Malnutrition, specifically under nutrition, refers to deficiencies or imbalances in the intake of energy and/or nutrients, which can lead to weight loss, muscle wasting and micronutrient deficiencies or insufficiencies( 1 ). In vascular surgery patients, studies have reported the rates of malnutrition as high as 60–90 % based on a variety of assessment methods and tools( Reference De Waele, Moerman and Van Bael2 – Reference Durkin, Mercer and McNulty4 ). Previous work by the authors revealed that 16 % of patients admitted to a tertiary acute care vascular surgery unit were malnourished according to the Patient-Generated Subjective Global Assessment (PG-SGA)( Reference Thomas, Delaney and Suen5 ).
The identification and management of malnutrition in patients admitted to a vascular surgery unit is critical due to its reported association with poorer clinical outcomes( Reference Westvik, Krause and Pradhan6 – Reference Ambler, Brooks and Al Zuhir8 ). Despite these consequences and the prevalence observed, malnutrition across clinical specialties remains under-recognised despite the availability of a number of validated malnutrition screening tools.
A malnutrition screening tool should be quick and simple to administer and able to be completed by an individual with minimal training or by the patients themselves. A variety of tools exist with commonly used ones being the Malnutrition Screening Tool (MST)( Reference Ferguson, Bauer and Banks9 ), Malnutrition Universal Screening Tool (MUST)( 10 ), the Nutrition Risk Screen-2002 (NRS-2002)( Reference Kondrup, Rasmussen and Hamburg11 ) and the Mini-Nutritional Assessment – Short Form (MNA-SF)( Reference Rubenstein, Harker and Salva12 ). A detailed description of each of these tools is available elsewhere( Reference Ferguson, Bauer and Banks9 – Reference Rubenstein, Harker and Salva12 ), but in summary, each tool consists of a number of items (2–6) pertaining to nutritional parameters known to be associated with malnutrition, with a weighted scoring system for each item and a defined cut-off score to indicate possible malnutrition, warranting further investigation by a dietitian. It is well recognised that malnutrition screening tools need to be validated for the population in which they are to be administered to expedite nutrition assessment and interventions where indicated and allow resources to be used efficiently( Reference Lacey and Prichett13 ). All four tools mentioned have been validated across a number of patient populations( Reference Ferguson, Bauer and Gallagher14 – Reference Chen, Xing and You20 ) and in a variety of settings( Reference Neelemaat, Meijers and Kruizenga17 , Reference Isenring, Banks and Ferguson21 – Reference Cohendy, Rubenstein and Eledjam24 ).
In some settings, a standardised approach to nutrition assessment is undertaken using a validated nutrition assessment tool. A commonly used tool is the scored PG-SGA, which awards patients with a score and a global rating of nutritional status. A detailed description of the PG-SGA can be found elsewhere( Reference Ottery, McCallulm and Polisena25 ). While originally developed in cancer patients( Reference Ottery26 ), the PG-SGA has since been validated in a number of patient groups( Reference Yoo, Oh and Youn27 – Reference Huang, Chi and Liu29 ) with high levels of sensitivity (92–100 % and specificity (84–96·7 %).
To date, neither malnutrition screening tools nor the PG-SGA has been validated specifically in patients with vascular disease, a group characterised by a heterogeneous aetiology of disease and presence of complex comorbidities that are growing in prevalence. Hence, it is critical that we investigate methods to identify those who are nutritionally vulnerable to optimise their nutritional health and overall clinical outcomes. Therefore, the aims of the present study were to (1) investigate the validity of the four commonly adopted malnutrition screening tools (the MST, MUST, NRS-2002 and the MNA-SF), and a commonly used nutritional assessment tool (PG-SGA) when compared against a dietitians clinical assessment in inpatients admitted to a vascular surgery unit and (2) evaluate the ability of the malnutrition screening tools and the PG-SGA to predict clinical outcomes, namely, length of hospital stay, in-hospital complications, quality of life and discharge destination in the same population.
Methods
Study sample
Participants were recruited consecutively from the Southern Adelaide Health Local Network Vascular Surgery Unit, Adelaide Australia. Participants were aged 18 years and over and were able to provide informed written consent or where this was not appropriate, consent was obtained from the participant’s legal next of kin/guardian. Participants were excluded if they presented to the emergency department without admission to the Vascular Surgery Unitor and were subsequently transferred to a private hospital, or if they were admitted for day procedures only. Those who were admitted to the vascular unit were excluded if they were unable to be recruited within 72 h of admission. Participants recruited to the study during previous admissions were also excluded. The present study received ethical approval from the Southern Adelaide Health Research and Ethics Committee (approval number 258.14) and governance approval from the Flinders Medical Centre.
