Introduction
The prevalence of epilepsy during pregnancy has been estimated at 0.3%–0.7%. Reference Olafsson, Hallgrimsson, Hauser, Ludvigsson and Gudmundsson1–Reference Farmen, Grundt, Nakling, Mowinckel, Nakken and Lossius9 Factors that make the care of pregnant patients with epilepsy (PPWE) distinct from pregnant patients without epilepsy (PPWoE) include the impact of seizures on maternal and fetal health, Reference Kaplan, Norwitz and Ben-Menachem10–Reference Adab, Kini and Vinten15 the recognized changes in antiseizure medication (ASM) pharmacokinetic that occur during pregnancy, Reference Nau, Zierer, Spielmann, Neubert and Ch16 and the possible teratogenicity of ASM, Reference Nau, Zierer, Spielmann, Neubert and Ch16–Reference Tomson, Battino and Bonizzoni18 which may be mitigated by the effects of folate supplementation on fetal development. Reference Tomson, Battino and Bromley19 These factors must be reconciled with the recommendation that ASM be continued throughout pregnancy. Reference Harden, Meador and Pennell20
PPWE also have unique demographics, when compared to PPWoE, higher rates of primiparity, Reference Yerby, Koepsell and Daling3,Reference Artama, Braumann and Raitanen21,Reference Viinikainen, Heinonen, Eriksson and Kälviäinen22 and a lower proportion of patients delivering at an advanced age Reference Yerby, Koepsell and Daling3,Reference Viinikainen, Heinonen, Eriksson and Kalviainen23 have both been reported. Additionally, PPWE were found to have higher rates of ectopic pregnancy, Reference Artama, Braumann and Raitanen21 hypertensive disorders of pregnancy, Reference Danielsson, Borthen, Morken and Gilhus5,Reference Viale, Allotey and Cheong-See24–Reference Richmond, Krishnamoorthy, Andermann and Benjamin30 preterm labor Reference Soontornpun, Choovanichvong and Tongsong2,Reference Artama, Braumann and Raitanen21,Reference Viale, Allotey and Cheong-See24–Reference MacDonald, Bateman, McElrath and Hernández-Díaz26,Reference Razaz, Tomson, Wikström and Cnattingius29 spontaneous and therapeutic termination, Reference Soontornpun, Choovanichvong and Tongsong2,Reference Artama, Braumann and Raitanen21,Reference Viale, Allotey and Cheong-See24,Reference Razaz, Tomson, Wikström and Cnattingius29 gestational diabetes, Reference Katz, Levy, Wiznitzer and Sheiner4,Reference Artama, Braumann and Raitanen21,Reference Mawer, Briggs and Baker31 cesarean section, Reference Olafsson, Hallgrimsson, Hauser, Ludvigsson and Gudmundsson1,Reference Soontornpun, Choovanichvong and Tongsong2,Reference Katz, Levy, Wiznitzer and Sheiner4,Reference Farmen, Grundt, Nakling, Mowinckel, Nakken and Lossius9,Reference Artama, Braumann and Raitanen21,Reference Viale, Allotey and Cheong-See24–Reference Razaz, Tomson, Wikström and Cnattingius29,Reference Yeh, Lussier, Sun, Lan, Yu and Chang32 peripartum hemorrhage, Reference Katz, Levy, Wiznitzer and Sheiner4,Reference Viale, Allotey and Cheong-See24,Reference Gyamfi-Bannerman, Huang and Bateman25,Reference Borthen, Eide, Daltveit and Gilhus27,Reference Pilo, Wide and Winbladh28,Reference Mawer, Briggs and Baker31 low neonatal birth weight, Reference Soontornpun, Choovanichvong and Tongsong2,Reference Yerby, Koepsell and Daling3,Reference Farmen, Grundt, Nakling, Mowinckel, Nakken and Lossius9,Reference Viinikainen, Heinonen, Eriksson and Kälviäinen22,Reference Viale, Allotey and Cheong-See24,Reference Razaz, Tomson, Wikström and Cnattingius29 postpartum depression, Reference H.Bjørk, Veiby, A.Engelsen and Gilhus33–Reference Reiter, Veiby, Daltveit, Engelsen and Gilhus35 and maternal mortality. Reference Edey, Moran and Nashef6,Reference Christensen, Vestergaard and Hammer Bech7,Reference MacDonald, Bateman, McElrath and Hernández-Díaz26 These findings were reported from studies performed in Scandinavia (Norway, Reference Danielsson, Borthen, Morken and Gilhus5,Reference Danielsson, Gilhus, Borthen, Lie, Morken and Lupattelli8,Reference Farmen, Grundt, Nakling, Mowinckel, Nakken