Colorectal cancer (CRC) is the third and second most prevalent cancer in men and women, respectively. Worldwide, countries in Asia have witnessed the sharpest increase in CRC rate. CRC is the fourth common cancer among Iranian people and its incidence has been increasing in both men and women in recent years(Reference Khosravi Shadmani, Ayubi and Khazaei1,Reference Rafiemanesh, Pakzad and Abedi2) .
A variety of genetic and environmental factors play a role in the development of CRC(Reference Kuipers, Grady and Lieberman3). CRC usually originates from lesions named colorectal adenomas (CRA). Prevalence of adenomas varies between 30 and 50 %, and its environmental risk factors are almost similar to CRC(Reference Øines, Helsingen and Bretthauer4). Various epidemiological studies indicated that dietary factors can contribute to or prevent from CRC development(Reference Aune, Chan and Lau5,Reference Baena and Salinas6) . Many of these studies investigated the relationship between nutrients, foods, food groups, dietary patterns and diet quality indices with CRC or CRA(Reference Randi, Edefonti and Ferraroni7–Reference Schwingshackl and Hoffmann11). The findings of these studies showed that adopting a diet high in plant-based foods and low in animal and processed food products was associated with lower risk of CRC and CRA(Reference Tayyem, Bawadi and Shehadah12–Reference Azeem, Gillani and Siddiqui14).
Recently, much attention has been given to the role of dietary acid–base balance in the development of chronic diseases including CVD, type 2 diabetes, cancers and overall mortality(Reference Kucharska, Szostak-Wegierek and Waskiewicz15–Reference Park, Steck and Fung17). Short-term imbalanced acid–base is quickly corrected without significant clinical effects. However, long-term diet-dependent acid load, as a consequence of high protein consumption, may raise the level of insulin-like growth factor-1 that is associated with increased risk of CRC(Reference Park, Steck and Fung17,Reference Furstenberger and Senn18) . Furthermore, some studies showed that extracellular acidity stimulated the invasive potential of cancer cells(Reference Kato, Ozawa and Miyamoto19,Reference Martinez-Zaguilan, Seftor and Seftor20) . However, the evidence for the relationship between diet-dependent acid load and the risk of cancers is limited(Reference Park, Steck and Fung17,Reference Fenton and Huang21) .
According to our knowledge, until now, no study has examined the association between diet-dependent acid load and the risk of CRC and CRA. Therefore, the aim of the current study is to examine the association of diet-dependent acid load and the risk of CRC and CRA (as an important precursor of CRC) in a sample of Iranian adults.
Methods
Study design and population
This was a hospital-based case–control study, conducted in three major referral hospitals for CRC (Taleghani, Shohadae Tajrish and Emam Hosein) in Tehran, Iran between December 2016 and September 2018. The study participants had already been recruited for another study(Reference Bahrami, Houshyari and Jafari22). To be included as CRC cases in original study, patients had to be diagnosed with CRC, no longer than 3 months before the interview date, confirmed by colonoscopy and pathology reports, with no history of cancers, polyps and inflammatory bowel diseases. CRA cases were either patients with rectal bleeding or asymptomatic individual with positive faecal occult blood tests who were referred to colonoscopy procedures. Patients with histologically confirmed adenomatous polyps were assigned to the CRA group. All cases were aged between 30 and 70 years old. Over the same period of time, age (±10 year) and sex-matched controls were recruited from patients admitted to same hospitals as cases for conditions unrelated to cancer, colorectal polyps, inflammatory bowel diseases and dietary restriction due to chronic diseases such as orthopedic disorders, traumas, acute surgical conditions, nose, and ears and skin problems. Overall, 536 participants (268 controls, 134 CRC and 134 CRA) were recruited in the current study. Subjects with incomplete FFQ and implausible energy intake estimates (outside the range of ±3 sd from the mean) were excluded (28 controls, five CRC and four CRA). The final sample consisted of 499 participants (240 controls, 129 CRC and 130 CRA).
