Epidemiological and clinical studies have identified a number of dietary compounds purported to influence the molecular events involved in the progression of prostate cancer. Carotenoids(Reference Giovannucci1–Reference Etminan, Takkouche and Caamano-Isorna3), lipid-soluble antioxidants(Reference Lee, Gaziano and Buring4, Reference Weinstein, Wright, Pietinen, King, Tan, Taylor, Virtamo and Albanes5), and fish oil-derived PUFA, EPA (20 : 5n-3) and DHA (22 : 6n-3)(Reference Kobayashi, Barnard and Henning6, Reference Leitzmann, Stampfer, Michaud, Augustsson, Colditz, Willett and Giovannucci7), are of particular interest. Cell-culture studies have affirmed the anticancer properties of these nutrients; however, the relevance of these experimental data to human diseases is limited. One limitation is that concentrations of nutrients used in experimental studies are typically much higher than established human plasma levels. Furthermore, the majority of investigations utilising in vitro cell models supplement culture media with fetal bovine serum (FBS). Extrapolations from these studies to human disease are erroneous because an absence or imbalance of nutrients in FBS compared with human serum (HS) affects cell growth, development and function. Important differences in the composition of HS and FBS exist including the amounts of n-3 PUFA, the n-6:n-3 fatty acid ratio and the PUFA:SFA ratio(Reference Ma8). FBS is also devoid of basal levels of testosterone, a regulator of the growth of many prostate cancer cell lines(Reference Soronen, Laiti and Torn9), as well as many other growth-regulating hormones found in HS derived from males. In the present study, initial work focused on establishing a range of prostate cancer cell lines in culture supplemented with HS and, subsequently, evaluating the effects of bioactive nutrients on cell growth and integrin expression.
The consumption of lycopene, abundant in tomato-based products, has long been associated with prostate health and a reduced risk of developing prostate cancer(Reference Giovannucci1, Reference Stacewicz-Sapuntzakis and Bowen10). In addition to exhibiting potent antioxidant activity(Reference Heber and Lu11), lycopene has been shown to inhibit the proliferation of normal prostate epithelial cells(Reference Obermuller-Jevic, Olano-Martin, Corbacho, Eiserich, van der Vliet, Valacchi, Cross and Packer12), as well as several prostate cancer cell lines(Reference Kotake-Nara, Kushiro, Zhang, Sugawara, Miyashita and Nagao13). Accumulating experimental evidence also indicates that lycopene is capable of influencing other aspects of prostate cell functioning, including cell-cycle inhibition, increased cellular differentiation, inhibition of the insulin-like growth factor-1 signalling pathway and increased gap-junction communication(Reference Wertz, Siler and Goralczyk14–Reference Kanagaraj, Vijayababu, Ravisankar, Anbalagan, Aruldhas and Arunakaran16), which would be expected to influence cell growth. The antioxidant activity of vitamin E has also been well documented(Reference Brigelius-Flohe and Traber17), with specific isomers demonstrating an ability to inhibit the growth of prostate cancer cells(Reference Jiang, Wong, Fyrst, Saba and Ames18, Reference Malafa, Fokum, Andoh, Neitzel, Bandyopadhyay, Zhan, Iiizumi, Furuta, Horvath and Watabe19). However, aside from antioxidant properties, little is known about other potential mechanisms of action of vitamin E on prostate cancer progression(Reference Fleshner and Kucuk20, Reference Willis and Wians21). Numerous epidemiological studies have indicated that high fish consumption may be an effective approach to reducing risk of several cancers, including prostate cancer(Reference Leitzmann, Stampfer, Michaud, Augustsson, Colditz, Willett and Giovannucci7, Reference Astorg22). These observations are supported by several experimental studies reporting inhibitory effects of EPA and/or DHA treatment on the growth of prostate cancer cells(Reference Rose and Connolly23, Reference Chung, Mitchell, Zhang and Young24). To date, most of the epidemiological and clinical prostate cancer research investigating specific dietary nutrient intakes has focused on the reduction of disease risk. While further understanding of preventative mechanisms is essential, research into how nutrients affect malignant cell growth and disease progression following diagnosis is desperately needed.