and Lossius9,Reference Borthen, Eide, Daltveit and Gilhus27 Finland, Reference Artama, Braumann and Raitanen21,Reference Viinikainen, Heinonen, Eriksson and Kalviainen23 Sweden, Reference Pilo, Wide and Winbladh28,Reference Razaz, Tomson, Wikström and Cnattingius36 Denmark, Reference Christensen, Vestergaard and Hammer Bech7 Iceland), Reference Olafsson, Hallgrimsson, Hauser, Ludvigsson and Gudmundsson1 the United Kingdom (UK), Reference Mawer, Briggs and Baker31 the United States of America (USA), Reference Yerby, Koepsell and Daling3,Reference Edey, Moran and Nashef6,Reference Gyamfi-Bannerman, Huang and Bateman25,Reference MacDonald, Bateman, McElrath and Hernández-Díaz26 Israel, Reference Katz, Levy, Wiznitzer and Sheiner4 Thailand, Reference Soontornpun, Choovanichvong and Tongsong2 Taiwan, Reference Yeh, Lussier, Sun, Lan, Yu and Chang32 and Canada. Reference Richmond, Krishnamoorthy, Andermann and Benjamin30 Such increases in the rates of obstetrical complications have not been uniformly reported worldwide, however. For instance, while Iceland Reference Olafsson, Hallgrimsson, Hauser, Ludvigsson and Gudmundsson1 and Finland Reference Hiilesmaa, Bardy and Teramo12,Reference Artama, Braumann and Raitanen21 reported similar rates of gestational hypertension among PPWE, studies from the USA reported an increase in this obstetrical complication among PPWE. Reference Gyamfi-Bannerman, Huang and Bateman25,Reference MacDonald, Bateman, McElrath and Hernández-Díaz26 In addition, a cohort study from the UK Reference Mawer, Briggs and Baker31 did not replicate findings from Finland, Reference Artama, Braumann and Raitanen21,Reference Viinikainen, Heinonen, Eriksson and Kalviainen23 Norway, Reference Danielsson, Borthen, Morken and Gilhus5,Reference Farmen, Grundt, Nakling, Mowinckel, Nakken and Lossius9 and Thailand Reference Soontornpun, Choovanichvong and Tongsong2 showing increased rates of low-birth weight among the neonates of PPWE.
Given these cross-border variations, further Canadian-specific data are needed. In this study, we report the obstetrical outcomes of a cohort of Canadian PPWE receiving specialized obstetrical care in an urban tertiary care center in Toronto (ON).
Methods
Patient Selection and Data Collection
PPWE were identified from a preexisting prospectively collected database of patients seen in the maternal-fetal medicine obstetrics program at Mount Sinai Hospital (Toronto, ON, Canada) between January 1, 2014, and November 20, 2020. Epilepsy was defined according to the International League Against Epilepsy 2014 operational definition of epilepsy. Reference Fisher, Acevedo and Arzimanoglou37
The following data were collected through review of patients’ records: maternal demographics (maternal age, level of education, ethnicity, prior deliveries, and births), pregnancy data (maternal smoking, folic acid supplementation, ASM usage in the first trimester, and termination of pregnancy, and diagnosis of gestational hypertension, diabetes, or bleeding), delivery data (use of instrumentation, mode of delivery, indications for cesarean section, as well as occurrence of and treatment received for postpartum hemorrhage), and neonatal data (stillbirth, prematurity, major congenital malformation, and Neonatal Intensive Care Unit admission). Patients who were lost to follow-up after a single maternal intake visit were excluded. A minimum of one six-week postpartum visit is considered standard of care in our institution. The following variables were obtained from Statistics Canada for all reported Canadian pregnancies between 2014 and 2020: maternal age, primiparity, gestational hypertension, gestational diabetes, cesarean delivery, postpartum hemorrhage, stillbirth, and prematurity. We performed a review of the English literature available in the PubMed database using the keywords “Epilepsy,” “Pregnancy,” and “Obstetrical outcomes” to retrieve studies on the obstetrical outcomes of Canadian PPWE.
