentration in the PBS solution was determined by measuring the protein concentration and was adjusted for the oxidation assay to 100 μg/ml protein. The LDL oxidation process was followed by determining the formation of conjugated dienes by measuring the absorption at λ = 234 nm in a microplate reader (Tecan safire2). The reaction was carried out in PBS (100 mmol/l; pH 6·0) and the final reaction mixture was 330 μl. In each well of a ninety-six-well plate 300 μl of a 100 μg/ml LDL solution were added. The reaction was started by the addition of CuCl2 (final concentration 20 μmol/l). The kinetics of oxidation was monitored by recording the absorption every 3 min for ≤ 6 h at 37°C. All reaction mixtures were prepared in triplicate. For quantification, the oxidation kinetics was analysed on the basis of the oxidation lag phase which was defined as the interval between the initiation of the oxidation and the intercept of the tangent for the slope of the absorbance curve during the propagation phase. Intra- and inter-assay variations were less than 5 and 8 %, respectively.
Methods of analyses in peripheral blood mononuclear cells
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation at 400 g for 20 min at 20°C, and the cells were counted using a Coulter Counter Z2 (Beckman Coulter, Krefeld, Germany). Autologous serum from each subject for the culture of PBMC was heat-inactivated for 30 min at 56°C.
Quantification of cytokine secretion
PBMC were cultured at 1 × 109 cells/l in Roswell Park Memorial Institute (RPMI) medium containing 10 % autologous serum +1 % penicillin/streptomycin and were stimulated by a mixture of 2·5 mg concanavalin A/l and 1 μg lipopolysaccharide (Escherichia coli 0111:B4)/l for 24 h at 37°C to measure the production of the T helper 1 and T helper 2 cytokines GM-CSF, interferon-γ, IL-2, IL-4, IL-5, IL-10, IL-12 (p70), IL-13 and TNF-α by a bead-based multiplex cytokine assay. Cell-free supernatant fraction fluid was collected and stored at − 80°C until analysis. The multiplex assay was performed according to the manufacturer's instructions (Bio-Plex Human Cytokine Th1/Th2 Panel; Bio-Rad Laboratories, Munich, Germany). Measurements and data analyses with five-parametric-curve fitting were performed on the Bio-Plex System in combination with the Bio-Plex Manager software version 4.1 (BioRad Laboratories).
Quantification of natural killer cells
The percentage of CD3− CD16+CD56+ natural killer (NK) cells was measured by flow cytometry (FACSCalibur; BectonDickinson, Heidelberg, Germany) as described earlier(Reference Watzl, Kulling and Möseneder24). Antibodies to quantify NK cells were purchased from Becton Dickinson (Simultest).
Peripheral blood mononuclear cell proliferation and lytic activiy of natural killer cells
Proliferation of PBMC and the NK cell activity of PBMC were determined as described previously(Reference Briviba, Schnäbele and Rechkemmer23, Reference Watzl, Bub and Brandstetter29).
Single cell microgel electrophoresis assay (comet assay)
DNA damage was measured by the single cell microgel electrophoresis assay (comet assay) described previously(Reference Briviba, Stracke and Rüfer18).
Carotenoid concentration in peripheral blood mononuclear cells
For analysis of the carotenoid concentration in PBMC 10 × 106 cells were used. Cells were lysed in an ultrasonic bath. Extraction and HPLC analysis were conducted in accordance with the procedure for plasma.
Statistical analysis
All statistical calculations were performed using the StatView program (version 5.0; SAS Institute, Inc., Cary, NC, USA). Results are reported as mean values and standard deviations. Differences between the mean values of the α-carotene, β-carotene and lutein concentrations and the antioxidant activity in carrots were statistically analysed using the unpaired Student's t test. Changes between the baseline (day 0) and the following time points among the three treatment groups were tested for significance by repeated-measures ANOVA and the Tukey–Kramer post hoc test. Plasma α-carotene concentrations were transformed twice logarithmically since the equal variance and normal assumptions of ANOVA were rejected by the Forsythe-Brown test. Differences were considered significant at P < 0·05.
Results
Carotenoid content and antioxidant activity in carrots
The total carotenoid content (sum of α-carotene, β-carotene and lutein) in organically and conventionally grown blanched carrots was 121 (sd 7) and 116 (sd 13) μg/g with a major contribution from β-carotene (Fig. 1). The results of the lipophilic antioxidant capacity of blanched carrots are listed in Table 1. No statistically significant difference was observed.

