- FP
fungiform papillae
- MT
medium taster
- NT
nontaster
- PROP
propylthiouracil
- PTC
phenylthiocarbamide
- SNP
single nucleotide polymorphisms
- ST
supertaster
A recent Irish study reported that the prevalence of overweight and obesity in 4–13-year olds is 24·6%(Reference Barron, Comiskey and Saris1), while in adults the prevalence is 61%(Reference Morgan, McGee and Watson2). These statistics reflect the current situation in the UK and most parts of Europe(Reference Berghöfer, Pischon and Reinhold3), and are approximately 5% behind levels in the USA(Reference Morgan, McGee and Watson2), where the prevalence is predicted to reach almost 90% by 2030(Reference Wang, Beydoun and Liang4). Obesity has been linked, among others, to increased risk of heart disease, cancer and depression(Reference Shelton and Miller5), with an estimated annual cost of €70 million to the Irish health service(Reference Barron, Comiskey and Saris1); between £2·4 billion and £2·6 billion to the UK and as much as $78·5 billion annually in the USA(Reference Wang, Beydoun and Liang4). Recognized contributory factors to the obesity ‘epidemic’ include decreased levels of physical activity and an increase in adverse eating behaviours(Reference Livingstone, McCaffrey and Rennie6) such as high consumption of energy-dense foods and low fruit and vegetable consumption(Reference Drewnowski and Specter7). The reasons for particular food choices are therefore of great importance to dietitians, health-care workers and nutritionists. Fruit and vegetables are vital for good health(Reference Van Duyn and Pivonka8, Reference Vig, Rampala and Thinda9), and the WHO recommends a minimum intake of 400 g of fruit and vegetables per day, preferably in five 80 g servings(10). However, almost 80% of adults fail to reach this amount, with an average daily intake in Ireland of 276 g, and in children an even lower average intake of just over 200 g(11).
In today's Western society, where most foods are available to most people, food choices are more than a simple matter of availability; they are governed by a multitude of complex processes(Reference Glanz, Basil and Maibach12–Reference Wardle, Carnell and Cooke15). Some of the factors involved include mood, the environment, health, allergies, convenience, hunger levels, cost, pregnancy, habit, cultural influences, sensory attributes such as colour and smell and of course taste(Reference Drewnowski and Specter7, Reference Drewnowski, Henderson and Levine16–Reference Scheibehenne, Miesler and Todd18). One model that demonstrates how the wide range of different factors involved interact is the Food Choice Process Model(Reference Connors, Bisogni and Sobal17) (Fig. 1).

Fig. 1. The Food Choice Process Model (taken from Connors et al.(Reference Connors, Bisogni and Sobal17)).
In this model, experiences over the life course influence the personal factors that govern an individual's unique personal food system. Within this system, people manage five main food-related values. These differ in relative importance from person to person and even between eating situations.
Taste is perceived as a highly influential factor in food choice decisions(Reference Glanz, Basil and Maibach12, Reference Connors, Bisogni and Sobal17, Reference Drewnowski and Rock19, Reference Kearney, Kearney and Dunne20). It is listed as one of the five main values in the Food Choice Process Model(Reference Connors, Bisogni and Sobal17), and has also been listed as the top reason for food choice by Americans(Reference Glanz, Basil and Maibach12). Taste preferences have significant impact on eating behaviours(Reference Mok21) and may be influenced by taste perception(Reference Tepper22–Reference Hayes, Sullivan and Duffy24). Perception of taste may vary between individuals depending on genetic variations in certain taste receptor genes. Genetically determined variation in taste sensitivity in human subjects was reported for four of the basic tastes: sweet(Reference Mainland and Matsunami25), bitter(Reference Kim and Drayna26, Reference Sugino, Umemoto and Mizutani27), sour(Reference Wise, Hansen and Reed28) and umami, the savoury taste exemplified by the taste of monosodium glutamate(Reference Drayna29, Reference Shigemura, Shirosaki and Sanematsu30). This review examines how genetic variation in taste may influence food choice and in turn impact on nutrient intake, focusing, in particular, on variation in bitter taste and the role of the receptor gene TAS2R38.