The progression of prostate cancer primarily involves the formation of secondary metastatic lesions to bone(Reference Keller, Zhang, Cooper, Smith, McCauley, Pienta and Taichman25, Reference Bubendorf, Schopfer, Wagner, Sauter, Moch, Willi, Gasser and Mihatsch26), a process partially mediated by integrin cell adhesion proteins. Integrins are a family of heterodimeric transmembrane proteins composed of α- and β-subunits that provide the physical link between proteins of the extracellular matrix and cell cytoskeleton. Integrins modulate many important signalling pathways and cellular events, including cell growth, survival, invasion, migration and differentiation(Reference Hood and Cheresh27, Reference Knudsen and Miranti28). Differences in the expression levels of specific integrins in normal and malignant prostate cells have been reported(Reference Stewart, Cooper and Sikes29); however, molecular events responsible for these differences remain largely unclear. Moreover, bone matrix contains several components, including type-I collagen, vitronectin, fibronectin and osteopontin, which are ligands for specific integrins implicated in the metastatic progression of prostate cancer(Reference Tantivejkul, Kalikin and Pienta30). Interactions of integrin α2β1 with type-I collagen have been implicated in the formation of bone metastasis(Reference Hall, Dai, van Golen, Keller and Long31). The integrins αvβ3 and αvβ5 mediate cell adhesion to a variety of extracellular matrix proteins, particularly vitronectin, and play a key role in tumour-mediated angiogenesis, as well as tumour cell migration, invasion and survival during the metastatic process(Reference McCabe, De, Vasanji, Brainard and Byzova32, Reference Nemeth, Nakada, Trikha, Lang, Gordon, Jayson, Corringham, Prabhakar, Davis and Beckman33). Expression of the integrins α2β1, αvβ3 and αvβ5 may facilitate migration and metastatic spread of cancer cells and correlate with increased tumour invasiveness(Reference Hall, Dai, van Golen, Keller and Long31, Reference McCabe, De, Vasanji, Brainard and Byzova32), making integrins a potential biomarker for cancer progression(Reference Knudsen and Miranti28) and enabling an assessment of the influence of bioactive nutrients on cancer growth and development.
For the present study, a range of cell lines representing the disease spectrum from normal prostate epithelial cells to highly invasive prostate cancer cells (RWPE-1>22Rv1>LNCaP>PC-3) were utilised. It was hypothesised that the addition of physiologically relevant concentrations of the nutrients lycopene, vitamin E or fish oil to cell-culture media would reduce prostate cell growth as well as α2β1, αvβ3 and αvβ5 integrin expression in this series of prostate cell lines.
Materials and methods
Human serum
Ethical approval was provided by the Faculty of Agriculture Forestry and Home Economics Health Research Ethics Board at the University of Alberta (Edmonton, Canada) to obtain HS from healthy male subjects between the ages 18 and 35. The subjects had no history of cancer, autoimmune disease or other existing medical conditions. Subjects receiving steroidal medications, hormone therapies or having undergone surgery within the last 3 months were excluded. Blood samples (50 ml) collected from the male subjects were separated by centrifugation and the serum fractions from all the donors were pooled and stored at − 20°C.
Cell culture
A range of cell lines representing the spectrum of human prostate cancer, from normal prostate epithelial cells to brain and bone metastases, were used in the experiments. RWPE-1, 22Rv1, LNCaP (androgen sensitive and prostate-specific antigen (PSA) positive), DU-145 and PC-3 (androgen insensitive and PSA negative) cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in 75 cm2 flasks (Fisher Scientific, Edmonton, AB, Canada) at 37°C in a humidified 5 % CO2 atmosphere. All the cell lines were maintained in culture media recommended by ATCC and contained 2 mm-l-glutamine supplemented with 1 % (v/v) antibiotic/antimycotic solution (100 U/ml penicillin, 100 μg/l streptomycin and 25 mg/l amphotericin B). All the culture media were further supplemented with either 10 % (v/v) heat-inactivated FBS or 1 % (v/v) HS. Serum concentrations were selected to standardise the total amount of fatty acids introduced into the cell cultures(Reference Ma8).
All experimental studies were performed on the cells after the fourth passage. The cell lines were maintained within two passages of each other. The cells were grown until they reached 90 % confluence and subcultured routinely, with the culture medium being changed two to three times a week. After culturing the cell lines in either HS or FBS for 4 d, gross cell morphology was determined by light microscopy. The cells were then trypsinised and counted using the Trypan Blue exclusion method to determine cell viability. Integrin expression was measured using monoclonal antibodies (α2β1, αvβ3 and αvβ5) combined with FACS flow cytometry analysis described later.
