Introduction
Topshells, limpets and winkles are among the most successful intertidal algal grazers in the North-eastern Atlantic Ocean and the Mediterranean Sea (Templado & Rolán, Reference Templado and Rolán2012). These marine gastropod molluscs are subject to one of the most extreme and dynamic natural environments, occurring from the supratidal to the subtidal zones in rocky shores. Topshells are exposed to different levels of thermal and hydric stress that result in specific morphological and biological characteristics related to adaptations to the harsh environmental conditions on rocky shores (Ramírez et al., Reference Ramírez, Tuya, Sánchez-Jerez, Fernández-Gil, Bergasa, Haroun and Hernández-Brito2005; Donald et al., Reference Donald, Preston, Williams, Reid, Winter, Álvarez, Buge, Hawkins, Templado and Spencer2012; Henriques et al., Reference Henriques, Delgado, Sousa and Ray2017). They play a pivotal role in the intertidal ecological balance and are frequently used as biological indicators of anthropogenic impacts (Sousa et al., Reference Sousa, Delgado, González, Freitas, Henriques and Ray2018).
The ecological importance of the genus Phorcus Risso, 1826 on the overall structure of intertidal communities, particularly their influence and control on algae, prompted intensive research over the past decades, highlighting that the removal of these grazers leads to imbalances on rocky shore communities (Creese, Reference Creese1988; Branch & Moreno, Reference Branch, Moreno and Siegfried1994). However, most of the studies concerning this genus focused on Phorcus lineatus (da Costa, 1778), and little information is available on Phorcus sauciatus (Koch, 1841). Nonetheless, local information on the population structure and distribution of P. sauciatus is available from the north-west Iberian Peninsula (Rubal et al., Reference Rubal, Veiga, Moreira and Sousa-Pinto2014) and the Canary Islands (Ramírez et al., Reference Ramírez, Tuya, Sánchez-Jerez, Fernández-Gil, Bergasa, Haroun and Hernández-Brito2005, Reference Ramírez, Tuya and Haroun2009; Alfonso et al., Reference Alfonso, Sarabia, Sancibrián, Alfaro, Adern and Hernández2015).
Phorcus sauciatus, a common temperate-subtropical grazer that inhabits extensive and gently sloping rocky shore platforms in the eastern Atlantic, including the Macaronesian archipelagos of Madeira, the Canaries and the Azores, reaches its northern boundary in the Iberian Peninsula (Rubal et al., Reference Rubal, Veiga, Moreira and Sousa-Pinto2014; Ávila et al., Reference Ávila, Madeira, Rebelo, Melo, Hipólito, Pombo, Botelho and Cordeiro2015). The life history traits of this species vary intraspecifically due to genetic differences and environmental effects. The size and age of these gastropods are positively related, thus allowing studies on population structure, reproductive strategy, growth rates, mortality and longevity (Crothers, Reference Crothers1998). These parameters depend on a complex combination of selective forces and are required to understand the distribution, abundance and adaptations of this species to an ever-changing environment. Additionally, the knowledge on the life history traits of these populations will play a pivotal role in providing proper background information for effective management of this important resource.
Exploitation of P. sauciatus in Madeira dates back to the 15th century when the archipelago was colonized by the Portuguese and has become more intensive due to the demographic increase of human settlement of the islands’ coast and the technological advances that facilitate access to the whole coast (Silva & Menezes, Reference Silva and Menezes1921; Sousa et al., Reference Sousa, Delgado, González, Freitas, Henriques and Ray2018).
The harvesting of these molluscs is not regulated in the Madeira archipelago except for coastal natural reserves where harvest is interdicted. The absence of proper harvest regulation results in the landings of this species not passing through auction, which prohibits us from knowing how much effort is exerted on the harvested populations. Knowledge gaps regarding the life traits and population dynamics of this species are one of the key factors contributing to the lack of harvest regulation (Sousa et al., Reference Sousa, Delgado, González, Freitas, Henriques and Ray2018). These gaps prompted an intensive collection of biological data in the scope of a comprehensive biological and ecological study that aimed to provide the scientific information required for the implementation of conservation measures and the proper regulation of harvesting activities.
Herein, we aim to (i) provide information on the biological parameters and exploitation rate of P. sauciatus in Madeira (NE Atlantic Ocean) and (ii) evaluate the effect of size at first capture on the exploited stock by applying a yield-per-recruit model. These objectives are pivotal for developing a series of conservation measures to preserve stocks of this mollusc, such as the establishment of a minimum catch size, the introduction of a closed season and the implementation of mandatory landings and first sale at auction of this commercially harvested species.
Materials and methods
Data collection
Monthly samples of P. sauciatus were collected from three locations throughout the coast of Madeira, located in the north-eastern Atlantic (32.00–33.05°N 15.05–18.00°W), between January and December 2017. Specimens were randomly collected from the mid-to-lower intertidal zone of the rocky shores during low tide, without selecting for size, for a period of 15 min. Shell length (L) was measured using a Vernier calliper to the nearest 0.01 mm, and total weight (W) to the nearest 0.01 g was measured using an electronic scale. Specimens were removed from the shell and dissected for sexing purposes by macro- and microscopic observation of the gonads (Desai, Reference Desai1966). Macro- and microscopic inspection of the gonads allowed for the assignment of each specimen to one of the five gonad maturation stages based on an adaptation of Desai's (Reference Desai1966) maturation scale. In stage I, female and male gonads present pink-brown pigmentation. The germinal epithelium in both sexes are ill-defined and oocytes are ~25 µm in diameter; in stage II, females present with greenish gonads, and males present with irregularly greenish gonads. Females present a well-defined germinal epithelium with numerous oocytes, measuring up to 45 µm in diameter, in each trabecula divided by connective tissue. Males present a germinal epithelium with rounded spermatogonia; in stage III, both female and male gonads are intumescent and present uniformly greenish pigmentation. Females have a well-defined honeycomb structure of connective tissue and oocytes up to 50 µm, and males present spermatocytes and spermatids; in stage IV, both sexes present fully developed gonads. Female gonads present with intense green pigmentation, and males present with pink and yellowish gonads. Females present numerous large oocytes that are freely present in the connective tissue. Oocytes up to 165 µm seem to remain intact when released into seawater. Males present spermatozoa; in stage V, females present with green gonads, and males present with pink and yellowish gonads. Female ovaries are filled almost exclusively with mature oocytes, and male gonads are filled with active spermatozoa. After macroscopic observation, the gonads were removed, damp-dried, weighed to 0.001 g accuracy and prepared for histological confirmation of the maturation stages. All measurements were performed on fresh samples.
The data were analysed for deviations to the parametric assumptions of analysis of variance (ANOVA). The data distribution was tested for normality using the Kolmogorov–Smirnov two-sample test. Homogeneity of variance was determined using Levene's statistics. ANOVA was used to test for differences in the shell length and total weight between the sexes (Sokal & Rohlf, Reference Sokal and Rohlf1995).