Evolution of taste and genetic variation in taste sensitivity
Taste is one of the primary means of determining the acceptability of a food and might have been critical to the survival of early human subjects(Reference Tepper31). Sweet and umami taste evolved as ‘energetic sensors’ to recognize carbohydrate and protein energy sources, whereas bitter taste evolved as a warning against toxin ingestion(Reference Tepper31–Reference Kim, Breslin and Reed33). This is supported by the fact that while just three genes exist in the T1R gene family (which is responsible for the receptors for both sweet and umami taste) over twenty-five genes exist in the T2R gene family of bitter receptors (Fig. 2) which appear to have evolved from gene duplication events that expanded the range of bitter compounds to which human subjects are sensitive(Reference Kaji, Karaki and Fukami34). Further, bitterness in food tends to trigger an innate negative response or aversion(Reference Glendinning35, Reference Drewnowski and Gomez-Carneros36) and can be detected at low levels(Reference Tepper31, Reference des Gachons, Beauchamp and Breslin37, Reference Meyerhof38) to protect against accidental ingestion of potential toxins, even in small amounts.

Fig. 2. Phylogenetic tree of members of the TAS2R gene family and some of the ligands which bind their receptors (taken from Behrens and Meyerhof(Reference Behrens and Meyerhof99)).
The perception of sweet, umami and bitter tastes are all mediated via G-coupled protein receptors, encoded by the TAS1R and TAS2R taste receptor gene families, while salty and sour tastes are transduced via ion channels(Reference Wise, Hansen and Reed28). A sixth possible taste, that of fat, is still under debate(Reference Mangold, Payne and Ma39). Salty tastes are elicited by NaCl as well as other salts and are most likely mediated by a highly specific Na channel in taste receptor cells(Reference Beauchamp, Stein, Basbaum, Kaneko and Shepherd100), and are a vital part of ion and water homoeostasis(Reference Kim, Breslin and Reed33). Because of this, it seems that sensitivity to and preference for salty taste are influenced more strongly by environmental cues, such as surrounding salt concentrations, rather than individual genetic variation(Reference Wise, Hansen and Reed28). Similarly, there is little variation in the detection threshold of sour taste (which indicates the presence of acids) because of genetic polymorphisms(Reference Wise, Hansen and Reed28). For these reasons, this review will concentrate on the variation in bitter, sweet and umami taste qualities, which have been demonstrated to some degree to associate with certain genetic variations, with a specific focus on bitter taste perception.
The products of the three genes within the TAS1 gene family are responsible in various combinations for both sweet and umami tastes(Reference Kim, Wooding and Riaz40). Sweeteners such as saccharin and naturally occurring sugars such as sucrose, glucose, fructose and alcohol sugars elicit a sweet taste mediated through a heterodimer of G-coupled protein receptors encoded by two genes, TAS1R2 and TAS1R3 (Reference Fushan, Simons and Slack41), while a heterodimer encoded by TAS1R1 and TAS1R3 forms the basis of the umami receptor(Reference Li, Staszewski and Xu42), responsible for the perception of L-glutamate(Reference Chen, Alarcon and Tharp43). A number of single nucleotide polymorphisms (SNP) have been identified in these genes in both the coding and non-coding parts of these genes. Some of these have been linked to variation in taste perception of both umami and sweet tastes (Table 1), although the mechanisms underlying most of these differences remain to be determined. Sequence analysis of the TAS1R gene family has shown that generally TAS1R3 is the most conserved, and so less variation in the ability to detect umami is envisaged than that of sweet taste(Reference Kim, Wooding and Riaz40). One recent study has identified two non-coding SNP in TAS1R3 (Reference Fushan, Simons and Slack41), and reported that a large amount of the variability in sucrose variation was explained by differences in promoter activity associated with these SNP. This is one of the first studies to show that non-coding SNP may also affect taste perceptions, and importantly, as the TAS1R3 subunit also forms part of the umami receptor, these SNP may also contribute to some of the variation observed in umami taste(Reference Fushan, Simons and Slack41), although this is yet to be confirmed.
