Introduction
There are many uncertainties in radiation therapy. These can relate to the volumes and targets outlined, patient positioning and reproducibility, movement during treatment, tumour and contour changes, planning algorithms, dose output fluctuations, imaging modalities and matching variations among observers. With conformal radiotherapy (CRT) and the increasing use of intensity modulated radiotherapy (IMRT) to treat prostate cancer, dose escalations and steep dose gradients require accurate definition of target volumes. Advances in online volumetric kilovoltage imaging have improved the delivery of radiation, however, if the contour is inadequately delineated the therapeutic ratio can still be compromised.
IMRT has allowed for dose escalation without increasing late toxicity and improve disease-free survival.Reference Teh, Woo and Woo1–Reference Dearnaley, Sydes and Graham5 This also extends to the organs at risk (OARs). According to ICRU 506 the planning target volume (PTV) includes the set-up margin and internal margin but differences in delineation are not always considered. Factors that may affect the variability includes the imaging modality, slice thickness of computed tomography (CT), experience of observer, resolution and contrast, size of the structure and use of contrast agents (CAs).Reference Fiorino, Reni and Bolognesi7
CT appears to be most commonly used in treatment planning of the prostate despite the increased accuracy of magnetic resonance imaging (MRI) with less inter-observer variability.Reference Rasch, Barillot and Remeijer8–Reference Rahmouni, Yang and Tempany11 CT provides volumetric information and electron density maps for dose calculations, but MRI has improved soft tissue contrast allowing for better definition of the apex as well as the rectal–prostate and bladder–prostate interfaces. MRI has been shown to consistently result in smaller prostate volumes and less variability in definition than CT. Thus, the CT apex and base were larger than on MRI and this may lead to increased dose to bladder, rectum and penile tissues.Reference Rasch, Barillot and Remeijer8, Reference Wachter, Wachter-Gerstner and Bock9, Reference Roach, Faillace-Akazawa and Malfatti12
Methods traditionally used to help improve the definition of these structures include the use of CA's, mostly retrograde urethrogram. Valicenti et al.Reference Valicenti, Sweet and Hauck13 showed a significant improvement with bladder contrast. It resulted in improved inter-observer reliability when contouring prostate volumes. Sharma et al.Reference Sharma, Duclos and Chuba14 went on to illustrate a dose disadvantage when bladder contrast was not used as the prostate bladder interface was difficult to distinguish without bladder contrast.
Rectal contrast
Few authors have studied the impact of rectal contrast. Accurate definition of the rectum will provide a ‘true’ dose volume histogram (DVH), which is more comparable between observers and radiotherapy centres. This will allow one to more accurately predict toxicity as studies have shown correlations between DVHs and rectal toxicity in prostate cancer.Reference Fiorino, Vavassori, Sanguineti and Bianchi15
Rectal contrast use is not standardised and Roach et al.Reference Lebesque, Bruce and Kroes16 suggests a nearly empty rectum with 15 cc of contrast to avoid changes in rectal volumes and prostate position. One studyReference Gao, Wilkins and Eapen17 examined the variability in prostate contouring on CT versus 1 mm anatomical photographs. An intra-observer variation of 2–8% SD and 18·8% was found among observers in relation to prostate volumes. A systematic error was found where observers missed an average of 2·8 mm of the prostate posteriorly and included more normal tissue anteriorly (average 5·8 mm). Although only performed on one patient, it indicates that there may be an uncertainty at the posterior aspect of the prostate. Inclusion of rectal contrast may provide the answer in limiting the systematic uncertainty by highlighting the anterior rectal wall.
Bladder and rectal contrast effect on dose
CAs mainly consist of higher atomic numbers (Z) which result in a change to the HUs and hence the electron densities used in calculations. This ‘misinterpretation’ of density may impact on the dose distribution and provide different DVHs and monitor units (MUs) than during treatment when CAs are not present.Reference Harvey and Blomley18 An initial systematic error may be introduced. The planned and delivered dose distribution can potentially differ and should be investigated and corrected for if significant.
When using CAs, the treatment planning system (TPS) accounts for them as high density tissues and higher attenuation for photon beams will be calculated. The MU calculations that a target volume requires for a given dose will also be distorted.Reference Ramm, Damrau and Mose19 Whether or not bladder and rectal contrast will significantly affect the dose distribution and calculation in prostate CRT is not well documented. Some studies have shown minimal change in doses mostly below 5% between contrast and non-contrast plans.Reference Weber, Rouzaud and Miralbell20–Reference Burridge, Rowbottom and Burt24 Unfortunately, comparisons are difficult as most studies refer to phantoms and other sites.