Growth and age
The shell weight-length relationship (W/L) was estimated by least-squares linear regression after the logarithmic transformation of both variables (logW = log a + b logL) using the potential relationship W = aL b (Bagenal & Tesch, Reference Bagenal, Tesch and Bagenal1978), where W is the total weight (g), L is the shell length (mm), a is the intercept (condition factor) and b is the slope (relative growth rate). The coefficient of determination, r 2, was used as an indicator of the quality of the regression. A Student's t-test (King, Reference King1995) was used to test the hypothesis of an isometric relationship (H 0: b = 3; H 1: b ≠ 3, at the 5% significance level).
Monthly length-frequency distributions were inferred through apparent shifts of the modes in the time series of the length-frequency samples by means of modal progression analysis (MPA) and used to estimate absolute growth using Bhattacharya's method, which is included in the package FISAT II (Fish Stock Assessment Tools FAO–ICLARM), VER 1.2.0 (Gayanilo et al., Reference Gayanilo, Sparre and Pauly2005). This method implies the identification of mean values through the decomposition of composite distributions into their components followed by the determination and linking of means perceived to belong to the same cohort, and finally, the estimation of growth parameters is determined using the growth increments and size-at-age resulting from the linking of the means. All of the identified size-age groups resulted from at least three consecutive points, and the selection of the best results was based on the separation index (SI) values (>2) for the different age groups and the number of individuals per age group. Only size-age groups with an SI greater than 2 were considered, since values below 2 are unreliable (Sparre & Venema, Reference Sparre and Venema1997). NORMSEP by Hasselblad & Tomlinson (Reference Hasselblad, Tomlinson and Abramson1971) was applied to decompose the mixtures of normal distributions based on Hasselblad's maximum likelihood method (Hasselblad, Reference Hasselblad1966).
The von Bertalanffy growth parameters were estimated by means of the Gulland and Holt method (Gulland & Holt, Reference Gulland and Holt1959) for non-linear parameter estimation in the routine ELEFAN I available in FISAT II (Gayanilo & Pauly, Reference Gayanilo and Pauly1997) using the equation L t = L ∞ {1–exp[−k (t–t 0)]} (Gulland & Holt, Reference Gulland and Holt1959), where L t is the mean shell length at age t (mm), L ∞ is the asymptotic shell length (mm), K is the growth coefficient (year−1), t is the age of P. sauciatus (years), and t 0 is the hypothetical age at which L t = 0 (years).
The growth performance index (φ′) is generally considered a better tool for comparing growth dynamics between species, phylogenetically related groups and intraspecific areas. The estimated parameters L ∞ and K were used to determine φ′ through the application of the equation φ′ = log10 (K) + 2 log10 (L ∞) (Pauly & Munro, Reference Pauly and Munro1984).
The inverse von Bertalanffy growth equation, t = t 0 (1/K) ln (1–L t/L ∞), was used to determine the age at length of P. sauciatus (King, Reference King1995), and the potential longevity (A 0.95) was estimated from the equation A 0.95 = t 0 + 2.996/K (Taylor, Reference Taylor1958).
Reproduction and recruitment
The sex ratio of P. sauciatus was determined, and the existence of differences in the proportion of sexes was tested using the chi-square goodness-of-fit statistic. A Pearson's χ2 test was applied to test for the presence of differences in sexual proportions between months. The gonadosomatic index (GSI) was estimated according to GSI = (wet gonad weight/total body wet weight) × 100. Differences in the mean GSI values between sexes among months were tested through ANOVA, considering a significance level of 0.05. To estimate the spawning season, the proportion of immature/mature specimens per month was plotted; all immature individuals between stages 1 and 3 and all mature individuals in stages 4 or 5 of gonadal development were considered (Desai, Reference Desai1966). The existence of a correlation between monthly sea surface temperature (SST) and the GSI was determined using the Pearson correlation.
The size at first maturity (i.e. the size at which 50% of all specimens in a stock are mature, Lm50) was estimated from the correlation between the proportion of mature individuals and length according to the logistic equation: P = 1/(1 + exp−(a+bL)) (Sparre & Venema, Reference Sparre and Venema1997), where P is the balanced probability, and a and b are the equation parameters estimated by the linear least square method using a logarithmic transformation. The mean size at maturity was defined as the size at which 50% of the population is mature, when P = 0.5, then Lm 50 = ( − a)/b (King, Reference King1995). The lengths at which 25% (Lm25) and 75% (Lm75) of the topshells were mature was also determined.
The recruitment pattern was determined through the projection of the length-frequency data backwards on the time axis using the estimated growth parameters (Moreau & Cuende, Reference Moreau and Cuende1991), and a normal distribution of this pattern was obtained by the NORMSEP routine (Pauly & Caddy, Reference Pauly and Caddy1985) in FISAT.
Mortality, exploitation rate, probability of capture and yield-per-recruit
Total mortality (Z) was estimated using the length-converted catch curve procedure, where the percentage of samples in the length groups are pooled to simulate a steady-state population. The natural mortality rate (M) was determined by Pauly's empirical model: log10M = −0.0066–0.279 (log10L ∞) + 0.6543 (log10K) + 0.4634 (log10T) (Pauly, Reference Pauly1980), where L ∞ is the asymptotic shell length (mm), K is the growth coefficient (year−1), and T is the annual mean environmental temperature, which was 20.5°C for the habitat of P. sauciatus in the study area. Harvesting mortality (F) was calculated as the difference between Z and M, and the exploitation rate (E) was estimated from E = F/Z (Gulland, Reference Gulland1971).
The probability of capture for P. sauciatus was calculated by using a logistic transformation of the probabilities obtained from the small topshells using the ascending left arm of the length-converted catch curve by plotting the cumulative probability of capture against the middle point of the length class intervals. The length at first capture (i.e. the cumulative probability of 50%, Lc50) was obtained from the resulting curve according to the equation: S L = 1/[1 + exp(S1–S2 × L)] (Sparre & Venema, Reference Sparre and Venema1997), where SL is the logistic curve, S1 and S2 are constants in the equation for the length-based logistic curve, and L is the topshell length. The lengths that correspond to the cumulative probabilities of 25% (Lc25) and 75% (Lc75) were also estimated.
Estimates of the relative yield-per-recruit (Y/R) and the harvesting mortality corresponding to the maximum production (FMAX) were estimated according to Beverton & Holt's (Reference Beverton and Holt1957) length-based method:
where Y/R is the catch in weight, w ∞, K and t 0 are growth parameters, T c is the age at first capture, T r the age at recruitment, F the harvesting mortality, M the natural mortality, Z the total mortality and ![]() $S = {\rm e}^{(-K(T_c-T_r))}$.
$S = {\rm e}^{(-K(T_c-T_r))}$.
To assess the effect of harvesting smaller and larger specimens on harvesting mortality and Y/R, the sizes of capture estimated previously (Lc25, Lc50 and Lc75) were applied to the Y/R model to simulate the effect of size.