Fig. 1 Carotenoid content of blanched carrots (Daucus carota ssp. sativus, variety Narbonne). (![]() ), Lutein; (
), Lutein; (![]() ), α-carotene; (
), α-carotene; (![]() ), β-carotene; (
), β-carotene; (![]() ), total carotenoids. Total carotenoids was calculated as the sum of the carotenoids lutein, α-carotene and β-carotene. Values are means (n 5), with standard deviations represented by vertical bars. There were no significant differences (unpaired Student's t test).
), total carotenoids. Total carotenoids was calculated as the sum of the carotenoids lutein, α-carotene and β-carotene. Values are means (n 5), with standard deviations represented by vertical bars. There were no significant differences (unpaired Student's t test).
Table 1 Lipophilic antioxidant activity of blanched carrots (Daucus carota ssp. sativus, variety Narbonne)*
(Mean values and standard deviations for five different samples)
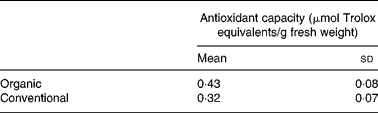
* There were no significant differences (unpaired Student's t test).
Dry matter content of carrots
The DM content of the analysed carrots ranged between 11·1 and 18·4 %. No significant differences were observed between the organically (13·3 (sd 2·9) %) and conventionally (13·7 (sd 2·6) %) produced carrots.
Intervention study
All thirty-six volunteers completed the study. The parameters age, BMI, blood glucose, lipid profile, as well as plasma vitamins C and E at day 0 are summarised in Table 2. No significant differences were seen between the three groups. Intervention with carrots did not affect plasma glucose, uric acid, TAG, cholesterol, vitamin C and vitamin E significantly (data not shown).
Table 2 Characteristics of study participants at day 0 of the intervention period*
(Mean values and standard deviations)

* No significant differences were observed between the groups (ANOVA).
In Figs. 2 (a) and (b) the changes in plasma α- and β-carotene concentrations are shown. After consumption of organically as well as conventionally produced carrots plasma α- and β-carotene in both intervention groups increased significantly compared with the control group. While plasma lutein increased significantly in the organic group, no differences were observed between groups for zeaxanthin and β-cryptoxanthin (data not shown). The lycopene concentration decreased in both intervention groups (data not shown). There were no significant differences between the organic and conventional groups in any of the carotenoids analysed.

Fig. 2 Changes in α-carotene (a) and β-carotene (b) concentrations during the intervention period in the organic carrot-fed group (–●–), the conventionally grown carrot-fed group (–○–) and the control group (–▾–). Values are means (n 12), with standard deviations represented by vertical bars. * Mean value was significantly different from that at day 0 (P < 0·001; repeated-measures ANOVA; Tukey–Kramer post hoc test).
The antioxidant status at day 0 and day 14 of the intervention period is shown in Tables 3 and 4. Carrot consumption had no effect on the antioxidant status in plasma, as determined by the FRAP, TEAC and ORAC assays. Additionally, no effect on the ex vivo LDL oxidation was found.
Table 3 Parameters of the antioxidant status of the subjects at day 0*
(Mean values and standard deviations)

FRAP, ferric-reducing ability of plasma; ORAC, oxygen radical absorbance capacity; TEAC, Trolox equivalent antioxidative capacity.
* No significant differences were observed between the groups (ANOVA).
Table 4 Parameters of the antioxidant status of the subjects at day 14*
(Mean values and standard deviations)

FRAP, ferric-reducing ability of plasma; ORAC, oxygen radical absorbance capacity; TEAC, Trolox equivalent antioxidative capacity.
* No significant intervention effects were observed compared with day 0 (group and time × group) (repeated-measures ANOVA).
Carotenoid concentrations in peripheral blood mononuclear cells
Carrot intake for 14 d did not significantly increase the α-carotene, β-carotene, lutein, zeaxanthin, β-cryptoxanthin or lycopene concentrations in the PBMC of the volunteers (Table 5). Furthermore, no statistically significant differences between the groups were observed.
Table 5 Carotenoid concentration of peripheral blood mononuclear cells before and after the 2-week intervention*
(Mean values and standard deviations for twelve men per group)

* No significant differences at day 0 and at day 14 were observed between groups (group, time × group) (repeated-measures ANOVA).
Immune parameters
No significant changes were observed with regard to PBMC proliferation, percentages of NK cells, lytic activity of NK cells and cytokine secretion at day 0 and after 14 d intervention with carrots (data not shown).
DNA damage in peripheral blood mononuclear cells
There were no statistically significant differences between the three groups at day 0 and after intervention (day 14) for levels of endogenous DNA damage (strand breaks, endonuclease III- and Fpg protein-sensitive sites), nor in the capacity to protect DNA against damage caused by H2O2 or iron chloride (Tables 3 and 4).