Statistical Analysis
Study sample characteristics were described using proportions and means with 95% confidence intervals. When comparing variables with the general Canadian population, we performed one-proportion Z-tests for nominal variables and one-sample T-tests for continuous variables. Multiple imputation, a statistical method that estimates the value of a missing variable based on other auxiliary variables available for a given patient, was performed with five iterations using the package mice. Reference Buuren and Groothuis-Oudshoorn38 Multiple imputation is favored against simply excluding the missing data, which is more likely to introduce censoring bias. Reference Madley-Dowd, Hughes, Tilling and Heron39 Statistical significance was determined as p < 0.05 unless otherwise specified. No correction for multiple comparisons was done due to the exploratory aims of this study. Study data were collected in REDCap (Research Electronic Data Capture). Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde40 Statistical analysis was performed with R Statistical Software version 4.1.2 (The R Foundation for Statistical Computing, 2021).
Ethical Conduct of Research
This study received approval from the Research Ethics Boards of Mount Sinai Hospital and the University Health Network (Toronto, ON, Canada).
Results
We identified 231 PPWE who had a total of 289 pregnancies (1.25 pregnancies per patient). Seven pregnancies from as many PPWE were excluded due to loss to follow-up (Fig. 1). Mean maternal age was 32.8 years (stand. dev. = 4.6). Most PPWEs were of white/European descent (Fig. 2a), married (Fig. 2b), and had some post-secondary education (Fig. 2c). Information on ethnicity was available in 67% (150/224) of patients, marital status in 90% (255/282) of pregnancies, and level of education in 57% (160/282) (Fig. 2d).
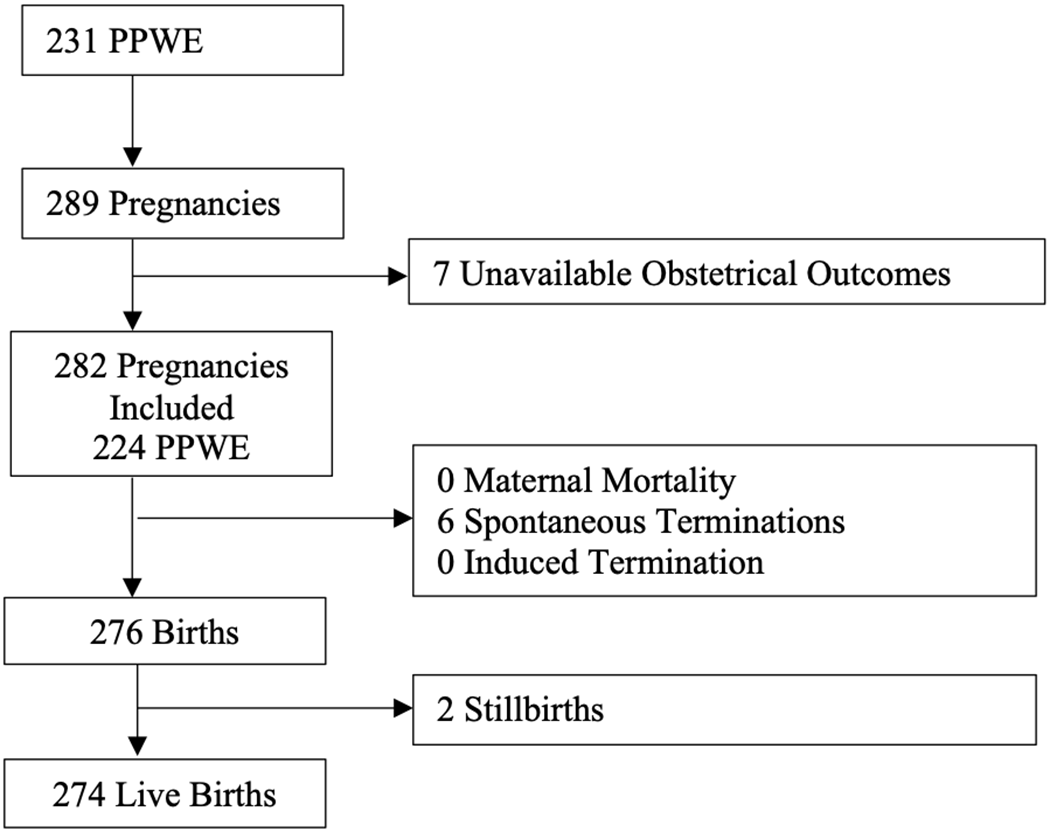
Figure 1: Study flowchart. PPWE=pregnant patients with epilepsy.