Data collection
Data were collected between October 2014 and August 2016. All demographic data and admission/discharge details were collected by the research team. Nutrition screening was completed by nursing staff on the vascular surgery unit within 24 h of recruitment. Where this was not possible, a member of the research team completed the screening. Nutrition assessment was completed by the research Accredited Practising Dietitian at the bedside within 72 h of admission to the ward, accompanied by blood test results relating to nutrient biochemistry.
Demographic data were collected from the medical records and included age, sex, and type of vascular disease. Vascular disease types were classified according to surgeon diagnosis as aneurysmal, peripheral arterial disease (encompassing aorto-iliac and infra-inguinal disease), occlusive other (encompassing carotid and upper limb ischaemia), venous, diabetic foot infection and other. Those classified as other included renal access, surgical management of thoracic outlet syndrome, trauma, ulcers of mixed or unknown aetiology, admission for postoperative complications and lower limb infection not attributed to occlusive disease or diabetes.
Malnutrition screening
Data required for completion of the four malnutrition screening tools (MST, MUST, NRS-2002 and MNA-SF) were completed on entry to the study. This included the collection of body weight, using a calibrated weigh chair (HVL-CS Hospital Chair Scale; A&D Mercury Pty Ltd) in light clothing and recorded to the nearest 0·1 kg, and ulna length to allow for the estimation of height (British Association for Parenteral and Enteral Nutrition). Following discharge, the research dietitian scored each of the four screening tools. Participants were classified as ‘at risk of malnutrition’ for each tool separately if they scored 2 or more on the MST or MUST, 3 or more on the NRS-2002, and 11 or less on the MNA-SF( Reference Ferguson, Bauer and Banks9 – Reference Rubenstein, Harker and Salva12 ).
Assessment of nutritional status
Nutritional status was assessed by an Accredited Practicing Dietitian within 72 h of admission during an in-person consultation, using the scored PG-SGA( Reference Ottery, McCallulm and Polisena25 ) with each participant being awarded a PG-SGA score and a PG-SGA global rating of A (well nourished), B (suspected or moderately malnourished) or C (severely malnourished).
Comprehensive dietetic assessment of nutritional status
The dietitian’s assessment was conducted retrospectively using all data collected during the data collection as described above inclusive of nutritional biochemistry. Fasting blood samples were collected by a phlebotomist and analysed by the hospital or state pathology service depending on the analytical test. Blood samples were analysed and determined as low, normal or high based on the reference ranges (shown in parentheses) provided by the analysing laboratory, for Fe (8–30 μmol/l), vitamin B12 (>260 mg/l), folate (6·5–45 μg/l), vitamin A (1–3·1 μmol/l), vitamin C (26–85 μmol/l), vitamin E (12–46 μmol/l) and vitamin D (60–160 nmol/l) and the trace elements Zn (9–21 μmol/l) and Se (0·8–1·64 μmol/l).
A participant was determined to be malnourished if they displayed a deficiency in any of the micronutrients according to the following guidelines, vitamin C ≤0·29 mg/dl( Reference Goebel30 ), vitamin B12 <200 mg/l( Reference Johnson31 ), folate <3 μg/l( Reference Johnson31 ), Zn <9·0 μmol/l, Se <0·7 μmol/l( Reference Poitou Bernert, Ciangura and Coupaye32 ), vitamin A <1 μmol/l( Reference Johnson31 ) or vitamin D <60 nmol/l or any of the following characteristics underweight (BMI of <22 kg/m2 for those aged 65 years or more( Reference Landi, Zuccala and Gambassi33 ) and <18·5 kgm2 for those aged under 65 years( 34 )), PG-SGA score ≥9( Reference Ottery, McCallulm and Polisena25 ), PG-SGA global rating B or C( Reference Ottery26 ), or Fe-deficiency anaemia (ferritin <15 μg/l plus Hb <130 g/l for males and <120 g/l for females)( Reference Pasricha, Flecknoe-Brown and Allen35 ).
Ability of the screening and assessment tools to predict clinical outcomes
Admission complications and discharge destination were collected from the medical records following discharge to enable the evaluation of the ability of the screening tools to predict clinical outcomes. Admission complications included infections, cardiovascular events, unplanned surgery or procedures, deterioration or development of an ulcer or wound and vascular restenosis/acute occlusion and acute renal failure.