Results
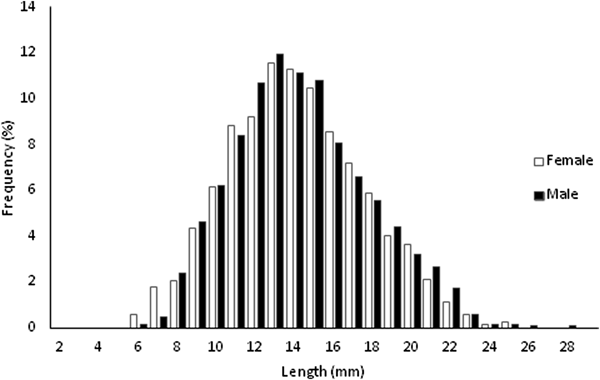
A total of 5480 specimens, 56.48% females and 43.52% males, were sampled throughout the study (Figure 1). Individual size varied between 6.09 and 29.25 mm L (![]() $\bar{x}$ = 14.64 ± 3.55 mm L) in females and between 6.73 and 28.78 mm L (
$\bar{x}$ = 14.64 ± 3.55 mm L) in females and between 6.73 and 28.78 mm L (![]() $\bar{x}$ = 14.74 ± 3.50 mm L) in males. The total weight ranged from 0.11 to 6.08 g (
$\bar{x}$ = 14.74 ± 3.50 mm L) in males. The total weight ranged from 0.11 to 6.08 g (![]() $\bar{x}$ = 1.06 ± 0.79 g) in females and from 0.13 to 5.68 g (
$\bar{x}$ = 1.06 ± 0.79 g) in females and from 0.13 to 5.68 g (![]() $\bar{x}$ = 1.11 ± 0.84 g) in males. The smallest sample specimen was collected in July at 2.34 mm L, and the largest specimen was collected in October at 29.25 mm L.
$\bar{x}$ = 1.11 ± 0.84 g) in males. The smallest sample specimen was collected in July at 2.34 mm L, and the largest specimen was collected in October at 29.25 mm L.
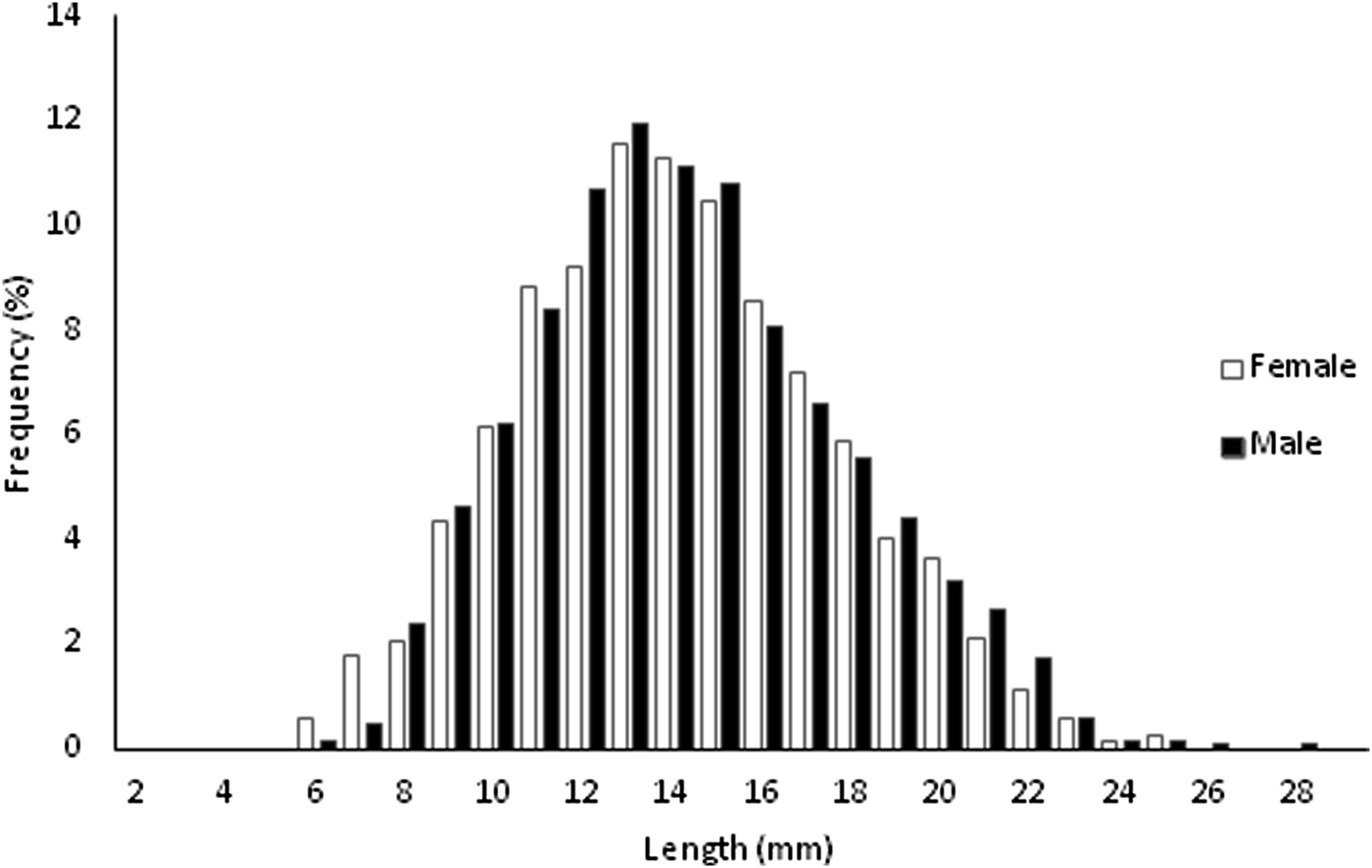
Fig. 1. Length-frequency distributions for females and males of Phorcus sauciatus, collected from January to December 2017.
Growth and age
Phorcus sauciatus size-frequency showed a normal distribution (Z = 1.861, P < 0.05) and a homogenous variance between the sexes (W = 0.145, P > 0.05); the same pattern was observed regarding weight, with a normal distribution (Z = 7.946, P < 0.05) and a homogenous variance between the sexes (W = 3.659, P > 0.05). No significant differences were found in the mean length (F = 0.749, P > 0.05) or in the mean weight (F = 2.668, P > 0.05) between females and males; thus, monthly length and weight frequency estimates were combined.
The correlation between shell length and total weight showed a negative relationship, and the parameters of the regression were estimated as W = −3.325 + L 2.815, r 2 = 0.95, P < 0.05. The b coefficient returned values less than 3, and statistical differences were highly significant (t = 21.762, P < 0.05).
The monthly length-frequency distributions of P. sauciatus are shown in Figure 2. The von Bertalanffy growth parameters were obtained for the best fit with L ∞ = 31.90 mm L and K = 0.31 year−1. The growth performance index (φ′) was calculated as 2.50. Phorcus sauciatus showed a predominance of individuals in the first age classes, with 88.94% of all specimens from the studied population being <4 years. The most representative age class was 2 years, representing 54.71% of the studied specimens. Potential longevity, assuming t 0 = 0, was determined to be 9.66 years.
