Gestational diabetes mellitus is the most common metabolic disorder in pregnancy. It was projected that almost 21.3 million live births worldwide were affected by some form of hyperglycaemia in pregnancy. Of these, an estimated 86.4% were due to gestational diabetes mellitus, and the rest were pre-gestational diabetes or gestational impaired glucose tolerance. Reference Cho, Shaw and Karuranga1 Gestational diabetes mellitus is a significant determinant for perinatal mortality and morbidity. 2 Gestational diabetes mellitus may either cause fetal macrosomia and polyhydramnios or placental insufficiency and fetal growth restriction. The measurement of peripheral vascular Doppler indices is a non-invasive fetal monitoring technique frequently used to evaluate fetoplacental haemodynamics, especially the degree of fetal deterioration associated with placental insufficiency and fetal growth restriction. Unlike pre-gestational diabetes, in gestational diabetes mellitus, fetal growth restriction is a less common finding compared to fetal macrosomia and polyhydramnios. Fetoplacental Doppler indices mostly remain normal throughout uncomplicated diabetic pregnancies. Reference Dicker, Goldman, Yeshaya and Peleg3,Reference Johnstone, Steel, Haddad, Hoskins, Greer and Chambers4 Maternal hyperglycaemia is associated with increased perinatal mortality and morbidity; even when the fetal growth and peripheral circulatory dynamics are normal. Reference Rudge, Calderon, Ramos, Abbade and Rugolo5
Fetal cardiac functions are usually affected in diabetic women. Reference Rizzo, Arduini, Capponi and Romanini6,Reference Ghaderian, Hemmat, Behdad, Saeedi and Shahsanaei7 The information obtained from these functional cardiac measurements can be used for making clinical decisions. The alterations in cardiac function are mainly attributed to either myocardial hypertrophy resulted from maternal hyperglycaemia and hyperinsulinemia or hypoxaemia secondary to increased oxidative metabolism due to hyperglycaemia. Reference Reller and Kaplan8,Reference Teramo9 It has been suggested that changes in fetal cardiac functions due to maternal hyperglycaemia initially started with changes in diastolic functions, followed by impairment in systolic functions. Reference Russell, Foley, Kinsley, Firth, Coffey and McAuliffe10,Reference Fouda, Abou ElKassem, Hefny, Fouda and Hashem11
Doppler analysis of flow velocities through the mitral and tricuspid valve by using early and late diastolic filling ratio is a conventional echocardiographic method for the assessment of fetal diastolic function. The early and late diastolic filling ratio of atrioventricular valves expresses both cardiac compliance and preload conditions. Reference Labovitz and Pearson12 A decrease in the early and late diastolic filling ratio of atrioventricular valves is related to ventricular diastolic dysfunction, mainly due to chronic hypoxia and cardiac overload. Reference Hernandez-Andrade, Benavides-Serralde, Cruz-Martinez, Welsh and Mancilla-Ramirez13 Another conventional method for evaluating fetal ventricular diastolic function is to analyse the flow dynamics of the precordial veins (ductus venosus, inferior vena cava, hepatic veins, and pulmonary veins) as they are in almost direct continuity with the atria. Reference Van Mieghem, DeKoninck, Steenhaut and Deprest14 Pulmonary venous flow reflects the change of the pressure of the left atrium and its response to systemic vascular status and mainly the diastolic functions of the left side of the heart. Reference Talbert and Johnson15 The pulmonary vein pulsatility index is a Doppler parameter easily obtained with high reproducibility.
This study aims to investigate whether fetal cardiac diastolic functions measured by selected conventional Doppler indices are altered in appropriate-for-gestational-age or macrosomic fetuses of gestational diabetic mothers with poor glycaemic control.