Health-related quality of life is commonly examined in the literature when investigating clinical outcomes and in the present study was included as an outcome in the predictive validity analyses. In the present study, health-related quality of life was assessed using the EuroQoL 5-dimension 5-level (EQ-5D-5L)( Reference Herdman, Gudex and Lloyd36 ), which includes five questions related to mobility, self-care, usual activities, pain/discomfort and anxiety/depression with five levels of impairment recognised in each domain: no, slight, moderate, severe and extreme problems in the relevant dimension of health. Using these responses, an EQ-5D-5L utility value was created using an evaluation algorithm( Reference Brazier, Roberts and Tsuchiya37 ). EQ-5D-5L utility values have a range of −0·624 to 1: the maximum score of 1 representing perfect health, a score of 0 representing death while scores less than 0 represent health states that are worse than death( Reference Kind, Hardman and Macran38 – Reference Post, Stiggelbout and Wakker40 ).
Statistical analysis
The calculation of sample size was based on determining the precision of the expected sensitivity and specificity of the proposed screening tools( Reference Ferguson, Bauer and Banks9 , Reference Rubenstein, Harker and Salva12 ). A prevalence of malnutrition of 61 % was determined from a prospective, observational, audit of vascular surgery patients in an elective setting( Reference De Waele, Moerman and Van Bael2 ). A total sample size of 322 participants would need to be recruited to obtain 197 participants with malnutrition (prevalence of the malnutrition is 61 %). The sample size calculation allows a point estimate of 85 % sensitivity and specificity to be measured with a precision of ±5 % with 95 % confidence. The sample size calculation was also based on investigating the effect of nutritional status on complications and health care outcomes. Although several outcomes have been addressed, patient’s mortality was chosen to justify the power and sample size calculation. Using a hierarchical cox regression model on a 3-year follow-up study of vascular patients with lower limb ulcers, Miller et al.( Reference Miller, Delaney and Penna41 ) demonstrated that those patients with BMI <30 kg/m2 were 4·6 times more likely to die than those with BMI ≥30 kg/m2 (95 % CI 1·04, 20·4; P = 0·04). As the CI was so wide, we used a risk of death at the lower end of the CI to detect a large sample size. A two-sided log rank test with an overall sample size of 266 subjects (133 in the BMI <30 kg/m2 group and 133 in the BMI ≥30 kg/m2 group) achieves 90·0 % power at a 0·05 significance level to detect a hazard ratio of 1·50. The Power Analysis & Sample Size Software was used to calculate the sample size( Reference Hintze42 ).
Statistical analysis was conducted using SPSS for Windows version 25 (SPSS Inc.) and Stata version 14.0 (StataCorp LLC). Significance was set at the P < 0·05 level. Continuous variables were assessed for normality using the Shapiro–Wilk test and reported as means and standard deviations. If not normally distributed, medians and interquartile ranges (IQR) are reported. Sample characteristics are expressed as frequencies and percentages. Contingent on the normality tests, the independent-samples t test or Mann–Whitney U test was used for testing differences across two groups for continuous variables.
To determine the concurrent validity of the five tools (four screening tools and the PG-SGA), measures of diagnostic accuracy were determined. Sensitivity (Sn), specificity (Sp), positive predictive value and negative predictive value were determined against the results of the dietitian’s clinical assessment (the reference standard). In the reference standard, respondents were classified as either ‘malnourished’ or ‘not malnourished’. To facilitate comparison with the reference standard and in keeping with clinical practice, two levels of risk were considered for each screening tool namely ‘at risk’ (aggregating participants with high or moderate risk of malnutrition) and ‘not at risk’. Similarly, the PG-SGA global rating was classified into ‘malnourished’ and ‘not malnourished’ with ratings B (moderately, suspected malnourished) and C (severely malnourished) aggregated into one group (malnourished) as is common practice in similar literature( Reference Stratton, Hackston and Longmore16 , Reference Chi, Yin and Zhu43 , Reference Donini, Poggiogalle and Molfino44 ). A priori values of ≥80 % for sensitivity and ≥60 % for specificity were used to indicate a valid instrument( Reference Ferguson, Bauer and Gallagher14 ). The dichotomous forms of each tool were used in all subsequent analyses to investigate validity in keeping with clinical practice where malnutrition screening tools have a defined cutoff to determine if a patient is ‘at risk’ or ‘not at risk’ of malnutrition. The diagnostic consistency between the five tools against the dietitians assessment was assessed by the κ statistic( Reference Pallant45 ). The value of κ varies from 0 to 1 with values <0·2 indicating poor, 0·21–0·4 fair, 0·41–0·6 moderate, 0·61–0·8 substantial and >0·8 as almost perfect concordance. Negative κ values indicate that the number of agreements observed is fewer than that would be expected by chance indicating poor consistency overall( Reference Landis and Koch46 ).