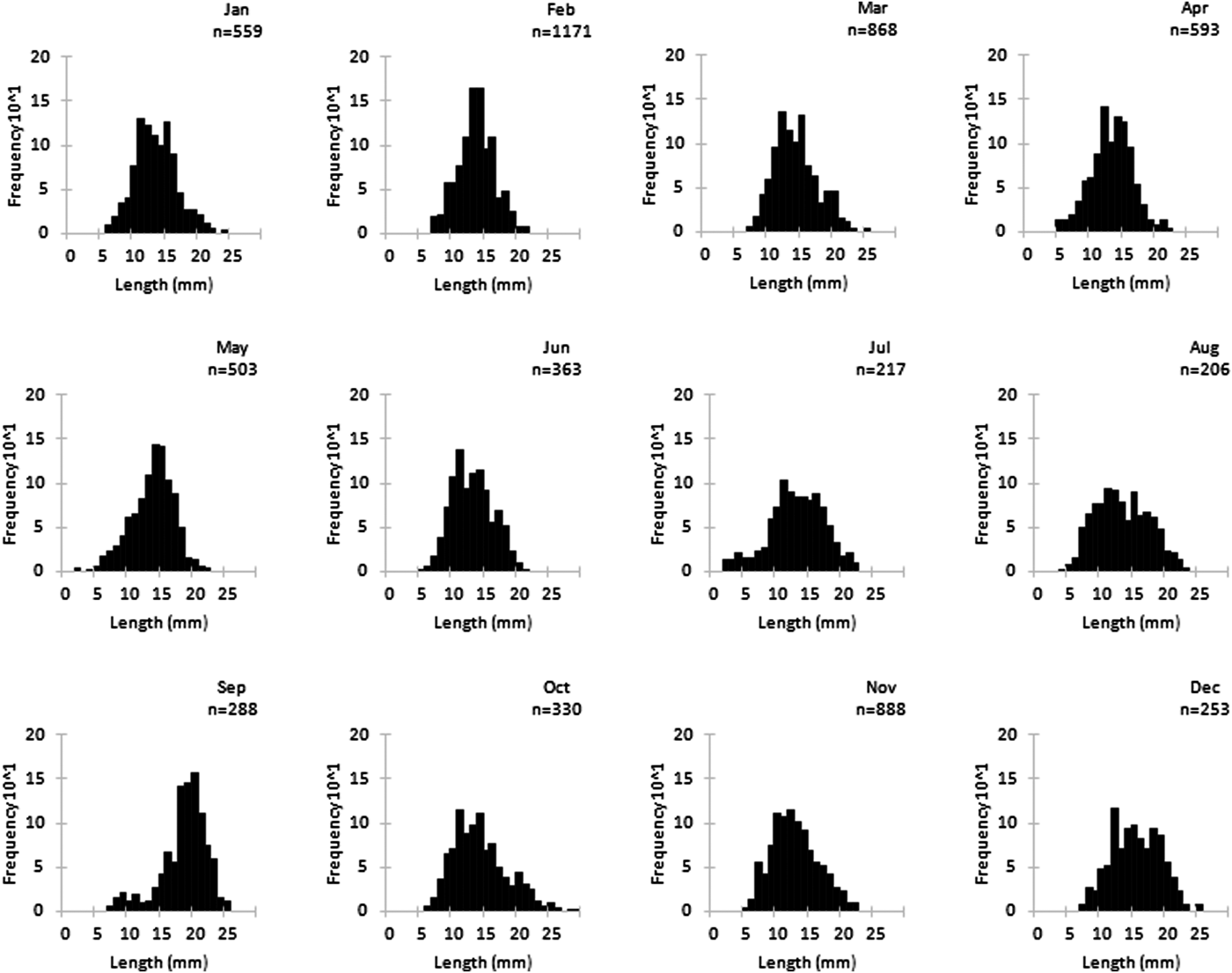
Fig. 2. Monthly length-frequency for Phorcus sauciatus, collected from January to December 2017.
Reproduction and recruitment
Phorcus sauciatus does not exhibit observable external sexual dimorphism. The overall sex ratio favoured females (1:1.30). The Chi-square goodness-of-fit test showed that the observed differences were significant (χ2 = 57.791, P <0.05). Monthly sex ratio analysis showed that females were predominant all year, except in February, when males were predominant (χ2 = 64.605, P <0.05).
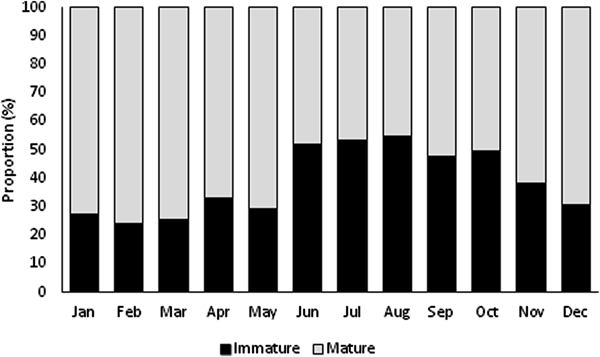
Immature and mature specimens were found all year (Figure 3). Immature specimens were more abundant in summer, and mature individuals were more abundant in the remaining seasons. The highest proportion of immature specimens occurred in August (54.92%), and the lowest proportion occurred in February (24.12%). Mature individuals were predominant (~61%) from September to May, with the highest proportion found in February (75.88%) and the lowest in August (45.08%).