Covariates’ assessment
Data regarding socio-demographic characteristics, family history of CRC and other cancers, tobacco use, medical history of diseases, medications and vitamin/mineral supplements and cooking techniques were obtained by a multicomponent questionnaire. Weight was measured with precision of 0·1 kg and height with the precision of 0·1 cm and then BMI was calculated. Alcohol intake consumption question was not included in the questionnaire due to prohibition of alcohol intake in Iran as an Islamic country. Furthermore, physical activity was assessed by a validated questionnaire in which participants were asked to specify the time of their leisure and occupational activity which were weighted based on intensity level(Reference Kriska, Knowler and LaPorte23,Reference Aadahl and Jorgensen24) . Metabolic equivalent task per 24 h was then calculated according to these specifications. Moreover, the reason for not considering possible dietary confounders such as whole grains, fibres, red and processed meat as confounders is that these are used in calculating amounts of nutrients which are part of potential renal acid load (PRAL) such as Mg and K.
Dietary assessment
Usual dietary intake of participants was assessed using a valid and reliable semi-quantitative FFQ, consisting of 148 foods and beverages(Reference Esfahani, Asghari and Mirmiran25). Dietary intake assessment referred to 1 year before diagnosis for cases and 1 year before interview for controls. Trained interviewers asked all participants to specify their intake frequency of given serving for each item on a daily, weekly and monthly basis. Collected data were then converted to daily frequencies. Daily gram intake of each item was calculated using the corresponding daily frequency and the portion size. Energy and nutrients for every participant were estimated using United States Department of Agriculture (USDA) food composition data. For traditional Iranian food items, such as traditional Iranian bread, which were not included in the USDA database, the Iranian food composition table was used(Reference Azar and Sarkisian26).
Diet-dependent acid load calculation
While various methodologies were proposed to estimate the diet-dependent acid load, PRAL, used in previous studies, was selected for the purpose of the current study(Reference Remer, Dimitriou and Manz27,Reference Frassetto, Todd and Morris28) . PRAL is an estimation of endogenous acid production which exceeds the amount of alkali produced from foods consumed daily. Also, animal protein to potassium ratio (A:K) and net endogenous acid production (NEAP) were calculated to see their linear trend across PRAL as simpler indicators of acid load. Calculations are detailed below:
PRAL (mEq/d) = [Protein (g/d) × 0·49] + [P (mg/d) × 0·037] – [K (mg/d) × 0·201] – [Ca (mg/d) × 0·013] – [Mg (mg/d)] × 0·026]
Negative PRAL values indicate a base-forming potential, while positive values of PRAL imply acid-forming potential of diet(Reference Remer and Manz29).
A:K ratio = animal protein (g/d)/potassium (g/d)(Reference Zwart, Hargens and Smith30)
Net endogenous acid production (mEq/d) = [54·5 × protein (g/d)]/[0·0366 × potassium (mEq/d)] – 1·02(Reference Frassetto, Todd and Morris28).
Statistical analysis
Data analysis was performed by Statistical Package Software for Social Science, version 24 (SPSS Inc.). Kolmogorov–Smirnov test was used to check the normality of data. To compare general characteristics of controls with CRA and CRC cases, the Mann–Whitney U test was used for continuous variables and χ 2 used for categorical variables. PRAL scores were categorised into tertiles based on 33·3th and 66·66th percentile values of PRAL. Dietary consumption across tertile of PRAL scores was assessed using median value for each tertile of PRAL and modelling this value as a continuous variable in general linear regression adjustment for age and energy intake. Unconditional logistic regression was used to estimate OR with 95 % CI of CRC and CRA according to tertiles of PRAL after adjustment for potential confounders in one model, adjusted for age, comorbidity, family history of cancer, common ways of cooking, level of salt intake, physical activity and Ca supplement use. A P-value < 0·05 was considered as statistically significant.
To designing figures, participants were categorised into four groups (group 1 = < –1 sd, group 2 = –1 sd – mean, group 3 = mean – +1 sd and group 4 = > +1 sd) based on mean and ±1 sd of PRAL. To estimate OR and 95 % CI, group 1 values were used as reference in regression models and other groups compared with group 1 and OR and CI calculated based on it and then logarithm (log) of OR were used for Y-axis and sd for X-axis.