The ability of the five tools to predict clinical and health-related quality of life outcomes was tested using multivariate regression analysis. In all regressions, dichotomous screening and assessment tool variables (at risk or malnutrition/malnourished or not at risk/well nourished) were entered as independent variables with age, sex, disease type and smoking status included as potential cofounders. To predict continuous dependent variables or outcomes (length of stay (LOS) and EQ-5D-5L scores), generalised linear models were fitted. To identify an appropriate family for the generalised linear model, a modified Park test was conducted following standard procedures( Reference Manning and Mullahy47 ). For generalised linear model models where the LOS was the dependent variable, coefficients of predicted dependent values in the modified park test indicated that the Poisson (for models including the MST and MUST) and inverse Gaussian (for models incorporating the NRS-2002, MNA-SF and PG-SGA) family of generalised linear model were appropriate for analysis. The ordinary least square regression model was appropriate for all models where the EQ-5D-5L index was the dependent variable. To predict binary outcomes (1=return to prior residence or to an institution such as residential aged care, rehabilitation or another hospital, 0=other discharge destination; 1=nosocomial complications, 0=no complications), binary regression models were fitted.
Results
A total of 2229 patients were admitted to the vascular surgery ward during the study period, all of whom were screened for study eligibility. Of these, 1327 (59·5 %) were ineligible, 568 (25·5 %) did not wish to participate, and 12 (0·5 %) participants withdrew before data collection resulting in 322 participants (14·4 %). Table 1 displays the participant demographics. The majority of study participants were male (69·3 %) and over 65 years old (61·6 %). Nearly all (95·7 %) lived independently, either alone or with another person/s with the majority (82·1 %) returning to their pre-admission residence on discharge. Of the participants, 21 % had at least one in-hospital complication and the median hospital LOS was 8 (IQR 5, 12) d. Median quality of life score was 0·72 (IQR 0·46, 0·82).
Table 1. Participant characteristics of 322 vascular surgery patients participating in a validation study of malnutrition screening and assessment tools (Numbers of patients and percentages; mean value and standard deviation; medians and interquartile ranges (IQR))

SCF, special care facility; RACF, residential aged care facilities; EQ-5D-5L, EuroQoL 5-dimension 5-level.
Table 2 shows the results of the malnutrition screening using the four screening tools, micronutrient status and the proportion of participants assessed as malnourished by the PG-SGA and by the dietitian’s clinical assessment. The malnutrition screening tools showed variable results ranging from 12·5 % at risk of malnutrition according to the MUST up to 47·5 % with the MNA-SF. According to the PG-SGA, 15·8 % of participants were assessed as either moderately/suspected malnourished (PG-SGA B) or severely malnourished (PG-SGA C). Suboptimal micronutrient status was prevalent with greater than 40 % having suboptimal Fe, Zn or vitamin B12 levels, 55·6 % showed low vitamin D levels, and approximately 18 % were low in selenium or vitamin A. Prevalence of suboptimal vitamin C was the greatest with 78·6 % classified as having suboptimal levels and 57·2 % being vitamin C deficient. The dietitian’s assessment of nutritional status revealed that 75·5 % of study participants had at least one nutritional parameter indicating that intervention may be warranted.
Table 2. Number and proportion of vascular surgery participants at risk of malnutrition according to the four screening tools and those assessed as malnourished according to the Patient-Generated Subjective Global Assessment (PG-SGA), and the dietitian’s clinical assessment
(Numbers of patients and percentages; n 322)
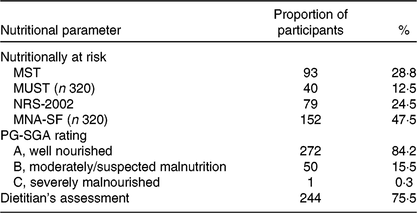
MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NRS-2002, Nutrition Risk Screen-2002; MNA-SF, Mini-Nutritional Assessment – Short Form.