Integrins
A range of prostate cell lines were assessed to determine α2β1, αvβ3 and αvβ5 expression. Cells were plated onto ninety-six-well plates (5·0 × 104 cells/well) and coated with a buffer solution (PBS:4 % FBS (Gibco Invitrogen Corporation, Burlington, Ont., Canada)) for 20 min. The plates were centrifuged for 2 min and then the supernatant was removed from each well. The plates were lightly vortexed to displace the cells from the bottom of the wells. Antibodies recognising the integrins α2β1, αvβ3 and αvβ5 (10 μg/ml; Chemicon Int., Cedarlane Laboratories Ltd, Hornby, ON, Canada) were added to treatment wells with the buffer being added to control wells. The plates were then incubated in the dark at 4°C for 30 min, and the cells were rinsed with the buffer and vortexed three times. Fluorescein isothiocyanate (20 μg/ml) was added to all the treatment wells and the buffer was added to the control wells. Plates were incubated in the dark at 4°C for 30 min, washed and vortexed three times. The cells were fixed in 300 μl of PBS:0·5 % paraformaldehyde fixative solution. Finally, integrin expression of the prostate cell lines was assayed using flow cytometry using a FACSCalibur instrument with CellQuest Software (BD Biosciences, Bedford, MA, USA). The cells were gated according to forward and side scatter characteristics and mean fluorescence of the gated cells was determined. Resulting percentages were corrected for background fluorescence (0–5 %) by incubating the cells with the appropriate isotype control (IgG1).
Lycopene and vitamin E media preparation
Preliminary experiments were carried out to define the concentrations of nutrients to be used in these experiments based on the physiological ranges reported in the literature at an amount that exhibited no toxicity (measured by lactate dehydrogenase assay, data not shown). Vitamin E ( ± α-tocopherol), lycopene (C40H56) and Tween 40 were supplied by Sigma-Aldrich (Canada Ltd, Oakville, Ont., Canada). Stock solutions of vitamin E and lycopene were solubilised in Tween 40 (100 μl), diluted with complete culture media to a concentration of 5 mm, vortexed and filter sterilised using a 0·22 μm filter (Millipore Corporation, Bedford, MA, USA). The filtered stock solutions were serially diluted with additional complete culture media to a working concentration of 5 μm for vitamin E and 10 nm for lycopene, which are within human physiological ranges(Reference Wu, Schwartz, Platz, Clinton, Erdman, Ferruzzi, Willett and Giovannucci34–Reference Riso, Visioli, Erba, Testolin and Porrini38).
Fish oil media preparation
Preparation of fish-oil-supplemented media was adapted from a procedure used by Schley et al. (Reference Schley, Brindley and Field39). A stock solution containing 10 mg/ml of fish oil was prepared in 95 % ethanol and stored at − 80°C under N2(g). The stock solution was reconstituted in HS and then incubated in a water bath at 37°C for 1 h with vortexing every 10 min. The HS and fish oil mixture was diluted with culture media to a final working concentration of 100 μm and used immediately.
Nutrient treatment in cell culture
All the experiments using nutrient-supplemented media were carried out in cells cultured with 1 % HS. The cells were seeded onto six-well plates at a concentration of 1·0 × 105 cells/well containing 3 ml of complete culture media. Then the cells were allowed to adhere for 48 h at which time the media were decanted and replaced with 3 ml of nutrient-supplemented media containing either 10 nm-lycopene, 5 μm-vitamin E, 100 μm-fish oil, the respective control vehicle or complete culture media. The cells were detached from the respective wells after the 48 h nutrient incubation by the addition of 1 ml of 0·25 % trypsin–0·03 % EDTA. The wells were rinsed with appropriate media and the cells were counted using a haemacytometer and cell viability was evaluated via Trypan Blue exclusion. The expression levels of integrins, α2β1, αvβ3 and αvβ5 were determined for each prostate cell line using flow cytometry as described under the integrins section.
Statistical analysis
Means and standard deviations were calculated for all integrins, and Student's unpaired t tests were conducted to determine the statistical significance (P < 0·05) between control vehicles and nutrient treatments for each cell line.
Results
Cell growth in human v. fetal bovine serum
The fatty acid and hormone composition of FBS is markedly different from HS(Reference Ma8). To determine the effect of these differences on prostate cell growth, RWPE-1, 22Rv1, LNCaP and PC-3 cell lines were cultured separately in either 10 % FBS or 1 % HS. Growth of the phenotypically normal RWPE-1 cell line was not significantly altered by culture in HS. However, all four malignant cell lines showed significant reductions in the growth of up to 50 % (P < 0·01; Fig. 1) when cultured in HS compared with FBS. Photographs comparing the growth and morphology of PC-3 cells in 1 % HS and 10 % FBS are shown in Fig. 2 (a), 2 (b).