Results
Characteristics of study population
Table 1 provides the main characteristics of study participants. Given that the selection of participants followed a frequency matched design, age and gender did not differ in controls and CRC and CRA cases. There was no statistically significant difference among participants with regard to BMI, smoking status, family history of CRC in first-degree relatives, vitamin D supplement, multivitamin use and energy intake. However, CRC cases were more likely to have family history of cancer in first-degree relatives than controls (51·2 % v. 32·9 %; P-value < 0·01). Compared with controls, adenoma cases were more likely to have at least one comorbidity (36·9 % v. 15·8 %; P-value < 0·01) and the use of Ca supplement was higher in adenoma cases than controls (24·6 % v. 14·6; P-value = 0·01). Moreover, the median value for physical activity was significantly higher in controls than adenomas (OR 39·02 (95 % CI 36·51–41·33) v. OR 37·75 (95 % CI 34·67–40·40); P-value < 0·01). Level of the salt intake was lower in controls compared with cancer and adenoma cases (P-value = 0·002). Common ways of cooking among controls differed significantly from cancer and adenomas patients (P-value < 0·05). More specifically, cancer cases consumed more fried and grilled foods compared with controls and consumption of steamed food was lower in adenomas than controls.
Table 1 Baseline characteristics of the study participants

MET, metabolic equivalent.
* P-value between cancers and controls.
† P-value between adenomas and controls.
‡ Matched variables of the study.
§ Comorbidities are defined as diabetes, hypertension and CHD.
¶ Significant difference (χ 2, P-value < 0·05).
‖ Significant difference (Mann–Whitney U, P-value <0·05).
Dietary intakes across tertiles
Table 2 shows dietary intake of energy, food groups and nutrients, according to tertiles of PRAL. Dietary intake of red meat (β = 0·23), poultry (β = 0·24), egg (β = 0·11), protein (β = 0·14), animal protein (β = 0·25) and net endogenous acid production (β = 0·82) and protein to potassium ratio (β = 0·65) increased significantly across increasing tertiles of PRAL (P for trend < 0·001), while dietary intake of dairy products (β = –0·11), legumes (β = –0·12), fruits (β = –0·48), vegetables (β = –0·35), carbohydrates (β = –0·67), Mg (β = –0·12) and K (β = –0·39) decreased significantly across the increasing tertiles of PRAL (P for trend <0·01). Intake of Ca (861·16 (sd 309·37) v. 873·92 (sd 297·56) mg/d) in third tertile was significantly lower compared with first tertile of PRAL (P for trend < 0·001). Compared with first tertile of PRAL, energy intake (10 327·58 (sd 3022·98) v. 9641·65 (sd 2628·30) kJ/d) was significantly higher in third tertile of PRAL (P for trend < 0·01).
Table 2 Dietary intake of study participants (controls, colorectal cancer cases and colorectal adenoma cases) across tertiles (T) of potential renal acid load score (PRAL)
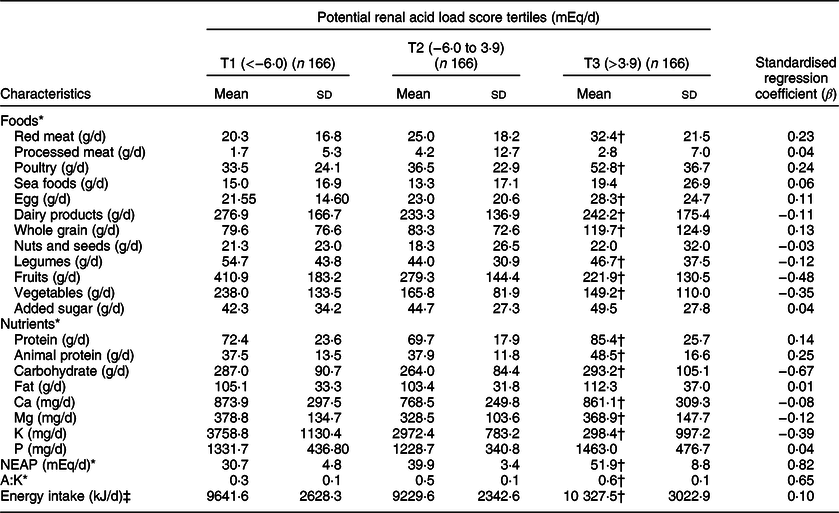
NEAP, net endogenous acid production; A:K ratio, the ratio of animal protein to potassium.