Discussion
Data on the influence of the farming system on phytochemical content, their bioavailability and physiological effects are scarce. Therefore, the aim of the present study was to evaluate the bioavailability of carotenoids from blanched carrots produced under defined conventional and organic conditions. Furthermore, the antioxidant status (FRAP, TEAC and ORAC assays, LDL lag-time test and DNA strand breaks) and immune parameters (for example, percentages and lytic activity of NK cells) were investigated in subjects consuming carrots from the two production systems.
The carotenoid levels and the antioxidant capacity of carrots (variety Narbonne) in the present study were similar to those previously reported(Reference Warman and Havard9). Additionally, no significant differences were found between the carotenoid concentration of organically and conventionally produced carrots in other studies(Reference Bourn and Prescott13, Reference Woese, Lange and Boess30), confirming our own observations. The present data indicate no differences between organically and conventionally produced carrots in antioxidant capacity. Therefore, other antioxidant substances in carrots (for example, polyphenols, vitamins) may also not be affected by the different agricultural systems(Reference Cao and Prior26, Reference Ziegler31, Reference Miller, Sampson and Candeias32).
During the 2-week intervention period, the volunteers ingested 24·3 (sd 1·4) mg (organic group) or 23·2 (sd 2·5) mg (conventional group) total carotenoids per d. These carotenoid intakes resulted in similar plasma concentrations of about 700 nmol/l for β-carotene, 350 nmol/l for α-carotene and 150 nmol/l for lutein. The total carotenoid plasma concentrations at day 0 as well as the increase after carrot consumption for 2 weeks are in agreement with previous reports(Reference Müller, Bub and Watzl33, Reference Briviba, Kulling and Möseneder34). The consumption of organic and conventional blanched carrots for 2 weeks significantly increased the plasma α-carotene and β-carotene concentrations compared with the control group. The intervention with carrots had no effect on the zeaxanthin and β-cryptoxanthin concentrations. This is reasonable because they are not contained in carrots. The compliance with the low-carotenoid diet of the volunteers was good, as reflected by the low plasma concentrations of the control group. The lycopene concentration decreased in both intervention groups, presumably because of the low intake of tomatoes and tomato products.
To date, no human intervention study has been conducted to analyse the bioavailability of carrot carotenoids (lutein, α-carotene and β-carotene) from organic and conventional agricultural systems. Caris-Veyrat et al. (Reference Caris-Veyrat, Amiot and Tyssandier5) conducted a human intervention study to investigate the bioavailability of carotenoids (lycopene, β-carotene) from organically and conventionally produced tomato purée after a consumption period of 3 weeks. The authors report that the carotenoid concentration in human plasma increased significantly. However, no differences were observed between the organic and conventional groups(Reference Caris-Veyrat, Amiot and Tyssandier5). This is in agreement with the present results. One might speculate that the similar carotenoid content (α-carotene, β-carotene, lutein) in carrots or tomatoes was responsible for the comparable bioavailability of carotenoids. Therefore, the difference in the plant matrix that might be caused by the different agricultural systems may not be relevant for the bioavailability of carotenoids(Reference Fjelkner-Modig, Bengtsson and Stegmark20, Reference Beckmann and Pestemer21).
Furthermore, possible physiological effects on human health were investigated. The plasma antioxidant capacity of the volunteers was measured using three different antioxidant assays (FRAP, ORAC and TEAC assays). The Cu2+-induced LDL oxidation (lag-time test) was used for the lipophilic antioxidant status. Oxidation of LDL and the subsequent generation of lipid hydroperoxides and other lipid peroxidation products are hypothesised to play a crucial role in the development of atherosclerosis and CVD(Reference Diaz, Frei and Vita35). The 2 weeks of intervention had no effect on the antioxidant status of the volunteers, despite the increase in plasma carotenoid concentration. Previous reports on the effects of carotenoids and carotenoid-rich foods on lipid peroxidation are inconsistent(Reference Krinsky36). The results of the present study are in agreement with our previous studies(Reference Briviba, Schnäbele and Rechkemmer23, Reference Pool-Zobel, Bub and Muller37, Reference Bub, Watzl and Abrahamse38). A possible explanation for the lack of an effect on the antioxidant status and LDL oxidation in the present study is that 2 weeks of intervention with blanched carrots may not be sufficient to enhance the antioxidative status and to protect the LDL against oxidation.