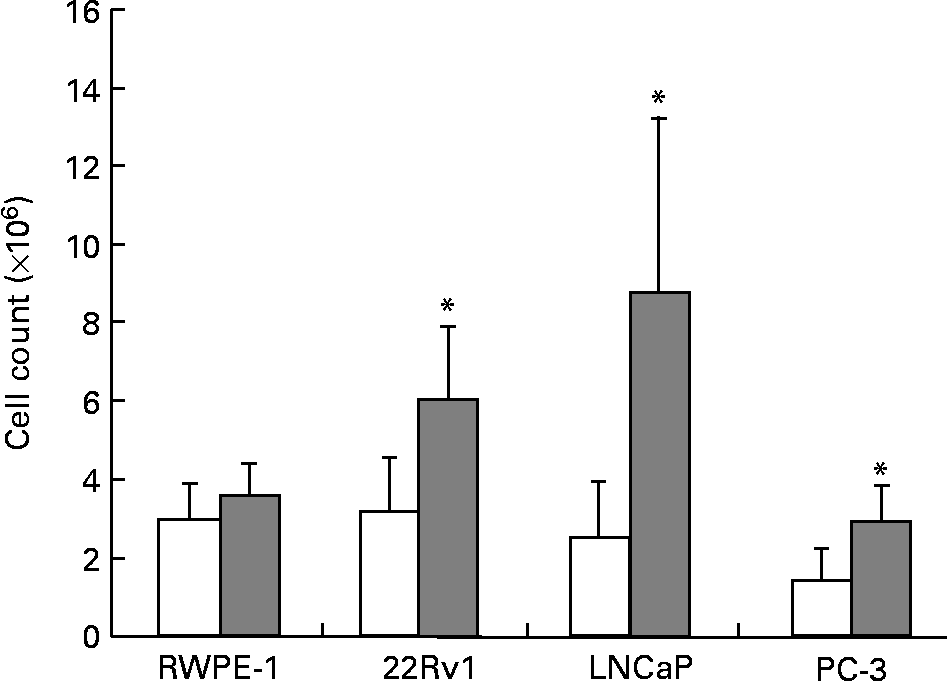
Fig. 1 Growth of RWPE-1, 22Rv1, LNCaP and PC-3 is greater when cells are cultured with fetal bovine serum (FBS; ![]() ) compared with human serum (HS; □). Viable cells were counted to determine cell population growth after 7 d in culture using Trypan Blue exclusion. Bars represent the means and standard errors (n ≥ 15). * Statistical significance (P < 0·01) between HS and FBS for the cell line as determined by Student's unpaired t test.
) compared with human serum (HS; □). Viable cells were counted to determine cell population growth after 7 d in culture using Trypan Blue exclusion. Bars represent the means and standard errors (n ≥ 15). * Statistical significance (P < 0·01) between HS and FBS for the cell line as determined by Student's unpaired t test.

Fig. 2 Photograph of PC-3 in 1 % human serum (HS) and 10 % fetal bovine serum (FBS). Cells were visualised by light microscopy following 7 d in culture with either (a) FBS or (b) HS (seeded at 1·0 × 106 cells).
Cell growth in response to lycopene, vitamin E and fish oil treatments
The ability of physiological concentrations of lycopene, vitamin E and fish oil to affect cell growth in RWPE-1, 22RV1, LNCaP and PC-3 cell lines was examined. Trypan Blue exclusion demonstrated that cell viability following nutrient exposure was greater than 99 %, and the lactate dehydrogenase assay confirmed that nutrient delivery vehicles and individual nutrients were non-cytotoxic (data not shown). Culture media containing lycopene (10 nm) resulted in no reductions in cell growth in any prostate cell lines tested (Table 1). An increase (P < 0·0001) in cell growth was observed in all the prostate cancer cell lines when vitamin E (5 μm) was added to the culture media; however, growth of the normal RWPE-1 cell line (Table 1) was not significantly affected by this nutrient. The addition of fish oil (100 μm) to the culture media produced a significant decrease (P < 0·004) in the cell growth of all prostate cell lines (Table 1). No marked changes in cell morphology were observed via light microscopy in response to lycopene, vitamin E or fish oil supplementation of the culture media (data not shown).
Table 1 Growth of cell lines with the addition of physiological levels of lycopene, vitamin E and fish oil (10 nm, 5 μm and 100 μm, respectively) was measured using Trypan Blue exclusion after 4 d in culture†
(Mean values and standard deviations for n≥9 for each nutrient)
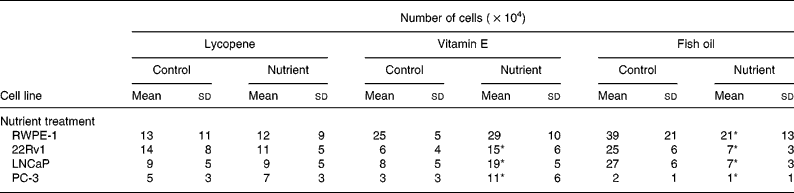
* P < 0·0001.