* Adjusted for age (linear) and energy intake (linear).
† P < 0·01, using general linear regression to compare the dietary intake of participant across tertiles of PRAL.
‡ Adjusted for age (linear).
Association of potential renal acid load with colorectal cancer and adenomas risk
Tables 3 and 4 present the OR and 95 % CI of CRC and CRA across tertiles of PRAL, respectively. After adjusting for potential confounding variables including age, comorbidities, family history of cancers, common ways of cooking, level of the salt intake, physical activity and Ca supplement consumption, the odds (95 % CI) of having CRC and CRA were 4·82 (2·51–9·25) and 2·47 (1·38–4·42) times higher at the highest compared with the lowest tertile of PRAL.
Table 3 OR and corresponding 95 % CI for the risk of colorectal cancer across tertiles (T) of potential renal acid load score (PRAL)
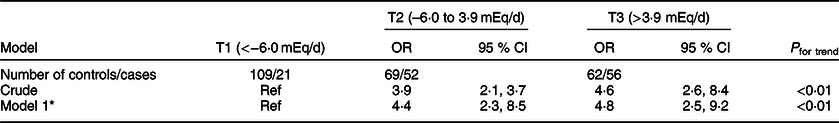
* OR adjusted for age (linear), comorbidity (in categories: diabetes, hypertension and CHD), cancer family history (in categories: yes, no), common ways of cooking (in categories: fried, boiled, grilled, steam cooked and combined), level of salt intake (in categories: low, normal and high), physical activity (linear) and Ca supplement use (in categories: yes, no).
Table 4 OR and 95 % CI for the risk of colorectal adenomas based on tertiles (T) of potential renal acid load score (PRAL)
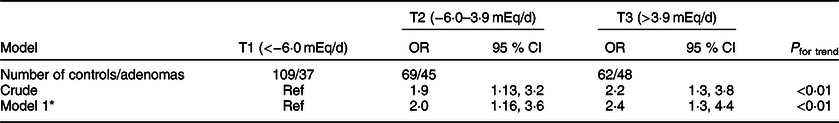
* OR adjusted for age (linear), comorbidity (in categories: diabetes, hypertension and CHD), cancer family history (in categories: yes, no), common ways of cooking (in categories: fried, boiled, grilled, steam cooked and combined), level of salt intake (in categories: low, normal and high), physical activity (linear) and Ca supplement use (in categories: yes, no).
Figures 1 and 2 show the logs of the odds (95 % CI) of CRC and CRA according to sd of PRAL, respectively. After adjusting for potential confounding variables, risk of having CRC and CRA increased through increasing PRAL.

Fig. 1 Participants categorised into four groups based on mean and ±1 sd of PRAL: <−1 sd (≤−14·62), from −1 sd to mean (from −14·61 to −1·42), mean to +1 sd (from −1·41 to 11·78) and >+1 sd: ≥11·79. To estimate logs of odds and 95 % CI, −1 sd values were used as reference in regression models and then logarithm (log) of odds was used for Y-axis and sd for X-axis
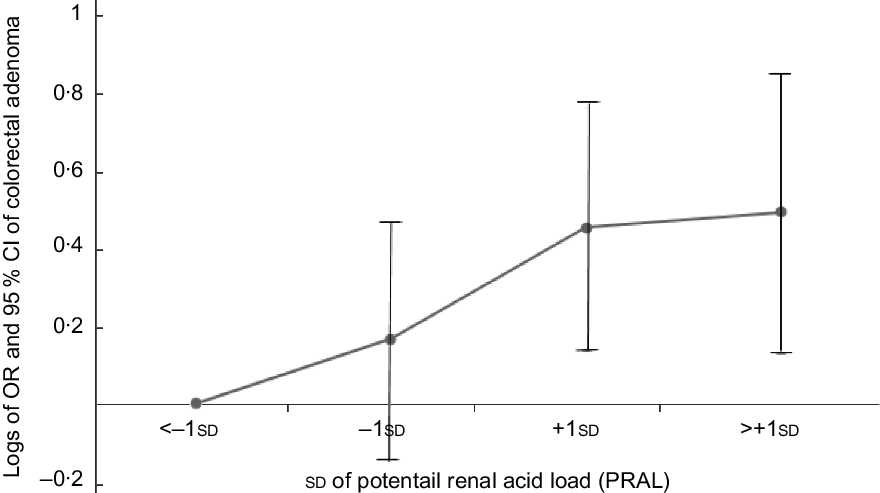
Fig. 2 Participants categorised into four groups based on mean and ±1 sd of PRAL: <−1 sd (≤−14·62), from −1 sd to mean (from −14·61 to −1·42), mean to +1 sd (from −1·41 to 11·78) and >+1 sd: ≥11·79. To estimate logs of odds and 95 % CI, −1 sd values were used as reference in regression models and then logarithm (log) of odds was used for Y-axis and sd for X-axis
Discussion
In this case–control study, we found that higher diet-dependent acid load scores were significantly and positively associated with increase in risk of CRC and CRA, among Iranian adults. In addition, the intake of animal products (red meat, poultry, egg, protein and animal protein) was positively associated with diet-dependent acid load while intakes of plant-based foods (legumes, fruits and vegetables) were negatively related to diet-dependent acid load.