Figure 2: Demographic features of pregnant patients with epilepsy (Mount Sinai Hospital, Toronto, Canada, 2014–2020). a ) Ethnicity. b ) Marital status. c ) Educational achievement/degree. d ) Available data for each demographic variable. *No secondary education diploma/degree obtained. **Per pregnancy. NA=Not available.
A complete set of obstetrical outcome data was available for 74% of pregnancies (210/282). The variables with the largest proportion of missing data were admission to the neonatal intensive care unit (36/274; 13%), folic acid supplementation status (11%, 30/282), occurrence of stillbirth (7.6%; 21/276), major congenital malformation (6.9%; 19/274), or peripartum hemorrhage (6.5%; 18/276) (See Supplementary Material 1). The obstetrical outcomes of the cohort, after accounting for missing variables with multiple imputations, are summarized in Table 1.
Table 1: Obstetrical outcomes of patients with epilepsy in an urban tertiary care center (Mount Sinai Hospital, Toronto, Canada, 2014–2020)

MCM=major congenital malformation; NICU=neonatal intensive care unit.
a Mean.
b Standard deviation.
c Forceps or vaccum.
d Gestational age at birth<37 weeks.
Most pregnancies occurred in mothers who had previously been pregnant (35% primigravid, 98/282), but, in most cases, never previously had a live birth (55% primiparous, 150/274). Forty-five percent of deliveries were done by cesarean section (123/276), including 25% (30/123) electively (Fig. 3). While most cases of postpartum hemorrhage responded to uterotonics, a minority (29%, 4/14) required a combination of blood transfusion, mechanical tamponade, and surgical intervention. Nine newborns were found to have major congenital malformations: three congenital heart defects, one common truncus arteriosus, one transposition of the great arteries, one neural tube defect, one limb abnormality, and two non-specified congenital heart defects.