† Lycopene had no effect on the growth of any of the cell lines, whereas fish oil reduced the growth of all the cell lines. Vitamin E reduced growth of the malignant cell lines.
Integrin expression in response to lycopene, vitamin E and fish oil treatments
Preliminary experiments measuring basal expression levels of integrins α2β1, αvβ3 and αvβ5 were conducted on the RWPE-1, 22Rv1, LNCaP, DU-145 and PC-3 cell lines (data not shown). Integrin α2β1 was highly expressed on all the five prostate cell lines. Integrins αvβ3 and αvβ5 were expressed only by the more invasive DU-145 and PC-3 cell lines. These results are consistent with other reports, indicating that the changes in the expression of specific integrins may correlate with the progression to a more invasive phenotype(Reference Stewart, Cooper and Sikes29, Reference Hall, Dai, van Golen, Keller and Long31). In accordance with these data, additional experiments measured only integrins shown to be expressed on each individual cell line.
α2β1 supplementation of the culture medium with 10 nm lycopene produced a significant increase (P < 0·0001) in integrin α2β1 expression by RWPE-1 cells, and a corresponding significant reduction (P < 0·04) in the expression by 22Rv1, LNCaP and PC-3 cells in comparison with vehicle control (Table 2). Supplementation of culture medium with 5 μm-vitamin E resulted in a decrease (P < 0·0001) of integrin α2β1 expression in 22Rv1 cells; however, the expression levels in RWPE-1, LNCaP and PC-3 cells were not significantly altered in comparison with vehicle control (Table 2). Supplementation of culture medium with 100 μm-fish oil increased (P < 0·02) α2β1 expression in RWPE-1 and 22Rv1 cells, and decreased α2β1 (P < 0·01) expression in LNCaP and PC-3 cells in comparison with cells incubated with the control vehicle (Table 2).
Table 2 Expression of the α2β1 integrin in cell lines with the addition of lycopene, vitamin E and fish oil (10 nm, 5 μm and 100 μm, respectively) shows nutrient-specific effects depending on the stage of the cancer each cell line represents†
(Mean values with their standard deviations for n 5–21 for each nutrient)
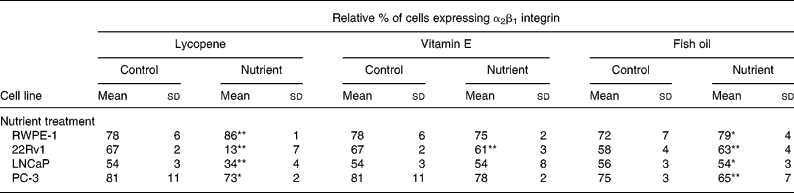
* P < 0·05.
** P < 0·0001.
† In the phenotypically normal cells, α2β1 was higher in the nutrient-treated cells. In most advanced cell types (PC-3), fish oil and lycopene reduced the expression of α2β1.
αvβ3 and αvβ5 are up-regulated in more aggressive prostate cancer cell types and thus were expressed at the highest levels in the PC-3 cell line, in agreement with a previous report(Reference Sun, Fang, Wang, Cooper, Pienta and Taichman40). The addition of 10 nm-lycopene, 5 μm-vitamin E or 100 μm-fish oil to the culture medium resulted in a significantly lower (P < 0·05) expression of both αvβ3 and αvβ5 integrins in the PC-3 cell line in comparison with the control vehicle (Figs. 3 and 4).
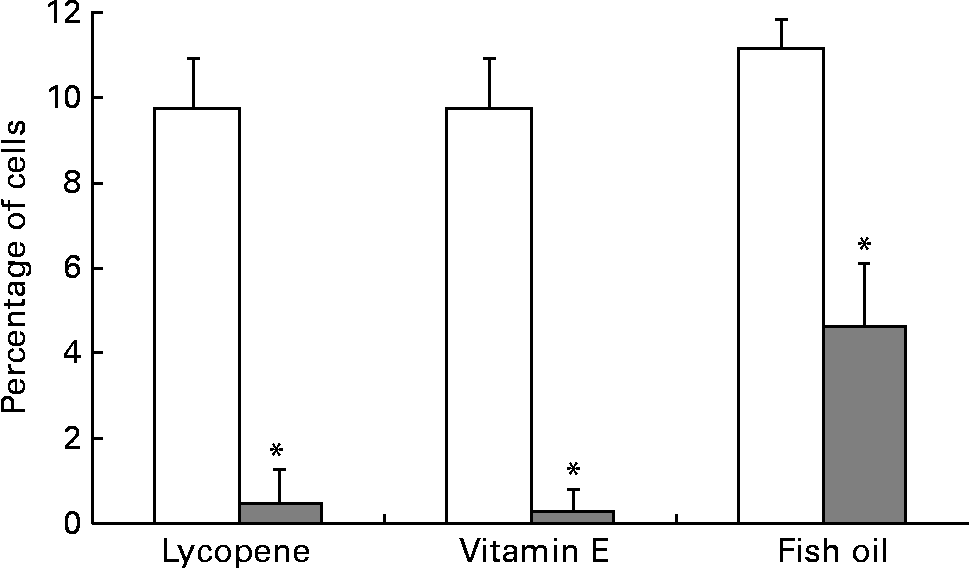
Fig. 3 PC-3 expressed less αvβ3 integrin when cells were cultured with lycopene, vitamin E or fish oil. The cells were cultured in media containing physiological levels of either lycopene, vitamin E or fish oil (10 nm, 5 μm and 100 μm, respectively) for 2 d. The cells were collected and incubated with integrin anitbodies and analysed using flow cytometry. Bars represent the means and standard errors (n 5–11 per treatment). (![]() ), Nutrient treatment; (□), vehicle control. * Statistical significance (P < 0·001) between nutrient treatment and vehicle control as determined by Student's unpaired t test.