A few epidemiological studies have evaluated the association between dietary acid–base disequilibrium and risk of cancers(Reference Fenton and Huang21). A prospective cohort study examined the relationship between urine pH (represented as net acid excretion) and bladder cancer based on a hypothesis that low urine PH is an important potential risk factor for bladder cancer. The current study indicated that there was no significant association between net acid excretion and bladder cancer, but in long-term smokers a non-significant increased risk was seen(Reference Wright, Michaud and Pietinen31). Another prospective cohort study showed that higher diet-dependent acid load (represented as PRAL) was significantly related to increased risk of breast cancer especially in estrogen reseptor (ER) negative and triple negative breast cancer(Reference Park, Steck and Fung17). The results of our study together with those of previous studies suggested a role of acid–base balance of diet in the development of cancer. Moreover, our findings showed a relationship between diet-dependent acid load and the risk of CRA as a main precursor of CRC.
The results of the present study showed that, among all food groups which have an impact on diet-dependent acid load, animal protein, total protein, poultry and red meat were positively associated with acidity, while fruits and vegetables had the highest impact on the base potential of diet. These findings are in line with the proposition that food groups can affect the acid–base balance. Meats, dairy products and eggs, which are high in sulphur containing amino acids, increase diet-dependent acid load by increasing hydrogen ion [H+] concentration, whereas fruits, vegetables and legumes have base-producing potential because they are rich in K and Mg which consume hydrogen ions when metabolised(Reference Robey32,Reference Remer33) .
Although there is no consensus on how diet-dependent acid-load affects the risk of CRC, several mechanisms have been proposed. Existing evidence indicated that short-term diet induced acid–base imbalance as a result of high protein meals is transient and does not have much clinical consequences(Reference Pizzorno, Frassetto and Katzinger34); however, on the long term, there are some potential hormonal and non-hormonal mechanisms to explain the effect of diet-dependent acidosis on CRC. Acidogenic diet contributes to mild metabolic acidosis which induces cortisol secretion excess(Reference Espino, Suarez and Santamarina35). Studies have reported that cortisol signalling may have a role in carcinogenesis. Chronic cortisol production leads to insulin resistance and activation of insulin-like growth factor-1, which may result in colorectal carcinogenesis and growth and malignant transformation of adenomatous polyps through mitogen-activated protein kinase (MAPK) pathway and phosphoinositide 3-kinase (PI3K) pathway(Reference Robey32,Reference Zhang, Xu and Li36,Reference Nosho, Yamamoto and Taniguchi37) . On the other hand, diet-dependent acid load could decrease circulating adiponectin(Reference Disthabanchong, Niticharoenpong and Radinahamed38) and prevent its anti-proliferative and apoptotic activities(Reference Gonullu, Kahraman and Bedir39). Evidence on the impact of protein intake, as a net acid producer, on leptin level is limited and the results are inconsistent(Reference Izadi, Saraf-Bank and Azadbakht40,Reference Weigle, Breen and Matthys41) . Leptin has proinflammatory, proliferative, anti-apoptotic and mitogenic activities, leading to CRC(Reference Rodríguez, Mastronardi and Paz-Filho42).