Validity of the screening and assessment tools
A higher prevalence of malnutrition (75·5 % overall) was observed as a result of the dietitian’s clinical assessment when compared with the PG-SGA results (Table 2). Concurrent validity and agreement of the malnutrition screening tools and the PG-SGA against the dietitian’s clinical assessment is displayed in Table 3. Overall, while the MNA-SF performed best, none of the four screening tools or the PG-SGA achieved the a priori levels for Sn and Sp and all showed poor negative predictive value. Negative κ values were observed between all four malnutrition screening tools and PG-SGA when compared with the dietitian’s assessment indicating poor diagnostic consistency between the dietitian’s clinical assessment and the tools (Table 3).
Table 3. Concurrent validity of four commonly used screening tools and the Patient-Generated Subjective Global Assessment (PG-SGA) against the clinical dietitian’s assessment of malnutrition in 322 vascular surgery patients*
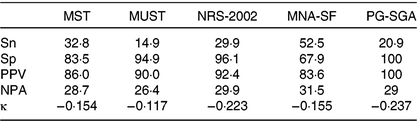
MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NRS-2002, Nutrition Risk Screen-2002; MNA-SF, Mini-Nutritional Assessment – Short Form; Sn, sensitivity; Sp, specificity; PPV, positive predictive value; NPA, negative predictive value.
* Desirable cut-offs: Sn ≥80, Sp ≥60, κ<0·2 poor agreement, 0·21–0·4 fair agreement, 0·41–0·6 moderate agreement, 0·61–0·8 substantial agreement, >0·8 excellent agreement.
Results of the regression analyses are displayed in Tables 4 and 5. A significant association was observed between LOS and four tools (the MST, MUST, NRS-2002 and PG-SGA). However, the direction of the relationship differed. The MST and PG-SGA had positive associations (coefficient 0·1061 (se 0·0376), P=0·005 and 5·02 (SE 1·33), P < 0·001, respectively) indicating that those at risk of malnutrition or already malnourished had a longer LOS while the reverse was true for the MUST (coefficient −0·00006 (SE 0·00003), P=0·029) and NRS-2002 (−0·004 (SE 0·002), P=0·045). No significant association was observed between LOS and MNA-SF. Associations were also observed between LOS and disease type and age in some of the models (Table 4). No significant associations