Table 1. Single nucleotide polymorphisms (SNP) in TAS1R and TAS2R gene family with known functional variation in sweet, umami and bitter perception

The TAS2R gene family is the largest family of taste receptors, and encodes the bitter taste receptors. Most of the bitter receptor genes are located on chromosomes 7 and 12, likely as a result of gene duplications. Variation has been observed in a number of bitter receptor genes, and in general, the variation observed in bitter receptors is higher than in most other genes(Reference Kim, Wooding and Riaz40), with a total of 151 non-synonymous SNP combinations or haplotypes identified within the members of the TAS2R gene family(Reference Kim, Wooding and Riaz40). It is not yet known if all of these correspond to variation in bitter sensitivity or to different taste receptors, but to date, variations in four of the bitter receptor genes TAS2R16, 38, 43 and 44, have been associated with differential bitter taste perception (Table 1). The area of bitterness sensitivity is the most extensively researched of all the taste qualities and dates back to the early 1930s, when ‘taste blindness’ to the bitter compounds phenylthiocarbamide (PTC) and propylthiouracil (PROP) was first noted(Reference Bufe, Breslin and Kuhn44). Both compounds contain a thiourea chemical group (N—C=S) and are recognised to a degree by the receptor encoded by the PTC gene TAS2R38 (Reference Bufe, Breslin and Kuhn44). PROP is now more commonly used in taste perception studies, as PTC has a slightly sulfurous odour(Reference Tepper45, Reference Reed, Tanaka and McDaniel46) and it has been reported to be toxic(Reference Wheatcroft and Thornburn47).
In 1992, the phrase ‘supertasters’ (ST), in relation to bitter taste perception, was coined(Reference Bartoshuk, Fast and Karrer48) following the research in PROP perception, when it was discovered that the ‘taster’ group could be further divided into ‘medium tasters’ (MT) and ‘ST’ of PROP, resulting in three groups of taster: ST, MT and nontasters (NT), depending on the perceived intensity of PROP, making up 20, 50 and 30% of the population, respectively(Reference Tepper22). ST perceive PROP as intensely bitter, and generally also dislike the taste. The TAS2R38 gene is responsible for the majority of the variation in bitter taste sensitivity of PTC, as well as a significant proportion of sensitivity to PROP(Reference Bufe, Breslin and Kuhn44, Reference Kim, Wooding and Ricci49, Reference Tepper, Koelliker and Zhao50). A number of SNP have been identified within this gene (Table 2), three of which give rise to the two main haplotypes observed in over 90% of the Caucasian population(Reference Kim, Wooding and Ricci49), PAV (proline–alanine–valine at amino acid positions 49, 262 and 296 respectively), the ‘taster’ form, and AVI (alanine–valine–isoleucine at amino acid positions 49, 262 and 296 respectively), the ‘NT’ form(Reference Drayna29, Reference Kim, Jorgenson and Coon51) and can be attributed to much of the variation observed in PTC and PROP sensitivity(Reference Kim, Jorgenson and Coon51).
Table 2. Single nucleotide polymorphisms (SNP) in TAS2R38
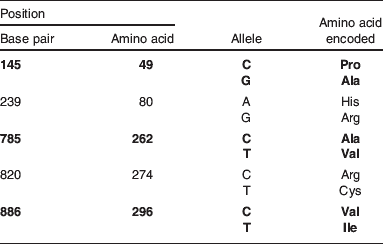
A total of five SNP have been observed in TAS2R38. The three most common, which give rise to the haplotypes PAV and AVI, are shown in bold. The less frequently observed sub-Saharan African SNP are shown in grey.