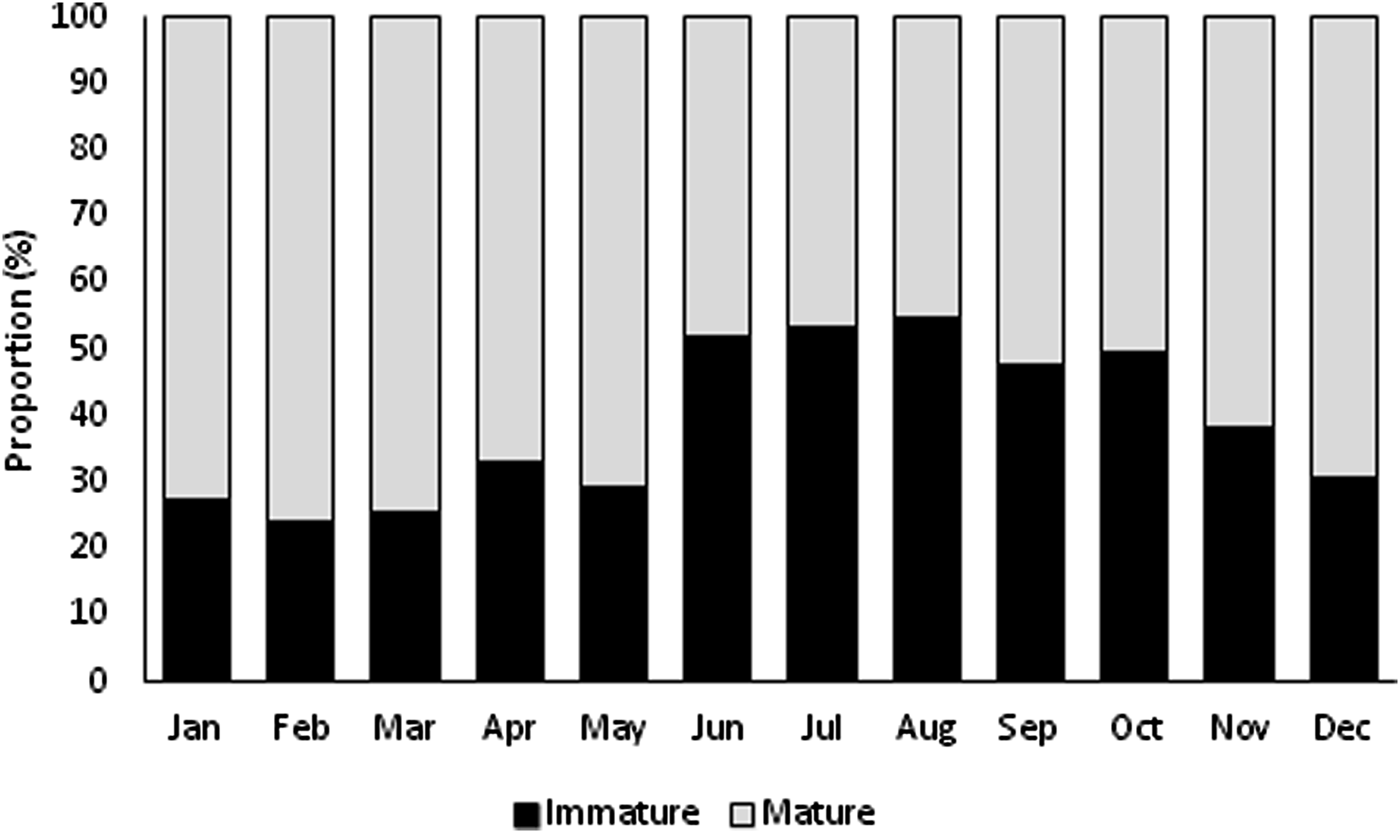
Fig. 3. Monthly distribution of immature and mature individuals of Phorcus sauciatus from Madeira archipelago, collected from January to December 2017.
GSI values only showed significant differences between sexes in March (F = 5.763, P < 0.05) and April (F = 6.764, P < 0.05) and monthly differences within females (F = 18.291, P < 0.05) and males (F = 22.053, P < 0.05) (Figure 4). Monthly proportions revealed an increase from October to March for both sexes. The highest GSI values occurred in March for both sexes, with 6.78% for females and 7.42% for males. After the observed peak in March, there was a decrease until August for both sexes, suggesting that the spawning season of P. sauciatus occurs between March and August with higher values from March to June. The observed monthly variations in the GSI were in accordance with the observed proportions of immature and mature individuals, increasing when the proportion of mature specimens was higher but decreasing when the proportion of immature specimens increased. The Pearson correlation factor (r 2 = 0.86) showed that the mean GSI of P. sauciatus was correlated with SST (F = 298.433, P < 0.05).
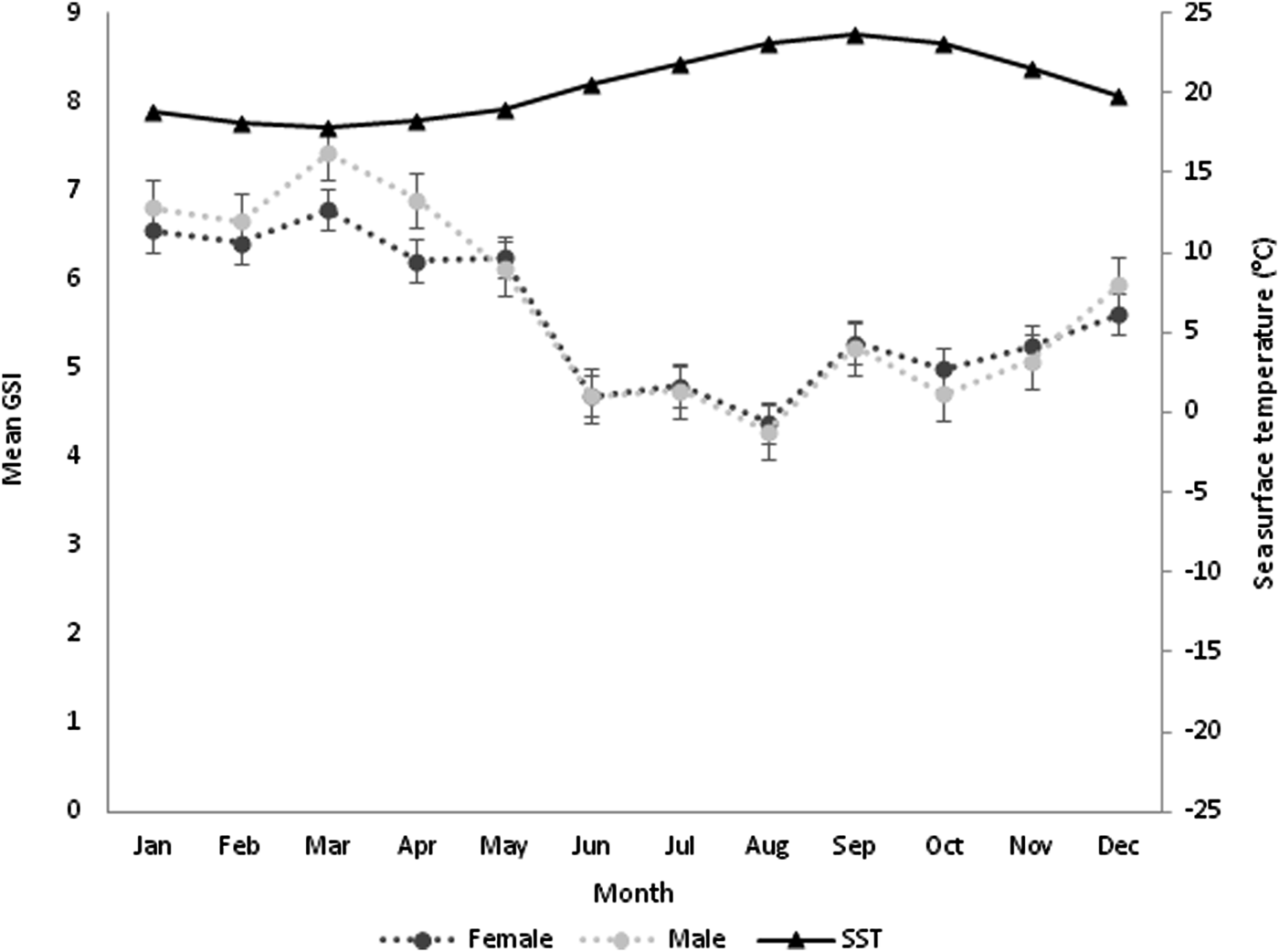
Fig. 4. Monthly variation in sea surface temperature and seasonal changes in gonadosomatic index (GSI) for females and males of Phorcus sauciatus from the Madeira archipelago collected from January to December 2017.