Study aim
To investigate the dosimetric effect of rectal and bladder contrast on the PTV and OARs in DVHs using a 15 MV four-field box technique. It is hypothesised that an increase in MUs may be required to deliver the prescribed dose to the isocentre to account for the higher attenuation when contrast is present. This may increase the dose significantly to the PTV and OARs when there is no CA present during treatment.
Method
CT simulation
Ten patients with prostate cancer were assessed in this study whose CT scans were anonymous to the lead investigator. Information regarding the scanning procedures was provided by the radiotherapy department to the investigator. The ten patients had intravesical and rectal contrast. All the patients were positioned supine with ankle immobilisation and underwent a CT scan using a Philips ACQSim scanner (Philips Healthcare, the Netherlands). Patients were instructed to empty their bladders and drink ∼500 ml of water before the scans. For contrast patients, a rectal catheter was inserted with the aid of KY jelly and 5 ml of urograffin was inserted into the catheter.
Following the necessary hygiene procedures, an anaesthetic was injected into the urethra. A catheter was then inserted until it reached the bladder and 15 ml of urograffin were injected. The catheter was then retracted as far as the prostatic urethra and a further 5 ml injected. The catheter was then clamped into position. The amount of contrast used was considered adequate for optimal visualisation of the bladder, prostatic urethra and rectal outline particularly at the level of the prostate. The patients underwent their CT scans for definition of the required volumes. The patients had 3 mm slice scans as per the centres scanning protocol. All the CT data was then imported to the TPS Oncentra Masterplan Version 1.4.3.1 (OTP) by Nucletron (Veenendaal, The Netherlands) in the treatment planning laboratory. These CT datasets were anonymous to the lead investigator and were uploaded by the supervisor of this study.
Delineation
On the ten CT datasets, the bladder, seminal vesicles, prostate, rectum and femoral heads were contoured by the lead investigator. The structures contoured consisted of the following:
• Rectum: the full rectum including contents was contoured from the recto-sigmoid junction to the anal canal.
• Bladder: the external wall from dome to base.
• Seminal vesicles: whole structure contoured separately.
• Prostate: whole prostate from base to apex.
• Femoral heads: included femoral neck and greater trochanter.
All the structures above were delineated on every slice using a mouse without any enhancing contrast tools or windowing. Only magnification was used.
Contrast impact on dose
Treatment plans were constructed to analyse the effect of rectal and bladder contrast on dose distributions. The clinical target volume (CTV) was the prostate only. The CTV was expanded by 0·5 cm posteriorly and 1 cm in the other dimensions to create the PTV. A dose of 74 Gy in 37 fractions, 2 Gy per fraction was prescribed to the 100% isodose, and the prescription point was placed at the geometric centre of the PTV. The prescription point was still checked to ensure that it was in an area that had a uniform tissue density within a 2 cm radius. None of the patients had prostate fiducial markers. The four-field box CRT plans were created with 15 MV only. The PTV was kept between 95% and 107% as per ICRU recommendations.6 Dose constraints in Gy for OARs used are listed in Table 1.
Table 1 OAR and DVC used in plans
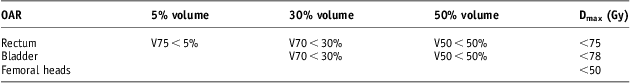
Abbreviations: OAR, organs at risk; DVC, dose volume constraints.
To simulate a ‘non-contrast scan’ without rescanning the patient, the bulk density of the rectal and bladder volumes were adjusted to mimic tissue density. This was changed to 1 g/cm3. No other change was made to the plan, and the DVHs were compared from both plans. A pencil beam algorithm was used on all plans with a matrix resolution of 0·5 cm.
Plan analysis
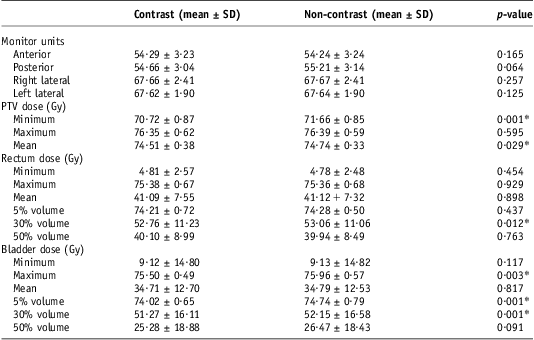
Table 2 shows the dose and volumes used to compare the contrast and non-contrast scans.
Table 2 Contrast versus non-contrast dose comparisons

Abbreviations: PTV, planning target volume; MUs, monitor units.