Furthermore, long-term diet high in red meat, processed meats, dairy and eggs results in sulphate and phosphate production, which are ultimately net-acid producers (H+). If the release of acids into the circulation exceeds the amount of bicarbonate (HCO3-) which is the final metabolite of K and Mg from vegetables and fruits, low grade diet-dependent acidosis occurs(Reference Robey32,Reference Pizzorno, Frassetto and Katzinger34) . Several studies have shown an inverse relationship between vegetables and fruits intake and CRC and CRA, while many studies have suggested that red meat and processed meats increase the risk of CRC and CRA(Reference Kunzmann, Coleman and Huang8,Reference Abid, Cross and Sinha43) . Considering that higher PRAL was associated with a higher risk of CRC and CRA in the present study and higher PRAL scores were associated with higher intake of red meat and lower intake of vegetables and fruits, our study confirms the protective role of high consumption of fruits and vegetables and low consumption of red meat for CRC cancer and CRA. According to mentioned associations between fruits, vegetables and red meats with CRC and CRA, it should be considered that the associations between PRAL and the outcomes could possibly be due to these risk factors and protective factors and PRAL may be one of the several mechanisms linking these foods with CRC. Several mechanisms have been proposed linking these foods to CRC. For example, for plant foods reducing the plasma concentration of inflammatory markers by antioxidant compounds, inhibiting CRC cell growth by holding down the activation of NF-κB pathway by phytochemical compounds(Reference Li, Niu and Sun44,Reference Jaganathan, Vellayappan and Narasimhan45) and promoting cell cycle arrest and apoptosis, inhibition of cell migration and invasion by fibre have been noted(Reference Zeng, Lazarova and Bordonaro46). Also, for red meats, high fat and polycyclic aromatic hydrocarbons and heterocyclic amines produced from cooking over high heat have been suggested(Reference Demeyer, Mertens and De Smet47).
Our study has some strengths. First, to our knowledge, the current study is the first to explore the association between diet-dependent acid load and the risk of CRC and CRA. Second, using a validated FFQ allowed for an accurate estimation of the main exposure of interest, which is dietary intake and gave us the opportunity to control for several important confounders(Reference Aadahl and Jorgensen24,Reference Asghari, Rezazadeh and Hosseini-Esfahani48) . On the other hand, there are some limitations in the present study. Since dietary intake was evaluated through a self-administered FFQ, measurement errors were inevitable. Selection and recall bias are inevitable in case–control studies. However, we tried to reduce these biases by including incident cases along with hospital controls and employing validated FFQ performed by trained interviewers. We did not have information about participants’ kidney function as an important determinant of acid–base equilibrium. However, we did not include patients with chronic kidney, liver and lung diseases in the study and the results were adjusted for comorbidities such as blood pressure and type 2 diabetes.
Conclusion
In conclusion, findings from this case–control study suggested that higher diet-dependent acid load is associated with higher risk of CRC and CRA. Further intervention studies are needed to determine whether diets with lower acid load could reduce the risk of CRC and CRA as a precursor of colorectal malignancies. Also, studies to confirm present findings in other populations are warranted.
Acknowledgements
Acknowledgements: The authors thank all study participants without whom the current study was impossible. Financial support: This investigation received no financial support. Conflict of interest: The authors declare no conflicts of interest. Authorship: In the current study, the author contributions are as follows: Conceptualisation and methodology: E.H. and A.S.; analysis: S.K.N., N.R. and B.R.; investigation: S.J.N.P.R. and A.B.; resources: A.S. and E.H.; writing: S.J.N., N.R., E.H. and A.S.; review and editing: G.S., B.R. and F.N.; visualisation: A.S. and E.H.; supervision: A.S. and E.H.; project administration: A.S. and E.H. Ethics of human subject participants: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (approval number: 0450/1118). Written informed consent was obtained from all subjects/patients.