The mean size at first maturity (Lm50) was 12.95 mm L, corresponding to 1.68 years. The size at which 25 and 75% of the study population reached sexual maturity was estimated at 12.17 and 13.73 mm L, respectively. The recruitment pattern was continuous throughout the year, reaching higher values between April (10.51%) and August (16.18%) and peaking in June (16.73%). Individuals with <5.00 mm shell length were only collected from April to August.
Mortality, exploitation rate, probability of capture and yield-per-recruit
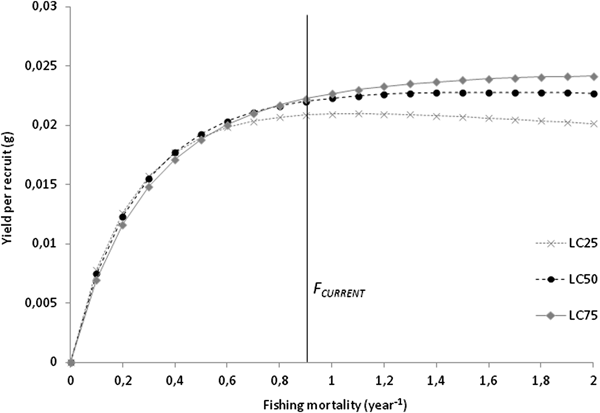
Total (Z) and natural mortality (M) were estimated at 1.61 and 0.71 per year, respectively. Harvesting mortality (F) was 0.90 per year, and the exploitation rate (E) was estimated to be 0.56. The length at first capture for the combined sexes (LC 50) was estimated as 13.19 mm L, corresponding to 1.72 years. The analysis of yield-per-recruit (Y/R) showed that, at the current exploitation rate, maximum production is achieved at F MAX of 1.7 per year, corresponding to a Y/R of 0.023 g. The simulation of Y/R varying the length-at-capture resulted in a decrease in the harvesting effort allowed to maintain a sustainable yield, with an F MAX of 1.1 per year. The simulation also showed a decrease in maximum production that can be achieved, with a Y/R of 0.021 g for LC 25; for LC 75, even though the harvesting effort can be maximized to an F MAX of 2.0 per year, the gains in maximum production are negligible, with a Y/R of 0.024 g (Figure 5).
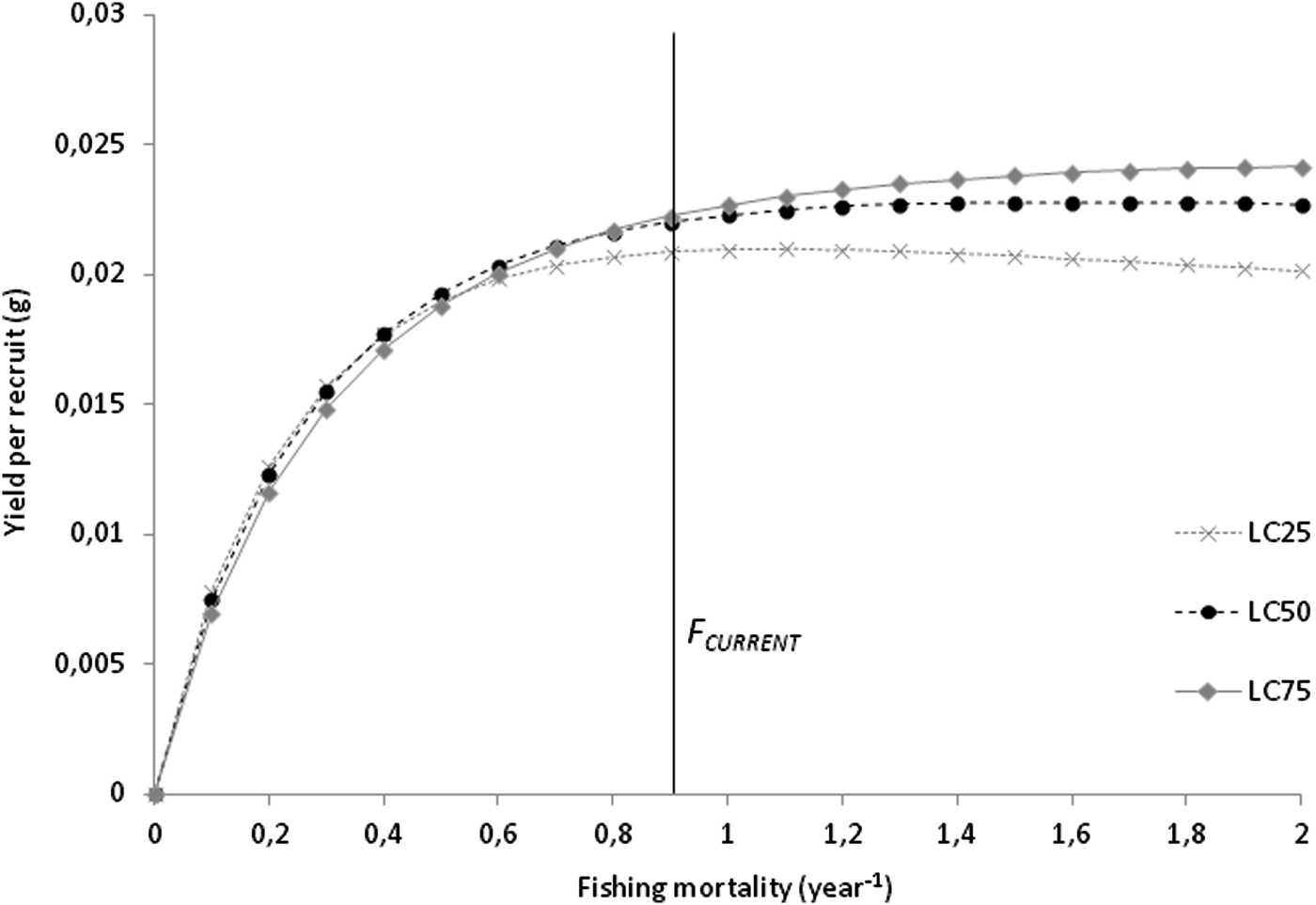
Fig. 5. Beverton–Holt yield-per-recruit curves on F for Phorcus sauciatus, considering LC 25, LC 50 and LC 75.
Discussion
Information on the life history traits of P. sauciatus provides significant knowledge, which is required to explain and understand the adaptations of this species to an ever-changing environment and to perceive how human activities such as fisheries, habitat disturbance or natural causes affect their abundance and population dynamics (Sousa et al., Reference Sousa, Delgado, González, Freitas, Henriques and Ray2018).