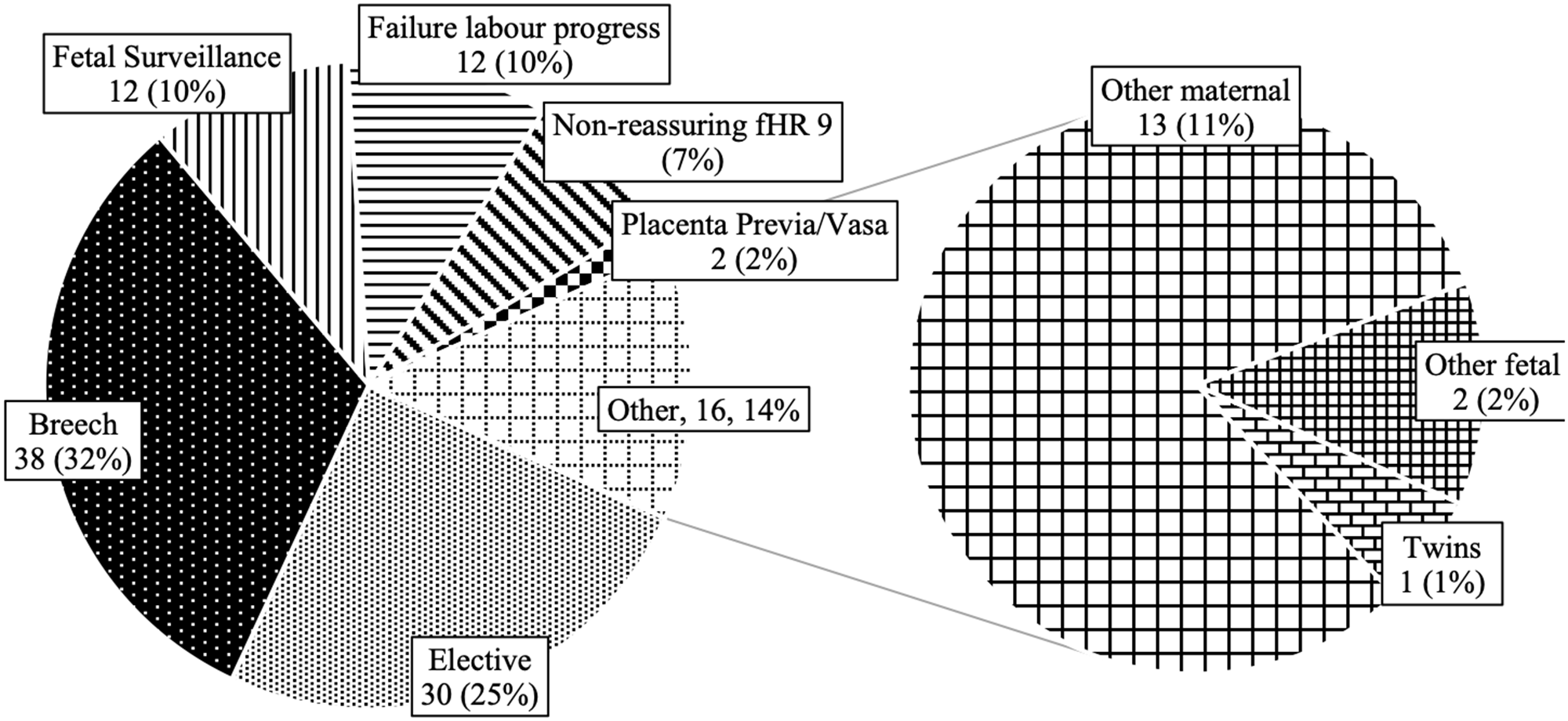
Figure 3: Indications for cesarean section delivery in pregnant patients with epilepsy (Mount Sinai Hospital, Toronto, Canada, 2014–2020). fHR=fetal heart rate.
Compared with the average Canadian pregnancy during the same period, PPWEs from this cohort were older, more likely to be primiparous, and more likely to deliver via cesarean section and have postpartum hemorrhage. They had similar incidences of gestational hypertension, stillbirth, prematurity, and a lower incidence of gestational diabetes (Table 2).
Table 2: Obstetrical outcomes of patients with epilepsy in an urban tertiary care center compared with the Canadian Population (2014–2020)
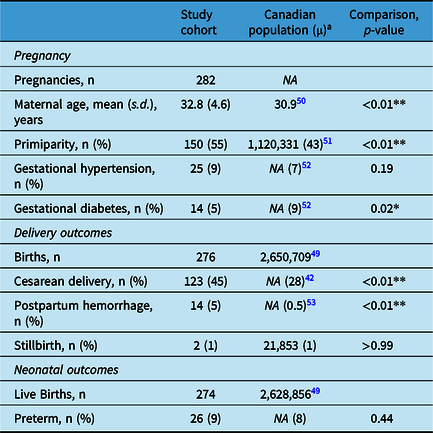
s.d.=standard deviation.
a For 2014–2020.
* p < 0.05.
** p < 0.01.
In our cohort, 85% of pregnancies were uncomplicated (241/282, excluding peripartum vaginal bleeding). Ninety-three percent (257/276) of labor and deliveries were uncomplicated, and eighty-five percent (235/276) of newborns were born at term, free from major congenital malformation, and did not require Neonatal Intensive Care Unit admission. Altogether, 68% (193/282) of pregnancies were, therefore, uncomplicated and free from obstetrical or neonatal complications.
Our review of the existing literature on the obstetrical outcomes of Canadian PPWE yielded two studies, both from Montreal (QC): one by Richmond et al. Reference Richmond, Krishnamoorthy, Andermann and Benjamin30 had a large sample size, which included a control group of PPWoE, and a second more recent by Li et al. Reference Li, Toffa and Nguyen41 had a significantly smaller sample size and included a limited number of obstetrical outcome measures (Table 3).