Materials and methods
Study population
This observational cross-sectional study was performed in the outpatient clinic of the Obstetrics and Gynecology Department of Izmir Katip Çelebi University Atatürk Training and Research Hospital between January, 2021 and April, 2021. Forty-five appropriate-for-gestational-age or macrosomic fetuses with gestational age between 28 weeks and 39 weeks whose mothers had gestational diabetes mellitus and poor glycaemic control were examined. The control group was formed by 49 appropriate-for-gestational-age fetuses of healthy pregnant women attending routine prenatal care between 29 and 41 weeks. The gestational age of the pregnant women was determined by the last menstrual period and confirmed by first-trimester obstetric ultrasound. The estimated fetal weight between 10th and 90th percentile was defined as appropriate-for-gestational-age. The estimated fetal weight >90th percentile was defined as fetal macrosomia (large for gestational age). The estimated fetal weight <10th percentile was defined as small for gestational age. Reference Salomon, Alfirevic and Da Silva Costa16 Fetal growth restriction was defined as the estimated fetal weight or abdominal circumference <10th percentile and umbilical artery pulsatility index >95th percentile and/or cerebra-placental index <5th percentile. Reference Salomon, Alfirevic and Da Silva Costa16,Reference Ciobanu, Wright, Syngelaki, Wright, Akolekar and Nicolaides17 The inclusion criteria for all the groups were singleton pregnancy and a normal mid-trimester fetal anatomic survey. Women who had a small for gestational age fetus or fetal growth restriction, pregnancy-induced hypertension, preeclampsia, coexisting systemic diseases, such as pre-gestational diabetes mellitus, renal diseases, connective tissue disorders, and history of smoking and alcohol consumption, were excluded. The diagnosis of gestational diabetes mellitus was made between 24 and 28 weeks of gestation based on a 75 g oral glucose tolerance test which was performed on both the study and the control groups for gestational diabetes mellitus screening. 18 Self-monitored capillary blood glucose levels were recorded by each woman with gestational diabetes mellitus four times daily: after an overnight fast and 1 hour after meals. Criteria for poor glycaemic control in patients under treatment either with diet alone or a combination of diet and insulin were as follows: blood glucose, >95 mg/dL after overnight fasting, and >140 mg/dL 1 hour after meals for at least 2 weeks.
Ultrasound and Doppler measurements
Ultrasound examinations were performed using a Voluson E6 system (GE Healthcare, Milwaukee, WI) with an RAB 4–8 MHz transabdominal probe. The analysis of Doppler measurements was always carried through by the same examiner (Seçil Karaca Kurtulmus) to reduce the inter-operator variability.
Fetal biometric measurements, including biparietal diameter, head circumference, abdominal circumference, and femur length, were obtained for each fetus to determine the fetal growth and estimated fetal weight (Hadlock formula; biparietal diameter, head circumference, abdominal circumference, femur length). Reference Melamed, Yogev, Meizner, Mashiach, Bardin and Ben-Haroush19 Fetoplacental circulation was assessed with the Doppler measurements including, middle cerebral artery pulsatility index, umblical artery pulsatility index, ductus venosus pulsatility index, and cerebra-placental index. Middle cerebral artery Doppler measurements were obtained through an axial view of the fetal cranium, including the thalamus and sphenoid bone wings. In this plane, the spectral Doppler gate is placed immediately after the origin of the middle cerebral artery. Umbilical artery Doppler measurements were obtained from a free-floating loop of the umbilical cord. The cerebra-placental index was calculated by the ratio of the middle cerebral artery pulsatility index and the umblical artery pulsatility index. Reference Oros, Ruiz-Martinez and Staines-Urias20 Percentile values of the pulsatility index measurements for umblical artery, middle cerebral artery, ductus venosus, and cerebra-placental index were calculated. Reference Ciobanu, Wright, Syngelaki, Wright, Akolekar and Nicolaides17 Fetuses with umblical artery pulsatility index >95th centile, middle cerebral artery pulsatility index <5th centile, cerebra-placental index <5th centile were noted.
For the cardiac functional measurements, the apical four-chamber view of the heart was obtained on a transverse cross-section of the fetal thorax. The following cardiac Doppler parameters were determined: tricuspid valve early and late diastolic filling ratio, mitral valve early and late diastolic filling ratio, pulmonary vein pulsatility index, and ductus venosus pulsatility index. For measuring the early and late diastolic filling ratio through the atrioventricular valves, the blood flow velocities through mitral and tricuspid valve orifices were obtained by placing the spectral Doppler gate inside the ventricle just under each valve (Figs 1 and 2). Subsequently, after visualisation of the pulmonary veins using coluor Doppler, the spectral Doppler gate was placed over the distal portion of the right upper pulmonary vein just before its entrance into the left atrium (Fig 3). Ductus venosus Doppler measurements were obtained through a sagittal plane of the upper fetal abdomen. After identifying the ductus with colour Doppler, the spectral Doppler gate is placed over the area of the fastest blood with the smallest insonation angle. Percentile values of the pulsatility index measurements for pulmonary veins and ductus venosus were calculated. Reference Lenz and Chaoui21,Reference Kessler, Rasmussen, Hanson and Kiserud22 Fetuses who have pulmonary vein pulsatility index >95th percentile and ductus venosus pulsatility index >95th percentile were noted.