The size distribution estimated for P. sauciatus from the Madeira archipelago is in accordance with what was previously reported for this species in the Canary Islands (Ramírez et al., Reference Ramírez, Tuya and Haroun2009). The relative growth coefficient obtained for the combined sexes revealed a negative allometric growth for this gastropod, which implies a disproportional increase in total weight in relation to the increase in individual shell growth. Thus, this species allocates more energy to growth than to reproduction in this geographic area. The value of the b parameter obtained for this species in the Madeira archipelago is well within the range of values usually obtained in other geographic areas (2.5–3.5) (Bagenal & Tesch, Reference Bagenal, Tesch and Bagenal1978; Froese, Reference Froese2006), indicating normal growth dimensions and/or the well-being of the studied population (Carlander, Reference Carlander1969; Bagenal & Tesch, Reference Bagenal, Tesch and Bagenal1978; King, Reference King1995).
Intertidal gastropods from different geographic areas in temperate seas frequently show intraspecific variation in shell size and shape (Reimchen, Reference Reimchen1982) as a result of adaptations to predation and wave action (Preston & Roberts, Reference Preston and Roberts2007) or to the conditions of their preferred habitats (Boulding et al., Reference Boulding, Holst and Pilon1999). The negative growth pattern obtained for P. sauciatus might be explained by the harvesting pressure on this resource, which leads to lower population densities. The reduction in population density results in an increase in food and substrate availability, promoting an increase in the population density of other intertidal gastropods, such as Patella aspera Röding, 1798 and Patelloida alticostata (Angas, 1865) (Black, Reference Black1977; Sousa et al., Reference Sousa, Delgado, Pinto and Henriques2017). This pattern may also result from the instability of the intertidal conditions, since P. sauciatus is highly vulnerable to sea temperature, wave action and desiccation (Thompson, Reference Thompson1979).
Growth in molluscs is influenced by several biotic factors (e.g. predation, competition and population density) (Haven, Reference Haven1973) and abiotic factors (e.g. temperature, insolation, photoperiod and food availability) (Crothers, Reference Crothers2001, Reference Crothers2012). Moreover, it seems that molluscs have a strategy of diverting energy to reproduction or to growth according to the organisms’ requirements, and this life history strategy will influence the growth rates of these organisms (Haven, Reference Haven1973; Branch, Reference Branch1974; Underwood, Reference Underwood1979; Clarke et al., Reference Clarke, Prothero-Thomas, Beaumont, Chapman and Brey2004). Growth in molluscs is generally conditioned by the allocation of the majority of their energy to shell production; once they reach sexual maturity, molluscs divert most of their energy to reproduction (Crothers, Reference Crothers1994).
The estimated asymptotic length of 31.90 mm L for P. sauciatus from Madeira is consistent with the length of the largest sampled specimen (29.25 mm L). This species had a moderately low growth rate of 0.31 year−1, which may be partially explained by the oligotrophic nature of the coastal waters of Madeira (Caldeira et al., Reference Caldeira, Groom, Miller, Pilgrim and Nezlin2002). Similar growth rates were observed in other intertidal gastropods, such as Patella candei d'Orbigny, 1840 (Henriques et al., Reference Henriques, Sousa, Pinto, Delgado, Faria, Alves and Khadem2012) and P. aspera (Sousa et al., Reference Sousa, Delgado, Pinto and Henriques2017), in the same geographic area.
Phorcus sauciatus in Madeira have a median lifespan of 9.66 years, meaning the population is moderately long-lived, which is in contrast to its congeneric species, P. lineatus, which may reach an age of 15 years at its northern/eastern limits in Great Britain (Crothers, Reference Crothers1998). The shorter lifespan observed for P. sauciatus in Madeira is in accordance with Clarke et al. (Reference Clarke, Prothero-Thomas, Beaumont, Chapman and Brey2004), who stated that molluscs in colder regions grow more slowly and reach a larger maximum size, thus achieving a longer lifespan than molluscs inhabiting warmer regions.
Phorcus sauciatus does not exhibit observable external sexual dimorphism in the studied populations, similarly to other topshells, such as P. lineatus, Steromphala umbilicalis (da Costa, 1778) and Steromphala cineraria (Linnaeus, 1758) (Desai, Reference Desai1966; Underwood, Reference Underwood1972; Crothers, Reference Crothers2001), and British trochids (Fretter & Graham, Reference Fretter and Graham1962); the sexes of P. sauciatus are only distinguishable by gonadal observation.
The gonadal cycle of P. sauciatus was established based on the monthly variation of the GSI, which reflects the periods of accumulation before the ripening and release of gonadal material during spawning as well as the use of stored energy reserves (Toro et al., Reference Toro, Thompson and Innes2002). Phorcus sauciatus seems to be reproductively active all year in Madeira, with mature and partially spawned individuals present throughout the year. The obtained results also showed a synchronous gametogenesis cycle between the sexes of P. sauciatus, with females and males exhibiting a similar pattern all year, with minor differences in the proportion of mature specimens. The reproductive cycle of this species seems to involve two key periods, namely, development and spawning. The gonadal development phase lasts from September to February when an increase in the GSI values and in the number of mature individuals can be observed. The main spawning pulse begins in March, when the highest GSI values for both sexes were found, indicating maximum development of the gonads, which consistently decreased until August, when the lowest GSI values for both sexes were observed; this finding agrees with the reduction in the proportion of ripe individuals. During this phase, gametes are released into the sea where fertilization occurs (Crothers, Reference Crothers2001). The occurrence of a noticeable spawning pulse between March and June indicates that, for this species, the majority of spawning occurs in the spring and extends more subtly throughout the summer. The observed reproductive cycle did not exhibit a resting phase, agreeing with Bode et al. (Reference Bode, Lombas and Anadón1986) for P. lineatus in northern Spain. These results are contrary to those observed from the northernmost populations in England, which exhibit a resting phase between the spawning phase and the gonadal development phase, which is typically shorter (Desai, Reference Desai1966; Underwood, Reference Underwood1972). This is probably related to differences in sea surface temperature; it is known that reproduction cycles in marine gastropods are influenced by temperature, with populations inhabiting colder habitats developing a more pronounced spawning period and a shorter development phase, while in warmer conditions, the phases of the reproductive cycle tend to be less pronounced (Crothers, Reference Crothers2001).
Underwood (Reference Underwood1972) observed that P. lineatus spawns throughout the summer and early autumn in England. Bode et al. (Reference Bode, Lombas and Anadón1986) verified that this period could be extended up to November for some individuals of the same species in Spain. In Madeira, the majority of P. sauciatus spawning seems to occur earlier in spring, suggesting that variations in reproductive seasons and in the duration of these periods are likely related to the geographic region, mainly due to the influence of temperature.
In Madeira, the spawning pulse of P. sauciatus is synchronized with the increase in seawater temperature, which is in concordance with Crothers (Reference Crothers2001), who stated that effective spawning appears to require an environmental trigger leading to gamete release at the same time with cascading effects but with sharp differences in the extent of the spawning seasons, with the northern populations characterized by short, mid-summer breeding periods and the southernmost populations with lengthened breeding periods that extend in some cases throughout much of the year but with little or no activity during mid-summer (Lewis, Reference Lewis1986).