), Nutrient treatment; (□), vehicle control. * Statistical significance (P < 0·001) between nutrient treatment and vehicle control as determined by Student's unpaired t test.
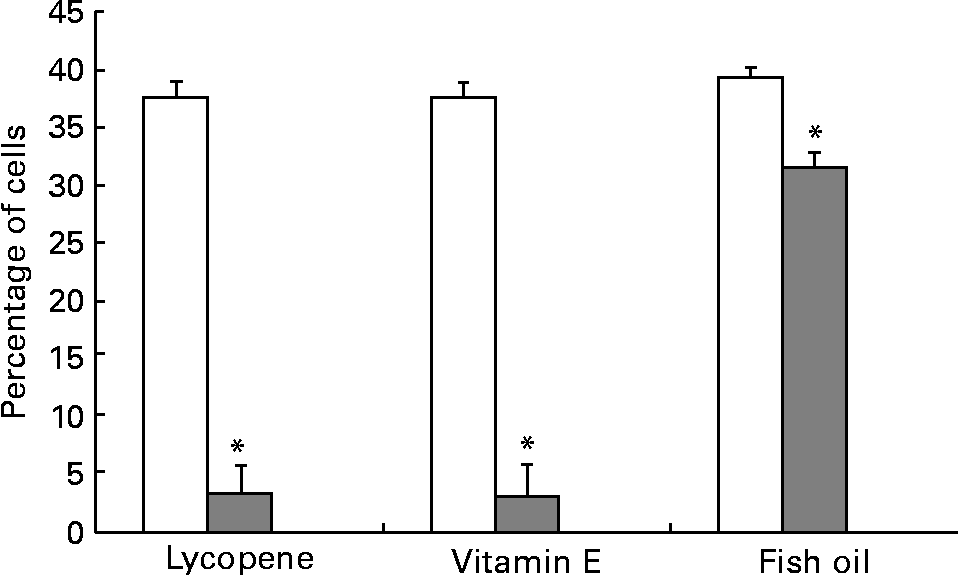
Fig. 4 PC-3 expressed less αvβ5 integrin when cells were cultured with lycopene, vitamin E or fish oil. Expression of αvβ5 was reduced when PC-3 cells were cultured with media containing physiological levels of lycopene, vitamin E or fish oil (10 nm, 5 μm and 100 μm, respectively) for 2 d. The cells were collected and incubated with integrin anitbodies and analysed using flow cytometry. Bars represent the means and standard errors (n 11). (![]() ), Nutrient treatment; (□), vehicle control. * Statistical significance (P < 0·05) between nutrient treatment and vehicle control.
), Nutrient treatment; (□), vehicle control. * Statistical significance (P < 0·05) between nutrient treatment and vehicle control.
Discussion
Currently, in vitro cell culture is the principal model system employed for the studies of human prostate cancer. A range of cell lines spanning the spectrum of this disease, from phenotypically normal prostate epithelial cells to aggressive metastatic tumour cells, have been established. Nearly all studies culture these cells in media supplemented with FBS. The relevance of these results to man may be misleading, due in part, to the major differences in the fatty acid composition which exist between HS and FBS(Reference Ma8). For example, the higher concentrations of n-6 fatty acids found in FBS have been repeatedly demonstrated to stimulate the growth of prostate cancer cells(Reference Brown, Hart, Gazi, Bagley and Clarke41, Reference Hughes-Fulford, Chen and Tjandrawinata42). Testosterone, present in HS but lacking in FBS, is an integral regulator of prostate cell growth and survival in the in vivo microenvironment(Reference Soronen, Laiti and Torn9). In the present study, significantly lower growth in all the malignant cell lines (22Rv1, LNCaP and PC-3) was observed when the cells were cultured in HS compared with FBS, whereas the growth of the phenotypically normal cell line RWPE-1 was not significantly affected by the culture in HS. Cell morphology of the prostate cell lines also differed between the two culture conditions, suggesting a reason for concern about the exclusive use of FBS as a media supplement.