Table 3: Canadian studies on obstetrical outcomes of patients with epilepsy
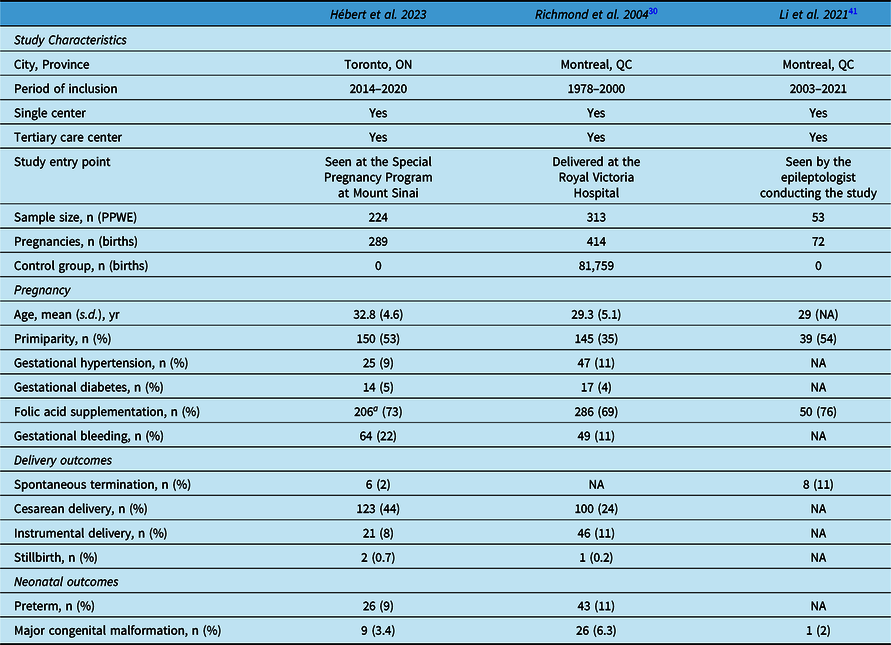
NA=not available; PPWE=pregnant patients with epilepsy; s.d.=standard deviation.
Discussion
In our cohort of PPWE from an urban obstetrical tertiary care center, the majority had uncomplicated pregnancies and deliveries. Stillbirth and major congenital malformation were rare neonatal outcomes, occurring at rates similar to that observed in national pregnancy registries from other high-income countries, Reference Danielsson, Borthen, Morken and Gilhus5,Reference Farmen, Grundt, Nakling, Mowinckel, Nakken and Lossius9,Reference Viale, Allotey and Cheong-See24,Reference Pilo, Wide and Winbladh28 and at a lower rate than previously reported in Canada between 1978–2000. Reference Richmond, Krishnamoorthy, Andermann and Benjamin30 In contrast to these encouraging data, the rates of cesarean section and postpartum hemorrhage were higher than in the general Canadian population. 42 The rate of cesarean section in our cohort of PPWE (45%) was also higher than recently reported in studies of PPWE from Norway (21%), Reference Farmen, Grundt, Nakling, Mowinckel, Nakken and Lossius9 Sweden (23%), Reference Razaz, Tomson, Wikström and Cnattingius36 and the USA (37%). Reference Gyamfi-Bannerman, Huang and Bateman25 This was also reflected in a higher rate of postpartum hemorrhage (5%) than reported in the USA (3%), Reference Gyamfi-Bannerman, Huang and Bateman25 but similar to that reported in Sweden (5%). Reference Razaz, Tomson, Wikström and Cnattingius36 The observed rate of cesarean section in our cohort of PPWE was also higher than previously reported among Canadian PPWE between 1978 and 2000. Reference Richmond, Krishnamoorthy, Andermann and Benjamin30 The incidence of deliveries by cesarean section has increased in the overall Canadian population over the last two decades 42 ; whether this increase has been more marked among PPWE than PPWoE remains to be clarified. Li et al. did not report on cesarean section rate in their more recent study. Reference Li, Toffa and Nguyen41 It is, furthermore, difficult to ascertain whether the higher rate of cesarean section observed in our cohort is representative of the overall Canadian PPWE population or, if instead, it is applicable only to the narrower subgroup of PPWE seen in tertiary care centers (i.e., referral bias). For example, the higher proportion of cesarean section may have been due to the higher maternal age of patients referred to our center, a known risk factor for cesarean section, rather than representing a true increase in the rates of cesarean section among PPWEs.
The mean maternal age of our cohort was indeed older than more recent studies of PPWE from Canada, Reference Li, Toffa and Nguyen41 the USA, Reference McElrath, Druzin and Van Marter43 and Finland. Reference Artama, Braumann and Raitanen21 Canadian PPWE may have delayed pregnancies to a larger extent than the general Canadian pregnant population, although we cannot exclude that this older maternal age is due to referral bias. Interestingly, Richmond et al. previously reported no such differences in maternal age between Canadian PPWE (mean age: 29.3 years) and PPWoE (mean age: 29.2).