Statistical analysis
All the recorded data were inputted into SPSS version 14 (SPSS Inc., Chicago, USA: now IBM). Paired t-tests performed evaluated whether the MUs, dose to the PTV, rectum and bladder differed significantly when the density was changed. The symbol * denotes a significant p-value (<0·05).
Results
Plan information
Table 3 shows descriptive statistics for the ten plans created with rectal and bladder contrast and for those when the contrast was given unit density.
Table 3 Mean and SD values for contrast and non-contrast plans

Note: *p < 0·05.
Abbreviation: PTV, planning target volume.
As expected, the MUs increased although not significantly by 0·09% (0·05) in the anterior beam, as the presence of the bladder contrast may have required more MUs to deliver the prescribed dose. This was, however, not replicated in the lateral fields which were very similar. The posterior beam gave a 1% (0·55 MU) increase in MU without contrast, and this was the maximum difference. These changes were not, however, statistically significantly different.
Although the minimum and mean PTV doses were statistically significantly higher without the contrast, this was only by an average of 0·94 Gy (1·3%) and 0·22 Gy (0·3%). The rectal dose did not differ statistically significantly with and without contrast except for the 30% volume dose. This was the largest, with an average increase of 0·3 Gy (0·57%) without the contrast.
The bladder showed a more consistent increase in dose when there was no contrast present than did the rectum. This was statistically significant for the maximum dose, 5% and 30% volume consisting of a dose increment of 0·46 Gy (0·6%), 0·72 Gy (0·98%) and 0·88 Gy (1·71%), respectively. Although the 50% volume was deemed not significant, the percentage dose increase without contrast was highest at 4·7% (Table 4).
Table 4 Mean MU and % dose difference (contrast – non-contrast)
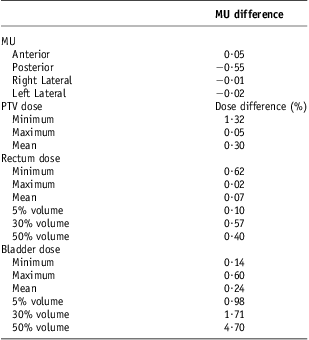
Abbreviation: MU, monitor units.
Discussion
The aim of this study was to investigate the effect of rectal and bladder contrast on MUs, and dose to the PTV, rectum and bladder. The hypothesis was based on the fact that contrast material, being of higher Z, attenuates the photon beam to a greater extent and therefore the MUs required may be higher with contrast. As contrast is absent during treatment, the effect of this may have clinical implications. Ramm et al.Reference Ramm, Damrau and Mose19 showed that the effect of introducing contrast materials increased with the concentration, volume and depth of the contrast material. The CT numbers increased with the concentration, illustrating the problem created for the dose calculation. The density and attenuation were therefore overestimated. Here the study showed an increased effect with a 6 versus a 25 MV beam. It was concluded that only 1–3% in dose changes was likely, provided the contrast was <500 HU and <5 cm diameter. It was also discussed that additional beams may reduce the overall effect. In this study the dose differences were also minimal, as expected due to the small amount of contrast used and high-energy 15 MV beams.
The insignificant effect of the high Z contrast media in this study and others may highlight the fact that only ∼25% of X-ray interactions are dominated by the pair production effect at 15 MV.Reference Johns and Cunningham25 As compton interactions are accounting for the majority of MV attenuation in this study (∼75%), this is dependant on electron density and not the atomic number. It is the electron density of the contrast that is therefore of more importance at 15 MV. Most materials have similar numbers of electrons per gram (e/g) apart from hydrogen, so physical density (g/cm3) becomes more important.Reference Johns and Cunningham25 Therefore, at this therapeutic energy, contrast materials with their high Z numbers will not effect the total mass attenuation that much. Increasing the energy higher than 15 MV will increase the pair production interactions which are dependent on Z and thus the high Z contrast may have a greater effect on the calculated dose and MU. Energies above 15 MV are, however, not very common for prostate radiotherapy.
This study only deals with 15 MV four-field CRT plans. It was felt that 15 MV is a very common energy used for prostate CRT. A possible further comparison of contrast and no contrast on 6 MV IMRT on other sites also may be something worth considering. However, it is of the author's opinion that the effect would also likely be clinically insignificant due to the X-ray interactions mostly dominated by the compton effect and the small volume of contrast media used. Studies as shown in Table 5 have also demonstrated minimal effect.