The immunomodulatory activity of carotenoids (namely β-carotene) has been demonstrated in several animal studies(Reference Bendich39), whereas data from human intervention studies with carotenoid supplements or carotenoid-rich foods are controversial. Carotenoids are able to modulate the primary immune response and moderate the activity of redox-sensitive transcription factors, also implicated in mutagenesis and carcinogenesis(Reference Chew and Park40). In the present study none of the analysed immune parameters after carrot consumption was modified. This finding is in line with results from our previous study dealing with the impact of carrot juice consumption on T-lymphocyte functions in healthy males where also no stimulatory effects of carrot juice consumption on immune parameters beyond baseline levels were detected(Reference Watzl, Bub and Brandstetter29). However, the 2-week depletion phase where study subjects only consumed a low-carotenoid diet, similar to the 4-week depletion period in the present study, led to a reduction of T-lymphocyte functions.
An explanation for the absence of effects on immune parameters might be that the carotenoid concentration in PBMC did not increase (Table 5). Data on the carotenoid concentration in PBMC are scarce. To date only four studies have been conducted; the PBMC carotenoid concentrations measured in the present study are comparable(Reference Porrini and Riso41–Reference Murata, Tamai and Morinobu44). In these studies 25 g tomato purée (containing 7 mg lycopene and 0·3 mg β-carotene) and the application of β-carotene supplementations (ranging between 50 and 580 mg/d) increased the β-carotene concentration in PBMC(Reference Porrini and Riso41–Reference Murata, Tamai and Morinobu44). Possibly the carotenoid content in the carrots of the present study was too low to induce an increase in carotenoids in the PBMC. As Hughes outlined in his review(Reference Hughes19), changes in immune functions especially after supplementation with β-carotene are more marked in the elderly. Probably, immunomodulatory events could have been detected in an older collective than used in the present study with subjects aged between 19 and 54 years (median age 32 years).
In the present study the endogenous DNA strand breaks were not affected by the 2-week intervention with 200 g blanched carrots daily. Conversely, we reported decreased levels on endogenous DNA strand breaks in subjects who followed 2 weeks' consumption of carrot juice (330 ml/d)(Reference Pool-Zobel, Bub and Muller37). The different results might be due to the different carotenoid uptakes. In the study by Pool-Zobel et al. (Reference Pool-Zobel, Bub and Muller37) 38·0 mg carotenoids/d in the form of carrot juice were ingested. In the present study the carotenoid uptake was 24·3 mg/d (organic group) and 23·2 mg/d (conventional group) in the form of blanched carrots, resulting in a 40 % lower intake than in our previous study. In addition, the bioavailability of carotenoids from carrots is affected by the processing method and differences in the food matrix(Reference Bryant, McCord and Unlu45). Heat treatment and also homogenisation of carrots improved the bioavailability of carotenoids(Reference Bryant, McCord and Unlu45, Reference Hedren, Diaz and Svanberg46). This was reflected also in the increase in the plasma carotenoid concentrations. In the present study, the α-carotene concentration increased by 81 % and the β-carotene concentration by 60 %. In the study by Pool-Zobel et al. the α-carotene concentration increased by 90 % and the β-carotene concentrations by 79 %(Reference Briviba, Schnäbele and Rechkemmer23, Reference Pool-Zobel, Bub and Muller37). Thus, the differences in carotenoid uptake, the processing method between blanched carrots and carrot juice, and the different plasma carotenoid concentrations could explain the controversial results.
Taken together, this is the first intervention study to have assessed the bioavailability, the antioxidant status, DNA strand breaks and the effect on immune parameters of organically and conventionally grown carrots. The agricultural system had no effect on the carotenoid concentration in the carrots as well as no effect on the bioavailability of carotenoids from blanched carrots. Finally, no antioxidant, antigenotoxic or immunological effects of the carrot supplementation were observed. Whether the different farming systems affect the nutritional quality of other foods in a similar way cannot be deduced from the present study.
Acknowledgements
The present study was supported by the Federal Agency for Agriculture and Food; Bundesprogramm ökologischer Landbau (04OE027). The authors thank the volunteers of the study as well as G. Rahmann, Heinrich von Thünen-Institute, Westerau, Germany, for providing the carrots. We gratefully acknowledge the excellent technical assistance of S. Delong, S. Demirel, S. Herrmann, E. Hoch, R. Lambertz, S. Merkel and G. Schultheiss, as well as L. Korn for his statistical consultations. B. W., K. B., C. K. and A. B. developed the initial idea. A. B. was responsible for the human intervention study, C. E. R. for the HPLC analyses and S. S. for the immune parameters. B. A. S. collected and analysed the data, performed the statistical analysis, recruited and checked the volunteers and also drafted the manuscript. All co-authors participated in critically revising the manuscript. None of the authors had any conflict of interest.