Figure 1. Blood flow through mitral valve. Mitral valve (MV), early diastolic filling (e), late diastolic filling (a).
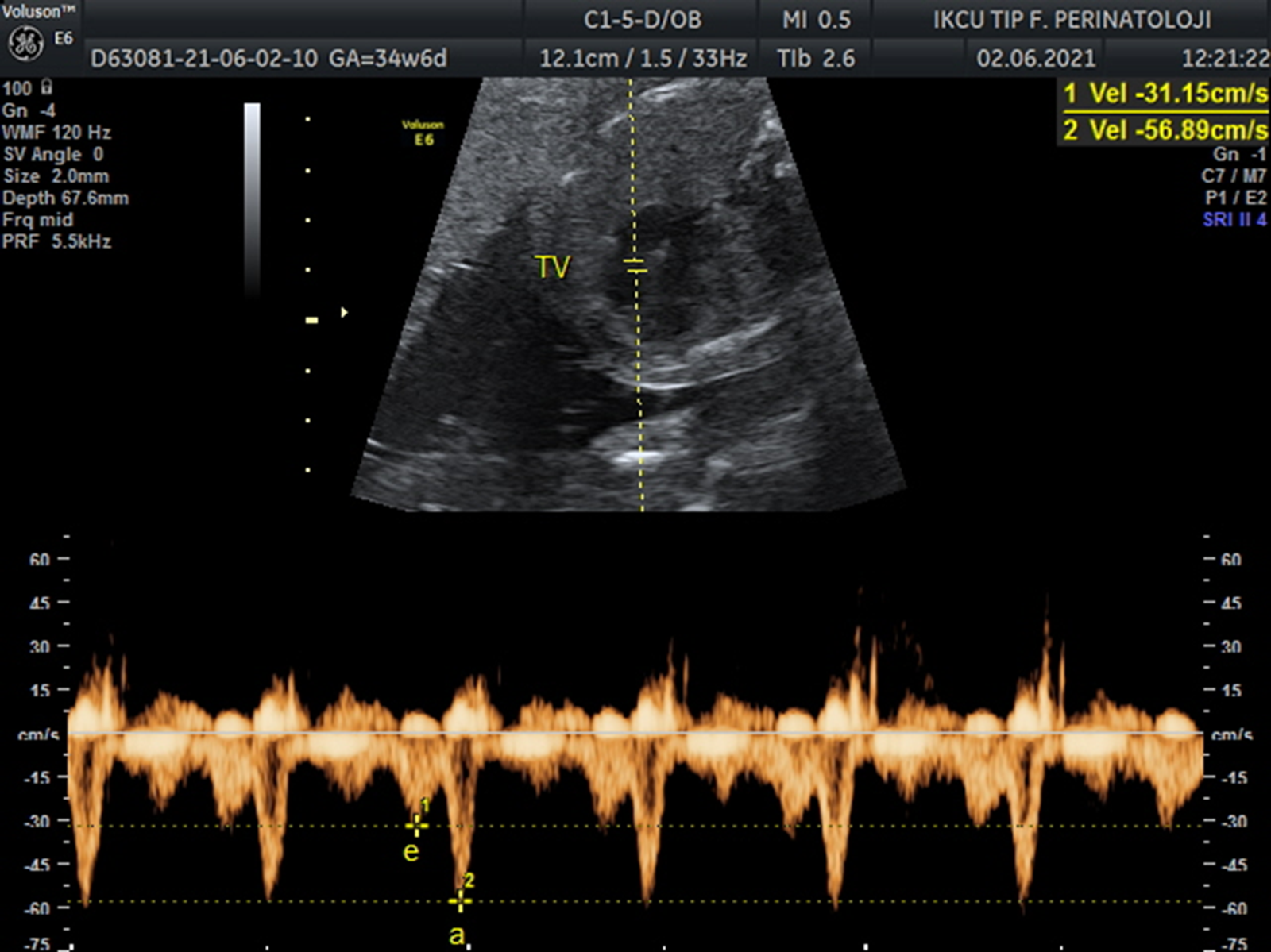
Figure 2. Blood flow through tricuspid valve. Tricuspid valve (TV), early diastolic filling (e), late diastolic filling (a).