In the approximately two-thirds of patients for whom data on ethnicity could be retrieved, 42% of our study participants were of non-white/European descent. By comparison, in the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs study, which took place in 20 epilepsy centers in the USA with a special focus on treating PPWE, the proportion of non-white/European patients was only 15%. Reference Pennell, French and May44 We could not retrieve comparable data on ethnicity for the overall Canadian pregnant population.
The high rate of primiparity (first live birth) observed in our cohort (53%) is consistent with observations among other studies of Canadian PPWE. Reference Richmond, Krishnamoorthy, Andermann and Benjamin30,Reference Li, Toffa and Nguyen41 It is, however, higher than recently reported among PPWE in Sweden (36.4%) Reference Razaz, Tomson, Wikström and Cnattingius36 and Norway (44.5%). Reference Farmen, Grundt, Nakling, Mowinckel, Nakken and Lossius9 This may be due to patients with epilepsy choosing to have smaller families because of, real or perceived, safety concerns and unease about “transmitting” epilepsy to their children. Delays in having a first child may also be engendered by the need to optimize ASM regimen before conception. Again, referral bias may play a role in the observed high rates of primiparity in our cohort, with patients who previously experienced difficulties conceiving being more likely to attend a tertiary obstetrical care center.
When compared with the only Canadian study reporting on this outcome among PPWE, the rates of gestational hypertension and diabetes were similar in our cohort. Reference Richmond, Krishnamoorthy, Andermann and Benjamin30 The proportion of active smokers observed in our cohort (5%) was lower than the population average among women of childbearing age during the same period (14%). 45,46 Whether this reflects a lower incidence of smoking in PPWE when compared with PPWoE, or a more widespread practice of tobacco weaning upon planning pregnancy is difficult to ascertain. Prior data in the general Canadian pregnant population indeed showed lower rates of smoking among pregnant patients than in the general population of women of childbearing age. Reference Al-Sahab, Saqib, Hauser and Tamim47,Reference Erickson and Arbour48 Maternal smoking prevalence in PPWE was not reported in the two aforementioned Canadian studies of PPWE. Reference Richmond, Krishnamoorthy, Andermann and Benjamin30,Reference Li, Toffa and Nguyen41
More patients were taking folic acid in our cohort than previously reported by Richmond et al. in 2004, Reference Richmond, Krishnamoorthy, Andermann and Benjamin30 but less than recently reported by Li et al. Reference Li, Toffa and Nguyen41 in 2021 from their cohort of patients seen by a single epileptologist at a specialized epilepsy program. This suggests an increased awareness of the role of folic acid supplementation since 2004, and the possible benefits of follow-up with specialized epilepsy care.
Limitations
Our cohort of patients was selected from an urban obstetrical tertiary care center via a high-risk pregnancy clinical program; our results may not be generalizable to the overall Canadian population of PPWE, especially PPWE living in rural settings whose care may be more likely to be provided by a primary care provider. Due to the primary obstetrical focus of the source database for this study, epilepsy-specific data (e.g., ASM usage and epilepsy type) were not available. A prospective study design may allow more comprehensive data collection. In particular, data on ethnicity, folic acid supplementation, or whether pregnancies were planned or unplanned could be collected prospectively. Finally, future studies should consider including a control group of PPWoE within their design rather than relying on population-based data, to avoid potential biases introduced when comparing outcomes from heterogenous populations.
Conclusions
In this cohort of Canadian PPWE from an urban tertiary care center, observed rates of obstetrical complications were low and, with the exception of cesarean section and postpartum hemorrhage, no higher than the average Canadian pregnancy over the same period. Future prospective studies are needed to complete a more accurate representation of the obstetrical care and outcomes of Canadian PPWE.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cjn.2023.254.
Competing interests
JH received salary support during his work on this study through a grant from the American Epilepsy Society. YI received a Women’s Health Bursary from the University of Toronto for his work on this study. There are no other conflicts of interest to report.
Statement of authorship
JH: Study conception and design, analysis and interpretation of data, manuscript drafting and revising.
YI: Data collection and interpretation, manuscript drafting and revising.
SN: Data collection and interpretation, manuscript revising.
JL: Data collection and interpretation, manuscript revising.
JS: Study conception and design, interpretation of data, critical manuscript revising.
EB: Study conception and design, interpretation of data, critical manuscript revising, funding acquisition.