Taste and fungiform papillae density
Another mechanism through which genetic variation might affect taste perception is through the density of fungiform papillae (FP), one of the three types of papilla structures which house the tastebuds on the tongue. The bitter TAS2R38 gene has been suggested to influence FP density, as PROP sensitivity has been generally reported as positively correlated with FP density(Reference Bartoshuk, Duffy and Miller52–Reference Yackinous and Guinard56) and thus tasters of PROP may exhibit higher densities of trigeminal (touch) fibres on the tongue than NT. This may explain reports that PROP intensity has been associated with perceived intensity of other bitter tastes such as quinine and caffeine(Reference Hansen, Reed and Wright57), and that PROP tasters experience a heightened perception of other taste qualities, including sweetness from sucrose(Reference Lucchina, Curtis and Putnam58) and saccharine(Reference Bartoshuk59, Reference Pronin, Xu and Tang60); and the perception of liquid fat(Reference Tepper and Nurse61), as well as heightened ‘oral burn’ from spices such as capsaicin(Reference Karrer and Bartoshuk62). However, the relationship between TAS2R38, PROP intensity and FP density is unclear: one study has reported that FP density does not predict PROP intensity in individuals heterozygous at TAS2R38 (Reference Hayes, Bartoshuk and Kidd63), while another study concluded that PROP intensity is not correlated to creaminess perception and therefore not related to FP density(Reference Lim, Urban and Green64). It has been suggested that the differences observed in oral sensitivity may be due to differences in central nervous system processes, rather than differences in FP density(Reference Green and George65). Further clarification in this area is needed between FP density, PROP taster status and TAS2R38.
Taste perception and food choice
Taste perception influences food preference, which is one of the strongest mediators of fruit and vegetable consumption in children and adolescents(Reference Rasmussen, Krølner and Klepp66, Reference Bere, Brug and Klepp67), and might represent a significant barrier to the consumption of high-fibre-foods and fruit and vegetables in these groups(Reference Neumark-Sztainer, Story and Perry13, Reference Berg, Jonsson and Conner68). The majority of taste perception studies focus on bitter taste perception and there is little information on the effect of either sweet or umami perception on food preferences. It is known that preference for sweet tastes is partly genetically mediated and in rodents variations in the T1R1 receptor contribute to differential preferences for sweeteners such as saccharin(Reference Sclafani69, Reference Meyers and Brewer70). In human subjects, preference for sweet tastes has been linked to a gene or genes located on chromosome 16p 11·2.(Reference Keskitalo, Knaapila and Kallela71). Unlike our murine counterparts, this is not because of variations in sweet taste receptor genes, as the TAS1R2 and TAS1R3 genes are both found on chromosome 1p 36(Reference Keskitalo, Knaapila and Kallela71). Instead this variation might influence sweet preference in some other way; possibly, the authors suggest through differential processing of sweet sensation(Reference Keskitalo, Knaapila and Kallela71). Sweet perception may influence food preferences, as individuals with an increased sweet perception tend to have a lower preference for sugar than less sensitive individuals(Reference Looy and Weingarten72). Sweet perception has been linked to alcoholism(Reference Mennella, Pepino and Lehmann-Castor73) and to increased BMI, with a reduced threshold observed in obese children(Reference Donaldson, Bennett and Baic74). While it is unclear whether preferences increase the risk of these conditions, or if over-consumption eventually influences preference, a heritable component does appear to be involved.
A variation in the perception of umami taste has also been observed, and this variation has been associated with SNP in umami taste receptor genes, although these SNP are yet to be linked with specific food preferences. Umami taste perception has, however, been linked to obesity, although with mixed results. One report suggested that a heightened umami perception was associated with an increased BMI(Reference Donaldson, Bennett and Baic74), while another found that obese women have a lower umami sensitivity, and prefer higher concentrations(Reference Pepino, Finkbeiner and Beauchamp75). One possible explanation for these disparate observations is that different mechanisms may be involved in the perception of threshold and suprathreshold (above the level needed for recognition) monosodium glutamate concentrations(Reference Pepino, Finkbeiner and Beauchamp75).