Table 5 Review of the effect of contrast agents on dose calculations in radiotherapy
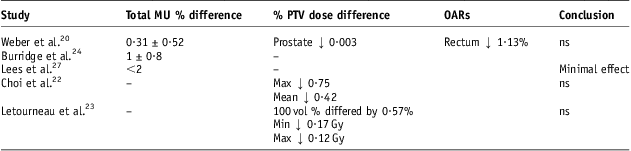
Abbreviations: ns, not clinically significant; MU, monitor units; PTV, planning target volume; OARs, organs at risk.
It was expected that an increase in the number of MUs would be required with contrast for the prescribed dose. This was true for the anterior beam which gave a very small increase of 0·05 MU (0·09%), but was unchanged in the lateral beams. The posterior beam required more MUs (mean 0·55) without contrast and this may be because of the presence of air in the rectum before the bulk density change. The anterior beam was likely more affected by the larger volume of contrast in the bladder and its proximity. The bulk density correction for the rectal volume included the full lumen and wall as contoured. This, however, may be an extreme scenario as the rectal contrast did not fully fill the rectum throughout all patients because of the small volume used. Individual beam MU changes were all minimal and <1% with a maximum change of 0·55 MU on the posterior beam.
Weber et al.Reference Weber, Rouzaud and Miralbell20 performed a similar study to the present one using bladder contrast only, 18 MV and a six-field technique. The total MU difference consisted of an average increase of 0·31 ± 0·52% with contrast with reported similar minimal changes in MU in the lateral beams. The dose to the prostate and rectum on average increased by 0·03% and 1·13%, respectively, when no contrast was used. The current study showed slight increases in the minimum, mean and maximum dose to the PTV without contrast. This was by 1·32% (0·94 Gy), 0·3% (0·22 Gy) and 0·05% (0·042 Gy), respectively. Choi et al.Reference Choi, Kim and Lee22 demonstrated comparable changes in IMRT head/neck patients. Increases of 0·4% (0·27 Gy), 0·42% (0·29 Gy) and 0·75% (0·59 Gy) were found in the non-contrast PTVs V95, mean and maximum doses. Letourneau et al.Reference Létourneau, Finlay and O'Sullivan23 showed changes of 0·17 Gy to the PTVs minimum dose and 0·12 Gy to the PTVs maximum dose among IMRT head/neck patients. This was concluded as having an insignificant effect. A summary of these studies is given in Table 5.
In this study, the dose to the bladder was more varied than the rectum. The non-contrast bladder dose increased statistically significantly at the maximum, 5% and 30% volume. This was by 0·6% (0·45 Gy), 0·98% (0·72 Gy) and 1·71% (0·88 Gy). All the measurements were <2% dose difference except for V50 with a difference of 4·7% (1·19 Gy). These changes were lower in the rectum perhaps due to amount of contrast used. The rectum had a maximum dose difference under 0·7%. The rectum did not show as much of an increase in dose without contrast similar to the bladder. This is likely due to the increased variation in density within the rectum itself (air pockets). Thus, changing the density to 1 g/cm3 may actually increase its overall density, as opposed to decreasing it and increase the compton effect attenuation. This may be reflected by the increased MU for the posterior beam (0·55 MU) in the non-contrast group.
Owing to the anatomical location of the prostate and the technique used, the presence of bladder and rectal contrast media appear to affect the anterior and posterior beams more than the laterals. A pencil beam algorithm was used for this study. This was very efficient and the insignificance of algorithm selection used in prostate planning has been shown in four-field box technique 15 MV by a study by Knoos et al.Reference Knöös, Wieslander and Cozzi26 It was concluded that for simple four-field box conformal techniques in the mostly uniform density pelvis that the selection of model/system is not critical for the final dose or dose distribution.
The major limitation of this study is that the same CT dataset was used with a bulk density correction, instead of scanning the patients with and without the CAs. It was felt, however, that to scan patients twice was not justified and to do so would also introduce possible variations in rectal and bladder volumes, as well as possible prostate positional changes. These may affect the DVHs and MUs required more so than the actual presence of small amounts of CAs and may make analysis of the contrast effect more difficult.
It is evident that any dosimetric changes that occur when contrast is introduced affect the dose minimally. Consideration must be given to the volumes and concentration used as advised by Ramm et al.Reference Ramm, Damrau and Mose19 The electron density is of importance at therapeutic energies due to the compton effect. When treatment planning dose volume constraints are very close to being met, one must consider the possibility that the dose to the structure can differ if density corrections are not accounted for. Despite these changes being small, they may in very rare occasions affect the acceptability of plans when adhering to constraints.
Acknowledgements
Special thanks to Michelle Leech, Head of Discipline of Radiation Therapy, Trinity College Dublin for all her help and support.
Conflicts of interest
There is no conflict of interest to report for this article.