were observed in the models for EQ-5D-5L index indicating no association between the predictor variables and health-related quality of life. The results of the logistic regression analyses are shown in Table 5. MST, NRS-2002 and PG-SGA all showed a significant association with discharge destination when all confounders were included with participants at risk of malnutrition or already malnourished being at least 2·3 times more likely to be discharged to another institution (OR 2·36 (se 0·71), P=0·004; OR 2·38 (se 0·74), P=0·005 and OR 2·91 (se 1·03), P=0·003, respectively). There were no other significant associations identified with discharge destination. When in-hospital complications were examined, only NRS-2002 had a significant association with at-risk participants being 1·85 (se 0·56) times more likely to have complications when confounders were controlled for.
Table 4. Generalised linear model (GLM) results
(Coefficients with their standard errors)
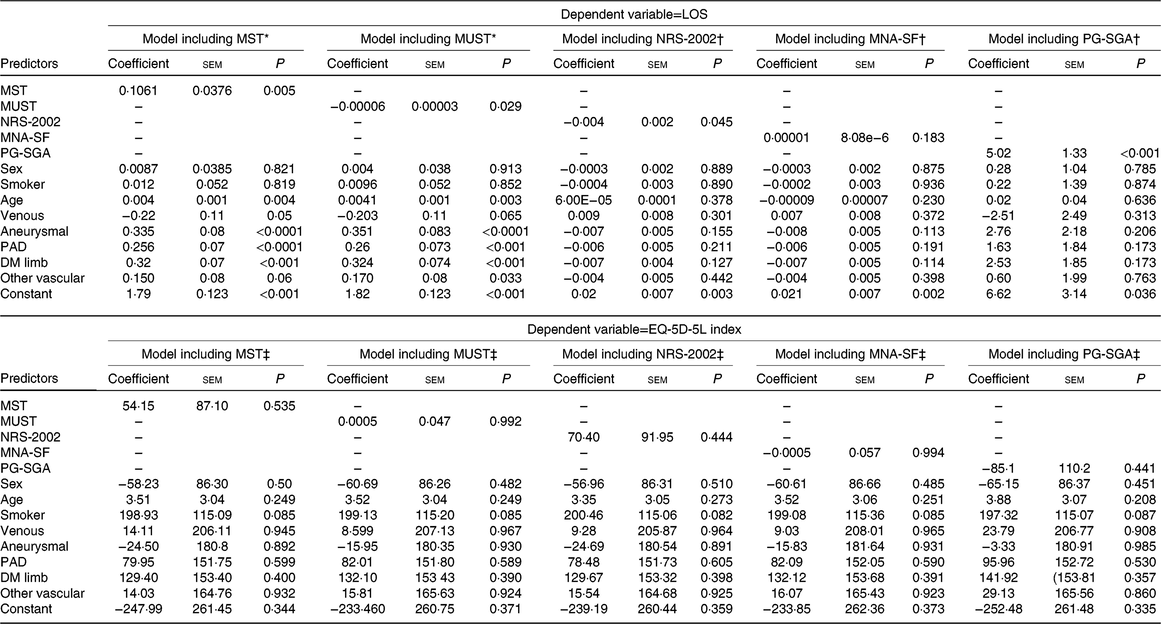
LOS, length of stay; MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NRS-2002, Nutrition Risk Screen-2002; MNA-SF, Mini-Nutritional Assessment – Short Form; PG-SGA, Patient-Generated Subjective Global Assessment; PAD, peripheral arterial disease; DM, diabetes mellitus; EQ-5D-5L, EuroQoL 5-dimension 5-level; OLS, ordinary least squares.
* GLM model family for LOS model that included results of the MST and MUST assessments (both coded as 1=at risk and 0=not at risk) was Poisson and link was log.
† GLM model family for LOS model that included results of the NRS-2002 and the MNA-SF assessments (both coded as 1=at risk and 0=not at risk) was inverse Gaussian and link was power−2.
‡ Regression model for EQ-5D-5L model that included results of the NRS-2002 and the MNA-SF assessments (both coded as 1=at risk and 0=not at risk) was OLS.
Table 5. Binary logistic regressions results
(Odds ratios and standard errors)
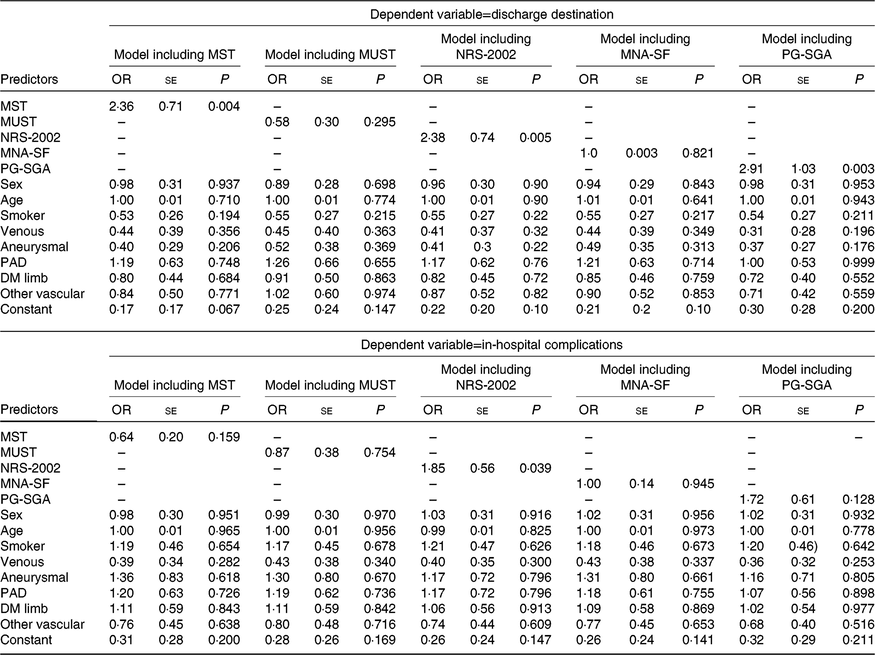
MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NRS-2002, Nutrition Risk Screen-2002; MNA-SF, Mini-Nutritional Assessment – Short Form; PG-SGA, Patient-Generated Subjective Global Assessment; PAD, peripheral arterial disease; DM, diabetes mellitus.