Within the same geographic region, other environmental factors, such as high wind speed allied to stimulation by wave action and the increase in phytoplankton concentrations, seem to act as triggers on the intertidal limpet species P. aspera (Sousa et al., Reference Sousa, Delgado, Pinto and Henriques2017) and P. candei (Henriques et al., Reference Henriques, Sousa, Pinto, Delgado, Faria, Alves and Khadem2012), which are winter breeders, suggesting that the trigger that stimulates reproduction is different between limpets and the topshells in Madeira.
The estimated length at first maturity for P. sauciatus in Madeira was 12.95 mm L, corresponding to 1.68 years. More than 60% of the sampled population was sexually mature. In the Canaries, the size at first maturity was estimated to be 9.50 mm (González et al., Reference González, Pajuelo, Lorenzo, Santana, Tuset, Jiménez, Perales-Raya, González-Lorenzo, Martín-Sosa and Lozano2012). The differences in abiotic conditions between the two archipelagos may explain the larger size at first maturity estimated for the population of Madeira. It is well known that a decrease in growth rate can be related to a decrease in temperature, resulting in delayed sexual maturation at larger sizes (Berrigan & Charnov, Reference Berrigan and Charnov1994). Another explanation may be due to the exploitation level of this species in the Canaries, where it is considered overexploited (González et al., Reference González, Pajuelo, Lorenzo, Santana, Tuset, Jiménez, Perales-Raya, González-Lorenzo, Martín-Sosa and Lozano2012). Additionally, since Phorcus harvest is size-dependent, as larger individuals are more visible and are thus more prone to being harvested, it is likely that the difference in the size at maturity between archipelagos is influenced by the different exploitation pressures. In overexploited populations, size at first maturity may suffer shifts as a result of changes in the population size structure, resulting in smaller sizes at first maturity (Fenberg & Roy, Reference Fenberg and Roy2008).
The recruitment pattern of P. sauciatus was continuous throughout the year, as indicated by the presence of immature individuals all year, but with higher levels of new recruits occurring during the main recruitment season from April to August. In the present study, recruits (2–5 mm L) were detected on rocky shores from April to August. Crothers (Reference Crothers2001) stated that settlement occurs when individuals reach lengths >1 mm L and verified that most settlement for P. lineatus in Great Britain occurs in early September, and by the end of that month, recruits could grow to 3 mm and even 4 mm. The recruitment trend obtained for P. sauciatus in Madeira is most likely a consequence of warmer temperatures, which result in continuous reproductive activity throughout the year with a period of intense reproduction. Therefore, the entrance of new recruits in the adult population follows the same trend, with a more prominent recruitment period occurring within a few months following the reproductive peak.
The analysis of the mortality rates estimated for P. sauciatus from Madeira suggests that harvesting mortality applies a high pressure on this resource compared with natural mortality. A combination of traditional and commercial fishing methods put harvesting pressure on the stocks of this topshell in the study area, which also occurs for the limpet P. candei (Henriques et al., Reference Henriques, Sousa, Pinto, Delgado, Faria, Alves and Khadem2012) and for P. sauciatus in the Canary Islands (Alfonso et al., Reference Alfonso, Sarabia, Sancibrián, Alfaro, Adern and Hernández2015). The relatively high total mortality together with the moderate slow growth rate estimated suggest that its biomass is maximized at an early age (King, Reference King1995), which is supported by the fact that ~89% of the sampled specimens were <4 years old. The low frequency of specimens in the age classes between 4 and 9 years (~11%) seems to be a consequence of intensive size-selective exploitation of the larger topshells, resulting in a decrease in the reproductive output. The reproduction of this species is gamete density-dependent, as occurs in other gastropods, such as the patellids Patella candei crenata and P. aspera (Riera et al., Reference Riera, Herrera, Pérez, Garrido, Álvarez, Monterroso and Núñez2016; Sousa et al., Reference Sousa, Delgado, Pinto and Henriques2017).
The catch selectivity analysis resulted in a shell length of 13.19 mm for the length at first capture, corresponding to an individual of 1.61 years old and indicating that most individuals are harvested after P. sauciatus achieves sexual maturity. The yield-per-recruit analysis suggests that this species’ stock is being moderately exploited in Madeira, considering the current levels of mortality are <1.7 year−1, which returns a yield of 0.023 g. The data showed that even if the harvesting effort is doubled, there will only be a slight increase of 0.001 g in the yield, and since the relationship between mortality and yield is essentially asymptotic, the harvesting effort required to take approximate mortality to F MAX would most likely be excessive for a profitable yield.
The simulation of the effect of size at first capture on the yield-per-recruit showed that the F MAX is 1.1 year−1 at LC 25, indicating that this fishery is vulnerable to the harvest of smaller specimens that will result in a decrease in yield, which is most likely due to lower levels of recruitment because of the lower reproductive output of smaller individuals. Increasing the size at first capture to LC 75 (F MAX increases to 2.0 year−1)) would produce negligible benefits in terms of yield.
Knowledge of the life history parameters on stocks of exploited marine species is one of the major contributors to the identification and implementation of harvesting management policies for sustainable exploitation. The present study showed that the urgent implementation of management measures is required to preserve the commercial stock of P. sauciatus in the medium and long term. As such, the data reported here will contribute to the establishment of a properly regulated harvest that is both profitable and sustainable. The recommended conservation measures are the following: (i) the definition of different harvesting typologies (e.g. non-commercial harvesters with catches for personal use and professional harvesters for commercialization) with the establishment of maximum catches per day (we suggest no more than 2 kg per day for non-commercial use and 20 kg per day for professionals); (ii) the implementation of landing obligations and first sale at auction of P. sauciatus in Madeira to gain exact knowledge about the status of the commercial catches and to monitor the harvesting effort by local authorities; (iii) the establishment of a minimum catch size of 15 mm shell length to ensure that a sufficient proportion of mature individuals contributes to the reproductive effort of the exploited population, and (iv) the establishment of a closed season between February and May to prevent all types of harvesting during the main spawning pulse.
Finally, the data provided in the present study come from a single time period, and some yearly variations may occur because of the particularities of abiotic and biotic conditions, e.g. temperature and turbidity. As such, the continuation of the monitoring of exploited populations and further studies focused on the reproduction of P. sauciatus are warranted to verify whether the observed patterns are consistent throughout time.
Acknowledgements
The authors are grateful to the Fisheries Research Service (DSI) from the Regional Directorate of Fisheries of the Autonomous Region of Madeira. We also acknowledge Jorge Lucas and André Pinto for their support, namely, in biological sampling and the fishing surveys.
Financial support
The first author was supported by a grant from ARDITI OOM/2016/010 (M1420-01-0145-FEDER-000001-Observatório Oceânico da Madeira-OOM). The present study was also supported by the Fundação para a Ciência e Tecnologia – FCT through the strategic project UID/MAR/04292/2013 granted to MARE and the European Regional Development Fund in the framework of the Project MARISCOMAC (MAC/2.3d/097) and the Regional Government of Madeira.