Figure 3. Blood flow through right upper pulmonary vein. Right pulmonary vein (PV), systolic flow (S), diastolic flow (D), atrial contraction (a).
For each Doppler parameter (fetoplacental, cardiac) to be measured, at least three similar consecutive cardiac cycle waveforms were obtained during periods with fetal apnea and by keeping the insonation angle with the examined vessel or atrioventricular valve below 30°.
For middle cerebral artery and umblical artery, the pulsatility index was calculated automatically by the ultrasound systems according to the formula: (Peak systolic velocity – Peak diastolic velocity /Mean velocity), which was described by Gosling et al. Reference Gosling, Dunbar and King23
For ductus venosus and pulmonary veins, the pulsatility index for veins was calculated automatically by the ultrasound systems according to the formula: (pulsatility index for veins = (Peak systolic velocity − The velocity during atrial contraction)/Mean velocity) which was described by Hecher et al. Reference Hecher, Campbell, Snijders and Nicolaides24
Statistical analysis
Data were analysed using SPSS version 20.0 (IBM. Armonk, NY, USA).
Normal distributions of continuous variables were assessed by Kolmogorov–Smirnov test. Independent samples t-test or Mann–Whitney U test was used to compare the numerical variables, and Fischer’s exact test was used to compare the categorical data. The descriptive variables were expressed as mean ± standard deviation for the normally distributed data, or median and the interquartile range for the non-normally distributed data. A p value equal to or less than 0.05 was considered to be statistically significant. The 95% confidence interval was calculated wherever appropriate.
Ethical approval
The University’s Research Ethics Committee approved the study protocol (Reference: 2021-KAE-0046). All participants were informed about the study and written informed consent was provided by each participant.
Results
Characteristics of the patient population
A total of 45 appropriate-for-gestational-age or macrosomic fetuses with poorly controlled gestational diabetes mellitus were matched by gestational age and maternal age to 47 controls. Mean maternal age, gestational weeks, pregnancy number, and parity were comparable between the two groups. The comparison of the maternal characteristics between the gestational diabetes group and controls is depicted in Table 1.
Table 1. Comparison of maternal demographic characteristics between the gestational diabetes mellitus and control groups

GDM, gestational diabetes mellitus.
Fetal placental Doppler measurements
The rate of fetuses who have abnormal fetoplacental Doppler measurements (umblical artery pulsatility index >95th percentile, middle cerebral artery pulsatility index <5th percentile, cerebra-placental index <5th percentile) were comparable in both groups. The comparison of the fetoplacental Doppler parameters between the gestational diabetes mellitus group and controls is depicted in Table 2.
Table 2. Comparison of fetoplacental Doppler measurements between the gestational diabetes mellitus and control groups

CPI = cerebra-placental index; GDM = gestational diabetes mellitus; MCA-PI = middle cerebral artery pulsatility index; UA-PI = umbilical artery pulsatility index.
Diastolic functions
In this study, no reverse flow was found during atrial contraction. Cardiac diastolic functions measured by mitral valve early and late diastolic filling ratio, tricuspid valve early and late diastolic filling ratio, pulmonary vein pulsatility index, and ductus venosus pulsatility index were not statistically different between the study and the control groups. Additionally, the rate of abnormal Doppler findings in pulmonary vein (pulmonary vein pulsatility index >95th centile), ductus venosus (ductus venosus pulsatility index >95th centile) were comparable between the groups.
The comparison of the cardiac Doppler parameters between the gestational diabetes mellitus group and controls is depicted in Table 3.
Table 3. Comparison of fetal diastolic functions between the gestational diabetes mellitus and control groups

CI = confidence interval; DV-PI = ductus venosus pulsatility index; GDM = gestational diabetes mellitus; PV-PI = pulmonary vein pulsatility index; MV = mitral valve; TV = tricuspid valve;.
Discussion
Fetal cardiovascular dynamics in diabetic pregnancies have been studied over the past years. However, less effort has been focused on cardiac functions in specific maternal and fetal subgroups of this vulnerable population. The data presented in this study revealed that compared to gestational age-matched normal controls, the fetal third trimester fetal cardiac diastolic functions measured by early and late diastolic filling ratio of both atrioventricular valves, pulmonary vein pulsatility index, and ductus venosus pulsatility index did not significantly differ in appropriate-for-gestational-age or macrosomic fetuses of gestational diabetic women who have poor glycaemic control.