Discussion
This is the first study to explore the validity of commonly used malnutrition screening tools as well as a nutrition assessment tool (PG-SGA) exclusively in vascular surgery patients. The MNA-SF achieved a better concurrent validity than the other screening tools when compared with the clinical dietitian’s assessment; however, none of the four malnutrition screening tools or the PG-SGA exhibited optimal concurrent validity as they did not achieve the a priori acceptable levels and had low negative predictive values indicating that all underestimated the presence of malnutrition in the participants. There was poor diagnostic consistency between each of the screening tools and the PG-SGA when compared with the dietitian’s assessment according to Kappa values.
Previous studies that have explored the validity of malnutrition screening tools have varied depending on the patient group in which the tools have been administered and the comparator/reference standard used. The MUST displayed excellent agreement (κ 0·783) with the Subjective Global Assessment in 50 medical inpatients (aged <65 years)( Reference Stratton, Hackston and Longmore16 ). However, in the current study, the MUST did not perform adequately (κ −0·117, Sn 14·9 %, Sp 94·9 %), which was similar to findings in renal inpatients when compared with the Subjective Global Assessment (Sn 53·8 %, Sp 78·3 %)( Reference Lawson, Campbell and Dimakopoulos48 ). Variable results have also been found with the MST, NRS-2002 and the MNA-SF with good validity in some settings( Reference Neelemaat, Meijers and Kruizenga17 ) and suboptimal( Reference Marshall, Young and Bauer22 , Reference Lawson, Campbell and Dimakopoulos48 , Reference Vandewoude and Van Gossum49 ) validity in others. The variable results lend support to the notion that there is no ‘one size fits all’ approach to malnutrition screening.
The investigation of the ability of the tools to predict short-term clinical outcomes yielded variable results. The NRS-2002 showed the best predictive ability, with significant associations observed with discharge destination, in-hospital complications and hospital LOS indicating poorer outcomes in those classified as at risk of malnutrition.
Existing literature that has looked at the ability of the MUST, MST, NRS-2002 and MNA-SF to predict outcomes has also reported variable results, depending on the population studied, sample size and setting. A study conducted by Wang & Tsai( Reference Wang and Tsai50 ) found the NRS-2002 to be predictive of LOS, non-infectious complications and higher cost and mortality in Chinese GI patients, whilst Raslan et al.( Reference Raslan, Gonzalez and Dias51 ) found that the NRS-2002 performed better than the MUST and MNA-SF despite it identifying the lowest proportion of nutritional risk out of the three tools studied. Both of these studies were conducted in acute care patients. However, when the NRS-2002, MNA-SF and MUST were studied in nursing home residents, the MNA-SF demonstrated the better predictive ability( Reference Donini, Poggiogalle and Molfino44 ). The authors postulated this was due to the inclusion of functional, cognitive and psychological parameters in the MNA-SF. The MNA-SF has been studied more extensively, particularly in the older age groups, showing that it is associated with increased risk of discharge to institutional care( Reference Neumann, Miller and Daniels52 ) and longer LOS in geriatric rehabilitation( Reference Neumann, Miller and Daniels52 , Reference Slattery, Wegener and James53 ) and also long-term mortality( Reference Wang and Tsai50 ). However, these results were contradicted by Marshall et al.( Reference Marshall, Young and Bauer28 ) who found that the MNA-SF was not able to detect a significant difference in similar outcomes in their sample of geriatric rehabilitation patients. In younger populations, the results are not clear cut. The MNA-SF was found to be strongly associated with mortality in younger Ugandan adults( Reference Asiimwe54 ), whereas a trend towards longer LOS and increased likelihood of readmission was observed in younger rehabilitation patients but the results failed to reach significance as the study was likely underpowered( Reference Wegener, James and Slattery55 ). In the current study, the MST was predictive of discharge destination and LOS. Similar to other screening tools, the literature is variable with the MST being predictive of LOS in acute care patients( Reference Ferguson, Bauer and Banks9 ) but not in renal patients( Reference Lawson, Campbell and Dimakopoulos48 ) and not predictive of any clinical outcomes in patients undergoing geriatric rehabilitation( Reference Marshall, Young and Bauer22 ). The variable results in the current study and also in the existing literature highlight further that no one screening tool is suitable for use across a range of population groups and selected tools need to be valid for the population for which it is intended.
The NRS-2002 is the only screening tool to have been examined in vascular patients to date. De Waele et al.( Reference De Waele, Moerman and Van Bael2 ) found that the NRS-2002 did not result in any false positives; however, the presence of false negatives was not mentioned, which was found to be high in the present study. Participants were limited to elective surgery patients and those needing urgent surgery and/or limb amputations were excluded whereas our sample included all surgery types, and both elective and emergency patients making it a more representative sample of a routinely heterogeneous acute vascular surgery unit.