As prostate cancer cells become more invasive, they acquire phenotypic changes that alter their affinity for the components of the extracellular matrix, as well as other cellular components(Reference Stewart, Cooper and Sikes29). In the present study, we focused on several members of the integrin family of transmembrane proteins, α2β1, αvβ3 and αvβ5, demonstrated to have different levels of expression in more aggressive v. less invasive cells(Reference Hall, Dai, van Golen, Keller and Long31, Reference Fornaro, Manes and Languino43). In addition to facilitating the migration of prostate cancer cells out of the primary site, the integrins also mediate adhesion to several bone matrix components including type-I collagen, fibronectin, laminin and osteopontin(Reference Edlund, Sung and Chung44). As a majority of prostate cancer metastases occur in bone, inhibiting the ability of tumour cells to adhere to bone matrix components represents an important therapeutic target(Reference Tantivejkul, Kalikin and Pienta30). Integrins αvβ3 and αvβ5 have been shown to be highly expressed by the tumour cells capable of forming bone metastases(Reference Stewart, Cooper and Sikes29, Reference Zheng, Woodard, Fornaro, Tallini and Languino45). In agreement with previous reports(Reference Hall, Dai, van Golen, Keller and Long31), we demonstrated that the androgen-responsive and PSA-producing (and less invasive) cell lines 22Rv1 and LNCaP exhibit lower basal expression of integrin α2β1 in comparison with the cell lines derived from metastasis. Furthermore, in the present study, integrins αvβ3 and αvβ5 were expressed in only 1 % of the less invasive cell lines, RWPE-1, 22Rv1 and LNCaP cells, whereas PC-3 cells exhibited higher expression levels of 10 and 35 %, respectively. Collectively, these results denote a shift in integrin expression and subsequently in the potential for prostate cancer cells to adhere to extracellular and bone matrix components, especially type-I collagen, during disease progression. These observations suggest that integrins may represent important markers of the metastatic capacity of prostate cancer.
Epidemiological studies have associated lycopene, vitamin E and n-3 PUFA with reduced prostate cancer risk(Reference Giovannucci1, Reference Weinstein, Wright, Pietinen, King, Tan, Taylor, Virtamo and Albanes5, Reference Leitzmann, Stampfer, Michaud, Augustsson, Colditz, Willett and Giovannucci7), although the mechanisms mediating these effects are not fully understood. Human plasma lycopene concentrations are approximately 0·5 μm(Reference Stahl and Sies46), but range from 0·01 to 1·8 μm depending on the region of study(Reference Wu, Schwartz, Platz, Clinton, Erdman, Ferruzzi, Willett and Giovannucci34–Reference Riso, Visioli, Erba, Testolin and Porrini38, Reference Goralczyk and Siler47, Reference Olmedilla, Granado and Southon48). Kim et al. (Reference Kim, Rao and Rao49) reported a dose-dependent effect of lycopene on the growth of LNCaP cells with complete inhibition of growth occurring at 100 μm lycopene. Tang et al. (Reference Tang, Jin, Zeng and Wang50) showed that the growth of DU-145, PC-3 and LNCaP cells was inhibited 50 % by lycopene concentrations of 26·6, 40·3 and 168·5 μm, respectively. However, these lycopene concentrations are far in excess of what is physiologically achievable in man. Ivanov et al. (Reference Ivanov, Cowell, Brown, Rennie, Guns and Cox15) reported the growth of LNCaP and PC-3 cells to be significantly inhibited by physiologically relevant lycopene concentrations of 0·5 μm or more. By contrast, the physiologically attainable lycopene concentration used in the present study did not alter the growth of any prostate cancer cell line, a result consistent with the recent reports in DU-145, PC-3(Reference Pastori, Pfander, Boscoboinik and Azzi51) and LNCaP cells(Reference Hantz, Young and Martin52). In addition to the growth-inhibitory effects, lycopene also influences other aspects of prostate cell functioning, including cell-cycle inhibition, increased cellular differentiation, inhibition of the IGF-1 signalling pathway and increased gap-junction communication, as reviewed by Wertz et al. (Reference Wertz, Siler and Goralczyk14). Much like gap junctions, the integrin α2β1 is involved in the regulation of cell-to-cell signalling for adhesion and migration(Reference Nelson and Lehninger53). In the present study, the addition of lycopene to media reduced α2β1 in the malignant 22Rv1, LNCaP and PC-3 cell lines and, likewise, decreased αvβ3 and αvβ5 integrins in the PC-3 cell line. Therefore, lycopene may impact the progression of prostate cancer by altering invasive and migratory potential, as suggested by integrin expression.