Many studies have focused on evaluating values of the fetal cardiac functions in diabetic pregnancies for predicting fetal deterioration. Reference Russell, Foley, Kinsley, Firth, Coffey and McAuliffe10,Reference Fouda, Abou ElKassem, Hefny, Fouda and Hashem11,Reference Aguilera, Semmler and Coronel25–Reference Jaeggi, Fouron and Proulx30 However, these studies have displayed inconsistent results. Although not performed to cover a specific fetal subgroup as our study, the results of the previous two studies were in line with the findings of our study. Reference Aguilera, Semmler and Coronel25,Reference Miranda, Cerqueira, Ramalho, Areias and Henriques-Coelho26 In both studies, no significant difference in conventional and tissue Doppler third trimester fetal diastolic cardiac functional measurements was reported between women with gestational diabetes mellitus and controls. Similarly, in another study, Kooijman et al. examined the associations of an adverse metabolic profile in early pregnancy with placental, fetal cerebral, and cardiac haemodynamic development. They reported that no associations of maternal glucose concentrations with third trimester fetal cerebral, placental, and cardiac haemodynamics (mitral valve early and late diastolic filling ratio, ascendant aorta peak systolic velocity and diameter, left cardiac output) were present. Reference Kooijman, Jaddoe, Steegers and Gaillard27
Unlike our study, in a previous study where the fetal cardiac diastolic functions of mothers with diabetes were assessed, Zielinsky et al reported that compared to normal controls, fetuses of diabetic mothers have significantly lower left atrial shortening fraction, higher pulmonary vein pulsatility index, and an impaired left ventricular diastolic function probably as a result of myocardial hypertrophy. In their study, the type of diabetes, whether gestational or pre-gestational, was not mentioned, no subgroup distinction was made regarding fetal characteristics, and the sample size was relatively small (14 fetuses of diabetic mothers and 26 fetuses of normal control mothers). Reference Zielinsky, Nicoloso and Firpo28 Similarly, in some other studies, those who are focused on fetal diastolic function in maternal diabetes mainly showed evidence of impaired diastolic function as a result of decreased relaxation and compliance of the fetal myocardium. Reference Russell, Foley, Kinsley, Firth, Coffey and McAuliffe10,Reference Fouda, Abou ElKassem, Hefny, Fouda and Hashem11,Reference Tsyvian, Malkin, Artemieva and Wladimiroff29,Reference Jaeggi, Fouron and Proulx30 Most of these studies assessed the effect of maternal diabetes in unspecified fetal subgroups or only in fetal growth restriction. We thought that, unlike these previous studies, the lack of difference observed in terms of diastolic functions between the study and the control group in our study might be due to the inclusion of only specific subgroup fetuses, those are appropriate-for-gestational-age or macrosomic and also who have comparable fetal–placental flow with the controls.
Previous studies showed that due to the dominance of the right heart in late gestation, the fetal cardiac morphological and functional changes secondary to gestational diabetes mellitus are mostly noted in the right ventricle. Reference Aguilera, Semmler and Coronel25,Reference Miranda, Cerqueira, Ramalho, Areias and Henriques-Coelho26,Reference Patey, Carvalho and Thilaganathan31 We thought that the lack of difference between the two groups in our study might also be related to the fact that the pulmonary vein pulsatility index, which was used for assessing diastolic function, mainly indicates left heart functions.
Cardiac functional changes in fetuses of women with gestational diabetes mellitus can be a reaction to the effects of fetal hypoxaemia. Reference Escobar, Teramo and Stefanovic32 It is known that fetal hypoxaemia is a more evident and specific finding in fetuses with placental dysfunction and fetal growth restriction. For this reason, diabetic women who have concomitant vasculopathy are at higher risk for the development of fetal hypoxaemia and fetal growth restriction, which fetoplacental Doppler measurements may identify. Previous studies show that in the absence of vasculopathy, normal fetal placental resistance can be expected in most pregnancies complicated by diabetes. Reference Dicker, Goldman, Yeshaya and Peleg3,Reference Johnstone, Steel, Haddad, Hoskins, Greer and Chambers4 We thought that the lack of the difference in diastolic functions between the study and the control group in our study might be related to the fact that fetuses likely to have impaired fetoplacental circulation due to fetal growth restriction were not included.