The suboptimal performance of the malnutrition screening tools and the PG-SGA is likely related to the parameters included in each of these tools, which are of less relevance to vascular surgery patients. Malnutrition screening tools traditionally focus on weight and/or BMI status, unintentional weight loss and reduced appetite/oral intake. The NRS-2002 also accounts for disease severity and age, while the MNA-SF incorporates parameters known to impact on nutritional status that may be more relevant to this patient group; suboptimal mobility( Reference McDermott, Guralnik and Criqui56 ), increased psychological stress and depression( Reference Grenon, Cohen and Smolderen57 – Reference Smolderen, Hoeks and Pedersen59 ). The participants in the present study were mostly overweight or obese with minimal reporting of unintentional weight loss; hence, they would not score highly on the tools that focus solely on these parameters. The MNA-SF identified the highest proportion of ‘at risk’ participants likely due to the inclusion of broader parameters.
Overall, the four malnutrition screening tools and the PG-SGA performed poorly as they do not account for micronutrient status which we found to be a key nutritional issue in the participants of the present study. Incorporating micronutrient status into the clinical dietitian’s assessment provides a more comprehensive determination of nutritional status, and the present study has demonstrated that malnutrition screening tools or assessment tools that neglect this important area will likely be inadequate for implementation in a vascular surgery setting.
Micronutrients are crucial in this patient group for wound healing( Reference Posthauer, Dorner and Collins60 ) and vascular health( Reference Gaddipati, Kuriacose and Copeland61 ); hence, ensuring adequate micronutrient status is critical to ensure optimal perioperative and long-term outcomes. The malnutrition screening tools that are currently available in the existing literature do not include biochemical assessment of micronutrients as this contravenes the premise that a screening tool should be quick and simple to administer by any trained person or the patient themselves due to the requirement for additional resources and time rendering it more costly, not quick, nor simple. A cost analysis would be important to support or refute the inclusion of nutritional biochemistry within a screening tool. It is important to consider the strengths and limitations of the present study to enable us to draw conclusions. The present study is the first of its kind to investigate a range of screening tools in the vascular surgery population. It has a large sample size of 322 participants that are heterogeneous and therefore representative of the spectrum of vascular disease likely to be encountered in a vascular surgery unit. Nutrition assessment bias was minimised by having an Accredited Practising Dietitian conduct the PG-SGA who was not involved in the screening process and all measurements were conducted by a trained Accredited Practising Dietitian. Nursing staff who conducted the nutrition screening were trained via in-service education sessions and individual support by research team members.
While the study has many strengths, it is not devoid of limitations. The clinical dietitian’s assessment was completed retrospectively utilising information collected via the screening and nutrition assessment processes, hence the dietitian was not able to clarify information with individual participants and this may have influenced the assessment results. However, this is not relevant to the biochemistry results and hence the impact on results is likely to be minimal. When investigating the validity of the tools, participants at medium and high risk of malnutrition according to the MNA-SF, MUST and the PG-SGA were merged for analysis so the relationship between the different levels of risk or malnutrition with clinical outcomes could not be explored. Due to the small proportion of severely malnourished participants in this sample, it is unlikely that any statistically significant relationship would have been observed.
In conclusion, vascular surgery patients are complex with a range of pathologies influenced by nutrition. The present study found a high prevalence of malnutrition secondary to suboptimal micronutrient status that was not identified by the four malnutrition screening tools investigated or the PG-SGA indicating that the development of vascular disease-specific screening and assessment tools that encompass additional parameters of relevance such as micronutrients and mobility measures are warranted to ensure that those at nutritional risk receive appropriate nutritional care to optimise patient and clinical outcomes.
Acknowledgements
The authors wish to acknowledge the nursing staff on the vascular surgery unit at Flinders Medical Centre, Bedford Park, Australia for their support with this project. We also wish to thank and acknowledge the study participants for their time and commitment.
No financial support was received to conduct this study.
J. T. was involved in the design, implementation, analysis of results and preparation of the manuscript. B. K. was involved in the analysis of results and preparation of the manuscript. C. D. was involved in the design of the study and review of the manuscript. M. M. was involved in the design of the study and preparation of the manuscript.
There were no conflicts of interest.