Reports published to date on the effects of vitamin E and prostate cancer are conflicting(Reference Lee, Gaziano and Buring4). Richards et al. (Reference Richards, Benghuzzi, Tucci and Hughes54) reported LNCaP cell growth to be reduced at concentrations of 0·2 and 2·0 μm. By contrast, Jiang et al. (Reference Jiang, Wong, Fyrst, Saba and Ames18) reported that α-tocopherol failed to inhibit the growth of PC-3 and LNCaP cells at a concentration of 11·6 mm. The physiological concentration of vitamin E in HS ranges from 1·16 to 116 μm(Reference Lagua55). The present study suggests that vitamin E within this range enhances cell growth in all malignant cell lines, but not the phenotypically normal RWPE-1 cell line. Although preliminary, this nutrient may be important for the prevention of prostate cancer, but less effective once tumours are established. Recent data derived from an erythroleukaemia cell line demonstrated the ability of α-tocopherol to reduce the expression of several integrins in this cell line(Reference Breyer and Azzi56). Expressions of integrins αvβ3 and αvβ5 were significantly reduced by vitamin E treatment of the PC-3 cell line in the present study, suggesting that these cells may be less able to adhere to and migrate on the extracellular matrix components(Reference Wang, Ferreira, Shao, Laird and Sandig57).
n-3 PUFA found in fish oils have documented the effects on several stages of prostate carcinogenesis(Reference Rose and Connolly23, Reference Brown, Hart, Gazi, Bagley and Clarke41). The present study demonstrated a significant reduction in the growth of all prostate cell lines in response to treatment with fish oil at human physiological levels. We based this concentration on plasma fatty acid data from healthy human subjects who were not supplementing with fish oil(Reference Pratt, Watanabe, Bruera, Mackey, Clandinin, Baracos and Field58), as well as the levels found in infants who were fed human milk(Reference Clandinin, Van Aerde, Parrott, Field, Euler and Lien59, Reference Clandinin, Van Aerde, Parrott, Field, Euler and Lien60). In vitro treatment with n-3 PUFA at similar concentrations resulted in growth reductions in a breast cancer cell line(Reference Schley, Jijon, Robinson and Field61). Demonstration that fish oil treatment significantly reduced the expression of integrins αvβ3 and αvβ5 in the PC-3 cell line is suggestive of decreased ability for this cell line to form metastatic lesions to bone(Reference Wang, Ferreira, Shao, Laird and Sandig57). Additionally, when introduced into cell-culture medium, n-3 fatty acids are incorporated into plasma membrane phospholipids of normal prostate cells, as well as prostate cancer cells, altering structural and chemical properties(Reference Kobayashi, Barnard and Henning6). The observed growth inhibition and alteration in the integrin expression observed in the present study may be due to the changes in plasma membrane structure caused by long-chain PUFA incorporation.
In conclusion, differences in cell growth, morphology and integrin expression were evident when the cells were grown in HS compared with FBS. Physiologically relevant concentrations of lycopene had no significant effect on the growth of normal and malignant prostate cells. Vitamin E had no apparent growth-inhibitory effect on phenotypically normal prostate cells, but rather enhanced the growth of prostate cancer cell lines. Fish oil inhibited the growth of all prostate cell lines tested, further supporting its beneficial role in cancer prevention and as an adjuvant nutrient. The ability of lycopene, vitamin E and fish oil to decrease the expression levels of the integrins αvβ3 and αvβ5 in most invasive prostate cancer cell lines represents a crucial finding. Integrins are mediators of the metastatic progression of prostate cancer, particularly to the bone microenvironment and represent an important therapeutic opportunity. Further work is required to determine functional changes associated with the observed differences in integrin expression when the cells are cultured with lycopene, vitamin E or fish oil.
Acknowledgements
The source of funding for the present work was provided by the Canadian Institutes for Health Research. H. H. was the recipient of the Elizabeth Donald MSc Scholarship. The authors have no conflict of interest to declare. T. B and H. H share primary authorship. T. B. analyzed the data and wrote the paper; H. H. planned, conducted and analyzed the experiments and contributed to the writing of the paper. J. B. M. provided scientific expertise and mentorship, M. T. C. provided scientific direction and expertise and V. C. M. serves as the corresponding author.