It is thought that the effect of the type of maternal diabetes, either pre-gestational diabetes mellitus or gestational diabetes mellitus, on fetal haemodynamic parameters may be different. Rizzo et al reported that, in insulin-dependent pre-gestational diabetic patients, evidence of progressive and dramatic changes in the intracardiac and venous flow patterns occurred during the first half of pregnancy. They thought that these changes were more significant than those occurring in late pregnancy, suggesting a period of rapid development of the fetal heart in which maternal hyperglycaemia may adversely affect the early development of the fetal heart in that period. Reference Rizzo, Arduini, Capponi and Romanini6 In another study, Fouda et al evaluated fetal cardiac functions in pregnant women with pre-existing diabetes, gestational diabetes, and normoglycaemia. They reported that fetal diastolic functions were impaired in the pre-existing diabetes group, whereas there was no impairment of diastolic function in the gestational diabetes group. They suggested that in pre-existing diabetes, fetuses are exposed to hyperinsulinemia during the whole pregnancy. In contrast, in gestational diabetes mellitus, hyperinsulinemia occurs only during the third trimester, and fetuses are not exposed to hyperglycaemia during early fetal cardiac development, where the effect of hyperglycaemia is maximum. Reference Fouda, Abou ElKassem, Hefny, Fouda and Hashem11 In our study, all of the patients in the study group have diabetes which was first diagnosed in pregnancy at more advanced gestational ages, so-called gestational diabetes mellitus. The fetal diastolic functions were not impaired compared to normal controls emphasising the idea that earlier gestational metabolic changes are more evident on fetal cardiac functions than those appearing later, as mentioned by the authors above.
In maternal diabetes, subclinical unfavourable changes in fetal myocardial function due to maternal hyperglycaemia may not be demonstrated by conventional echocardiography. This is because conventional techniques mainly provide indirect information about cardiac functions, whereas advanced echocardiographic modalities can measure the deformation of the myocardium itself. In their study, Miranda et al assessed third trimester fetal cardiac functions in maternal diabetes and control group by conventional echocardiography and speckle-tracking echocardiography. They found no significant difference in conventional systolic and diastolic functional parameters for the fetal left and right ventricle, except for lower left ventricular cardiac output in the maternal diabetes group. However, they showed that fetuses of mothers with diabetes present signs of biventricular diastolic dysfunction and right ventricular systolic dysfunction with the deformation analysis measured using speckle-tracking echocardiography. Reference Miranda, Cerqueira, Ramalho, Areias and Henriques-Coelho26
Similarly, Bayoumy et al. showed that global longitudinal strain of the left ventricle, the tricuspid annular plane systolic excursion, and annular tissue Doppler velocity were significantly lower in fetuses of diabetic and obese women compared to fetuses of normal women. However, using standard echocardiography to assess fetal cardiac performance (Mitral and tricuspid annular plane systolic excursions, mitral and tricuspid early velocity, late velocitiy, and early and late diastolic filling ratio), they demonstrated that all fetuses seem to be healthy. Reference Bayoumy, Habib and Abdelmageed33 Additionally, Kulkarni et al also reported that two-dimensional speckle-tracking echocardiography measures of myocardial function in fetuses of diabetic and obese women were generally more consistent than traditional Doppler echocardiography parameters. Reference Kulkarni, Li and Craft34 In this study, conventional echocardiographic techniques were used to assess fetal diastolic functions. We thought that the subclinical cardiac changes in cardiac function in fetuses women with gestational diabetes mellitus could be measured by more advanced echocardiographic techniques such as speckle-tracking echocardiography and tissue Doppler imaging.
This study has several limitations. First, the sample size is small, and fetal cardiac functions are studied only in a specific subgroup of maternal and fetal conditions of diabetic women. A more extensive study, including women with pre-gestational diabetes and fetal growth restriction, is needed to corroborate our results and evaluate conventional Doppler indices' role (The early and late diastolic filling ratio of the atrioventricular valves, precordial vein pulsatility indices) in the prediction of fetal diastolic function. Second, due to the cross-sectional design of the study, the relationship between cardiac functions and advancing gestational week could not be evaluated. However, gestational week differences between patients were reduced by selecting the entire study group among the third trimester pregnant women. Third, the cross-sectional nature of this study does not allow the evaluation of the outcome of the fetuses
In conclusion, the data presented in this study revealed that the third-trimester fetal diastolic functions measured by selected conventional Doppler techniques did not significantly differ in appropriately grown or macrosomic fetuses of gestational diabetic women who have poor glycaemic control compared to gestational age-matched normal controls.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors state that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.