Variation in bitter taste perception has been linked to preference for many different foods. Thiourea-containing compounds such as PTC and PROP are bitter-tasting dietary goitrogens, which inhibit the amount of biologically available iodine, and can affect energy balance(Reference Reed, Li and Li76). Although PROP itself is not found in nature, PROP-related compounds occur in many fruit and vegetables and bitter-tasting foods, including the brassica family of vegetables which contain glucosinolates, and are hydrolysed to isothiocyanates(Reference Vig, Rampala and Thinda9). Isoflavones are bitter-tasting phenolic compounds found in soya and green tea, which might also taste bitter to PROP-sensitive individuals(Reference Akella, Henderson and Drewnowski77). Therefore, PROP-sensitive individuals may be more sensitive to the bitter tastes of certain ‘healthy’ foods with known chemopreventive effects(Reference Vig, Rampala and Thinda9), which may affect their preferences, and ultimately, health status(Reference Drewnowski, Henderson and Levine16, Reference Tepper31, Reference Drewnowski and Gomez-Carneros36, Reference Looy and Weingarten72, Reference Keller, Steinmann and Nurse78). Furthermore, PROP taster status and differences in oral sensitivities may also affect preference for fat and sugar (NT were reported as more likely to be ‘sweet likers’ than PROP-sensitive individuals(Reference Looy and Weingarten72)) and to prefer higher-fat foods(Reference Tepper and Nurse61), while ST have shown lower acceptance of whole-grain breads(Reference Bakke and Vickers79). This may be due to the link with the FP density which can affect oral sensitivity.
The perception of bitter taste has also been associated with a number of adverse health effects, such as a higher risk of alcoholism in PROP NT(Reference Duffy, Davidson and Kidd80), increased BMI in female NT(Reference Tepper, Koelliker and Zhao50, Reference Tepper and Nurse61, Reference Goldstein, Daun and Tepper81), a possible increased risk of colon cancer in male ST (Reference Basson, Bartoshuk and Dichello82), and in children it has been shown that NT have a higher risk of developing dental caries (tooth decay), presumably as they prefer sugar-containing foods to PROP MT and ST (Reference Rupesh and Nayak83). However, while it has been conclusively demonstrated that PROP tasters detect more bitterness from glucosinolate-containing vegetables than do NT(Reference Sandell and Breslin84), there is a general lack of agreement between reports on the extent of the link between taster status and food preference or intake. A number of studies have shown no correlation between taster status and food preferences(Reference Anliker, Bartoshuk and Ferris85–Reference Mattes and Labov87), while many others have reported links between taster status and preference for and/or consumption of various fruit and vegetables. Lower preference has been reported in PROP tasters (both MT and ST) for citrus fruit(Reference Drewnowski, Henderson and Shore88, Reference Tepper, Keller, Ullrich and Hofmann89) and this group appears to consume less fruit in general than NT(Reference Yackinous and Guinard56). Lower preference has also been reported for Brussels sprouts, cabbage and spinach(Reference Drewnowski, Henderson and Levine16), asparagus and curly kale(Reference Dinehart, Hayes and Bartoshuk90) and lower overall vegetable consumption(Reference Dinehart, Hayes and Bartoshuk90, Reference Jerzsa-Latta, Krondl and Coleman91) in PROP-sensitive individuals. One caveat with the majority of these studies is that they have examined differences in preference or intake in small population subgroups, which are not necessarily representative of the true extent of the effect of this trait in the general population. Additionally, various methods have been used to determine PROP taster status, making it difficult to compare them. Another drawback to earlier studies is that the PTC gene has been identified relatively recently(Reference Kim, Jorgenson and Coon51), meaning that few studies have examined the effects of both TAS2R38 and PROP taster status on dietary intakes. Finally, while it is clear that genetic predispositions are simply one of the range of factors involved in the determination of food preferences, many studies fail to account for this, and this may have contributed to the mixed set of results yielded from past studies(Reference Tepper31, Reference Mattes, Prescott and Tepper92). Understanding how taste influences fruit and vegetable preference and consumption may help develop an effective programme to mediate increased intake.
Current research aims and initial findings
Studies have shown a link between genetic variations and food preferences and intake. While many studies have examined the TAS2R38 gene or used PROP taster status as a marker of this gene, there is a lack of information examining both food preferences and habitual dietary intake in relation to both PROP taster status and TAS2R38 genotype. This study aimed at examining the extent of the link between TAS2R38 genotype and PROP taster phenotype, and to establish the extent of the influence of these traits separately on nutrient intakes in a group of Irish children and adults. A range of other factors were also considered including gender, age, perceived barriers to healthy eating and general awareness of healthy eating. A total of 525 children (225 male, 300 female) aged 7–12 (mean male age 10·39 (sd 1·84) years; mean female age 10·07 (sd 1·43) years and 165 of their parents (36 males; mean age 44·85 (sd 7·07) years and 129 female; mean age 41·09 (sd 6·06) years were recruited to the study through a number of schools which were contacted in Counties Dublin, Louth and Westmeath in the Republic of Ireland, from an even split of socio-economic areas, as determined by school location.
A proportion (84·2%) of the group (n 581) was genotyped for the three common alleles found at TAS2R38. This is the first time that this trait has been examined in the Irish population at this scale, and the allele and haplotype frequencies observed were as expected for a mainly Caucasian population, and were comparable to previously reported frequencies (Table 3). When genotypes were examined, the overall percentages of PAV/PAV, PAV/AVI and AVI/AVI in the cohort were similar to the percentages of ST, MT and NT of PROP, determined by the perceived intensity of a PROP-impregnated paper disc, as previously described(Reference Zhao, Kirkmeyer and Tepper93). However, there was an overlap between categories, such that those PAV/PAV individuals were not necessarily all PROP ST. Multivariate analyses revealed that genotype was a significant predictor of PROP intensity, and the overall contribution of genotype to phenotype was approximately 40% (R 2 0·41, P<0·001). This is lower than previous reports (55–85%)(Reference Tepper, Koelliker and Zhao50, Reference Kim, Jorgenson and Coon51) but may represent a truer estimation of the extent of the influence of this single gene on taste perception in a genetically diverse population, and is consistent with the recent finding that supertasting is dependent on more than simply the TAS2R38 gene(Reference Hayes, Bartoshuk and Kidd63).
Table 3. Allele frequencies at variant loci TAS2R38
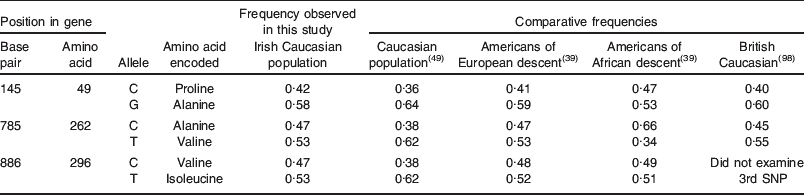
SNP, single nucleotide polymorphism.
Genotype, phenotype, food preferences and nutrient intakes
Participants were shown pictures of a range of different vegetables which they rated on a five-point hedonic scale, as described previously(Reference Looy and Weingarten72). These were grouped into ‘bitter vegetables’, from the average preference ratings for broccoli, cauliflower, cabbage and Brussels sprouts, and ‘non-bitter vegetables’ containing the remaining vegetables, peas and carrots, based on their glucosinolate contents. Multiple regression analyses were carried out to measure the percentage of variation in preferences owing to various factors. A linear model containing five variables was created: PROP taster status, genotype, sweetness perception, FP density, age and gender. This model was a significant predictor of vegetable preference (P<0·01), explaining 15·3% of the variation in males and 18·9% in females, with the most significant influences being PROP taster status, age and gender, while genotype was less important.
A 3-d diet history was obtained from both the adults and children through interviews with the researchers using a food atlas as a guide for portion size estimates and probing for any brand information. Dietary data were analysed using WISP © (Tinuviel Software, Llanfechell, Anglesey, UK) for overall macro and micronutrient intakes in the different genotype and phenotype groups. This was performed for boys, girls, men and women separately. While there were no BMI differences in either the men or the children, mean BMI was significantly higher in NT women (28·44, sd 6·96) compared to MT and ST (24·67 (sd 3·94) and 25·33 (sd 4·18)) BMI units, respectively; P<0·01). Energy intake was slightly higher in NT women, but not significantly so, and when micronutrients were examined, vitamin B6 intakes were significantly higher in NT (P<0·05). Interestingly, there was no significant difference between mean BMI in the three genotype groups in the women, consistent with one previous report that has examined both genotype phenotype and BMI in older females(Reference Tepper, Koelliker and Zhao50) and found that phenotype was more influential.
Upon examination of nutrient intakes by genotype, AVI/AVI females had significantly higher intakes of thiamine, vitamin B6 and folate, and lower intakes of vitamin B12 (P<0·05). The main dietary sources of folate are green leafy vegetables and fortified cereals, whereas common sources of vitamin B12 are animal products such as meat and dairy, as well as fortified breakfast cereals. This nutrient pattern observed in AVI/AVI females is similar to a pattern of intakes observed in the European Prospective Investigation into Cancer and Nutrition study, where a ‘health conscious’ group had significantly higher thiamine and lower vitamin B12 levels compared with others(Reference Olsen, Halkjaer and van Gils94). Additionally, AVI/AVI individuals consumed more fibre. These findings may be extrapolated to show that TAS2R38 is associated with healthy eating patterns in females. However, there were no significant differences in vitamin C, carotene or biotin intakes, which would be expected if fruit and vegetable intakes differed between genotype groups, and so the differences observed in fibre may be derived from increased whole-grain and lower animal product consumption. Whether certain types of fruit and vegetables intake differ between genotype groups is not clear from these analyses.
Conclusion
Food choices are affected by a wealth of factors, one of which is taste. Taste perception for various taste qualities may vary genetically and lead to differential preferences for certain types of foods. PROP taster status is linked to TAS2R38 genotype, and both of them can separately influence food preferences. However, in this study, phenotype (PROP taster status) was a greater source of variation in food preferences than TAS2R38 genotype, and may also predict BMI, which was higher in NT women. Over 20% of the variation in food preferences could be contributed to taster status. Notably, this influence was found to vary with gender for some vegetables, which was not expected. Clear differences were observed between both genotype and phenotype groups in certain vegetable preferences, although overall, differences in macro and micronutrients were relatively minor in children and in males. Further, AVI/AVI genotype was associated with increased markers of a healthful diet in adult females, yet conversely, overall energy intakes were also increased in this group. This indicates that while female AVI/AVI individuals may be eating more than their PAV/AVI or PAV/PAV counterparts, their choices may be more healthy. Therefore, in conclusion, while genetic variation in taste perception does indeed appear to have a small role in healthy eating, other factors such as age and gender are also important contributors to both food preferences and dietary intakes.
Acknowledgements
This work was supported by The Department of Agriculture, Fisheries and Food through FIRM (the Food Institutional Research Measure), funded under the National Development Plan 2007–2013. The authors declare no conflict of interest. E. F. wrote the manuscript and collected and analysed the data. S. O'B. collected and analysed the data. A. S. and A. M. contributed to the study design and data analysis. E. R. G. designed and supervised the study and revised the manuscript.







